Abstract
Neuroimaging studies have shown that the visual cortex of visually impaired humans is active during tactile tasks. We sought to determine if this cross-modal activation in the primary visual cortex is correlated with vision loss in individuals with retinitis pigmentosa (RP), an inherited degenerative photoreceptor disease that progressively diminishes vision later in life. RP and sighted subjects completed three tactile tasks: a symmetry discrimination task, a Braille-dot counting task, and a sandpaper roughness discrimination task. We measured tactile-evoked blood oxygenation level dependent (BOLD) responses using functional magnetic resonance imaging (fMRI). For each subject, we quantified the cortical extent of the tactile-evoked response by the proportion of modulated voxels within the primary visual cortex (V1) and its strength by the mean absolute modulation amplitude of the modulated voxels. We characterized vision loss in terms of visual acuity and the areal proportion of V1 that corresponds to the preserved visual field. Visual acuity and proportion of the preserved visual field both had a highly significant effect on the cortical extent of the V1 BOLD response to tactile stimulation, while visual acuity also had a significant effect on the strength of the V1 response. These effects of vision loss on cross-modal responses were reliable despite high inter-subject variability. Controlling for task-evoked responses in the primary somatosensory cortex (S1) across subjects further strengthened the effects of vision loss on cross-model responses in V1. We propose that such cross-modal responses in V1 and other visual areas may be used as a cortically localized biomarker to account for individual differences in visual performance following sight recovery treatments.
Keywords: Retinitis pigmentosa, fMRI, cross-modal plasticity, primary visual cortex, primary somatosensory cortex, tactile-evoked response
1 Introduction
The human visual cortex responds to vision loss in remarkable ways. Even in adults, cross-modal responses develop in partially-sighted individuals, where their visual cortex becomes active during non-visual tasks. Characterizing these responses can provide us with a cortically localized biomarker for vision loss, which may be useful for assessing sight restoration treatments. Since it is probable that cross-modal plasticity will have a negative impact on an individual’s ability to adapt to vision restoration (Lee et al., 2001), understanding the relationship between vision loss and cross-modal responses may help us predict how an individual will respond to treatment.
Cross-modal activations have been described in early and late-blind groups (Amedi et al., 2003, 2010; Buchel et al., 1998; Burton 2003; Cheung et al., 2009; Merabet et al., 2006; Ptito et al., 2005; Sadato et al. 1996, 2004; Sathian 2005). However, variability among individuals and correlations between severity of vision loss and amount of cross-modal responses is poorly understood. Tactile discrimination studies of blind have shown increased activation of occipital areas when compared to their sighted counterparts (Amedi et al., 2010; Cheung et al., 2009). Sadato et al. (1996) described activation of the primary and secondary visual cortical areas in early-blind subjects during a Braille reading task, and deactivation of those regions in a sighted control group. A similar pattern of tactile-evoked visual cortex activation was observed in sighted subjects after being blindfolded for five days and was reversed within 24 hours of removing the blindfold (Merabet et al., 2008). The presence of these occipital tactile-evoked activations is well-established, but the cause is in dispute (Burton 2003; Merabet et al., 2008; Pascual-Leone et al., 2001; Smirnakis et al., 2007; Wandell and Smirnakis, 2009). Nevertheless, tactile-evoked response in visual cortex may still be valuable as a cortically localizable physiological marker for assessing the effect of vision loss on V1. For such applications, cross-modal responses should correlate with vision loss, and the effect size should be larger than the individual variation of cross-modal responses in the normally-sighted population.
The state of V1 and other visual areas after vision loss can affect the efficacy of sight recovery treatment. The outcome of sight restoration procedures (such as implantation with a retinal prosthesis) often varies for reasons that are not fully understood (Humayun et al., 2012). Ocular imaging can measure the position of a prosthesis in the eye and psychophysics can record patient perceptions during stimulation, but no direct measures of brain activity are available. Functional imaging of cross-modal responses may provide part of the critical data that could account for the individual differences to treatment. For example, a decrease in tactile-evoked responses in the part of V1 that corresponds retinotopically to the implant would suggest that V1 is effectively driven by the signals evoked by the retinal stimulation. Conversely, undiminished tactile-evoked responses in V1 may indicate less effective stimulation. Similar analysis may be applied to other visual areas, provided these areas can be sufficiently localized in a blind subject (Benson et al., 2012; Henriksson et al., 2012).
In this study, we took the first step towards these goals. We sought to determine if a basic relationship exists between severity of vision loss and the extent and strength of tactile-evoked V1 responses in late-blind individuals with retinitis pigmentosa (Hamel 2006). We used three simple tactile experiments to elicit a strong cross-modal response in primary visual cortex and found that the cross-modal response is significantly linked to degrees of vision loss across individuals with retinitis pigmentosa (RP), a family of inherited degenerative photoreceptor disorders that has been a target for several experimental sight-recovery treatments.
2 Materials and Methods
2.1 Participants
Eighteen subjects participated in the study (9 sighted individuals and 9 individuals diagnosed with retinitis pigmentosa) having a mean ± SD age of 45.11 ± 13.78 years (range: 21–67 years); sighted control subjects were sex-matched and had a similar age range (24–66 years) to the RP subjects (21–67 years, Table 1). Five additional individuals with RP were also enrolled in the study but were excluded from our data analysis for the following reasons: two subjects were unable to perform the specified tasks correctly; a third subject was removed from the scanner after he exhibited claustrophobic symptoms; a fourth subject had posture restrictions that forced the use of a larger single-channel circular-polarization coil in place of our typical multi-channel coil, resulting in poor data quality; and a fifth subject was excluded from all analyses after we were unable to obtain consistent visual field measurements. Individuals were recruited from the community and received monetary compensation for their participation. The study received approval from the University of Southern California’s University Park Campus Institutional Review Board and all subjects provided written informed consent after explanation of the nature and possible consequences of the study. MRI experiments were conducted at the USC David and Dana Dornsife Cognitive Neuroscience Imaging Center, while additional visual acuity and Goldmann visual field measurements were obtained by ophthalmologists and study staff at the USC Doheny Vision Research Center. This research followed the tenets of the Declaration of Helsinki.
Table 1.
Normally sighted subjects S1-S9 served as sighted, sex-matched control subjects for RP subjects RP1-RP9. L.P. = light perception only. Subjects RP2 and RP4 (described as having a R/L handedness) were ambidextrous and chose to use their right and left hand, respectively, to complete the tasks.
| Subject ID |
Age, Sex |
Visual Acuity |
Years since onset of symptoms |
Can Subject Read Braille? |
Handedness (R/L) |
Diagnosis | Additional Description of Vision |
|---|---|---|---|---|---|---|---|
| RP1 | 41, F | L.P. | 22 | Yes | R | RP | Light perception only |
| RP2 | 52, M | 20/60 | 46 | No | R/L | RP | Partial tunnel vision; cataract removal from both eyes |
| RP3 | 67, M | L.P. | 24 | No | R | RP | Light perception only |
| RP4 | 43, M | 20/800 | 38 | Yes | R/L | RP | Partial tunnel vision in right eye |
| RP5 | 58, M | 3/200 | 23 | No | R | RP | Cataract removal from both eyes |
| RP6 | 57, F | 20/50 | 32 | Yes | R | RP | Cataract removal from both eyes |
| RP7 | 24, F | 20/25 | 11 | No | L | RP | Partial tunnel vision |
| RP8 | 51, F | 20/40 | 44 | No | R | RP | Loss of peripheral vision, night blindness, blurred vision in right eye |
| RP9 | 21, M | 20/20 | 1 | No | L | RP | Beginning loss of peripheral vision and some night blindness |
| S1 | M, 53 | 20/20 | --- | No | R | --- | Sighted |
| S2 | 43, M | 20/20 | --- | No | R | --- | Sighted |
| S3 | 51, F | 20/20 | --- | No | R | --- | Sighted |
| S4 | 24, M | 20/20 | --- | No | R | --- | Sighted |
| S5 | 30, M | 20/20 | --- | No | R | --- | Sighted |
| S6 | 34, F | 20/20 | --- | No | R | --- | Sighted |
| S7 | 40, F | 20/20 | --- | No | R | --- | Sighted |
| S8 | 57, F | 20/20 | --- | No | R | --- | Sighted |
| S9 | 66, M | 20/20 | --- | No | R | --- | Sighted |
All RP subjects were diagnosed with a typical form of retinitis pigmentosa, in which degradation of photoreceptors leads to varying degrees of peripheral vision loss. Diagnosis of RP was confirmed by each individual’s primary ophthalmologist and additional information regarding the individuals’ vision was obtained from their most recent medical records after receiving HIPAA authorization from the subjects. Subject RP8 (female, age 51) also had untreated cataract in her left eye, while subjects RP2 (male, age 52), RP5 (male, age 58) and RP6 (female, age 57) underwent successful cataract removal surgery prior to participating in the study. Following informed consent, the visual field of all RP subjects were measured during a Goldmann visual field examination (except subject RP6 whose visual field information was obtained from her recent medical records). Four RP subjects completed visual acuity exams on-site, while visual acuity information for the other five RP subjects was obtained from their recent medical records. RP subjects with minimal light perception only (whose visual acuity could not be measured) were assigned a visual acuity of 20/20000 (LogMAR = 3) for analysis purposes.
The RP group was divided into “Low Vision” (n = 5) and “Blind” (n = 4) subgroups. Those in the “Blind” subgroup were subjects whose visual acuity was worse than 20/200 (LogMAR = 1), a definition of legally blind. All remaining RP subjects were placed in the “Low Vision” subgroup.
2.2 Experimental stimuli and tasks
Both sighted and RP groups completed the same three tactile tasks in the following order: 1) a shapes task requiring subjects to determine if any of a series of raised-line shapes was bilaterally symmetric, 2) a Braille-dot counting task in which subjects counted the number of dots in a series of random Braille letters (subjects were not asked to read the letters), and 3) a sandpaper task requiring individuals to determine the relative roughness between a strip of sandpaper and the sandpaper disc surrounding it (Figure 2D). Each subject was given a sheet composed of 4 columns and 5 rows of tactile elements spaced approximately 25 mm apart, for a total of 20 tactile elements per sheet. Each sheet was attached to a plastic clipboard and handed to the subject by the experimenter before each functional scan, after which the subject placed his/her dominant hand in a “ready position” on the bottom left-hand corner of the sheet until the task began. Subjects RP2 and RP4 were ambidextrous and chose to use their right and left hand, respectively, to complete the tasks. Subjects completed two sheets for each task, where the second sheet consisted of the same tactile elements as the first in a rearranged order. Design of the shapes and Braille elements were based on a tactile stimuli setup used by Cheung et al. (2009) during tactile experiments in one low-vision subject.
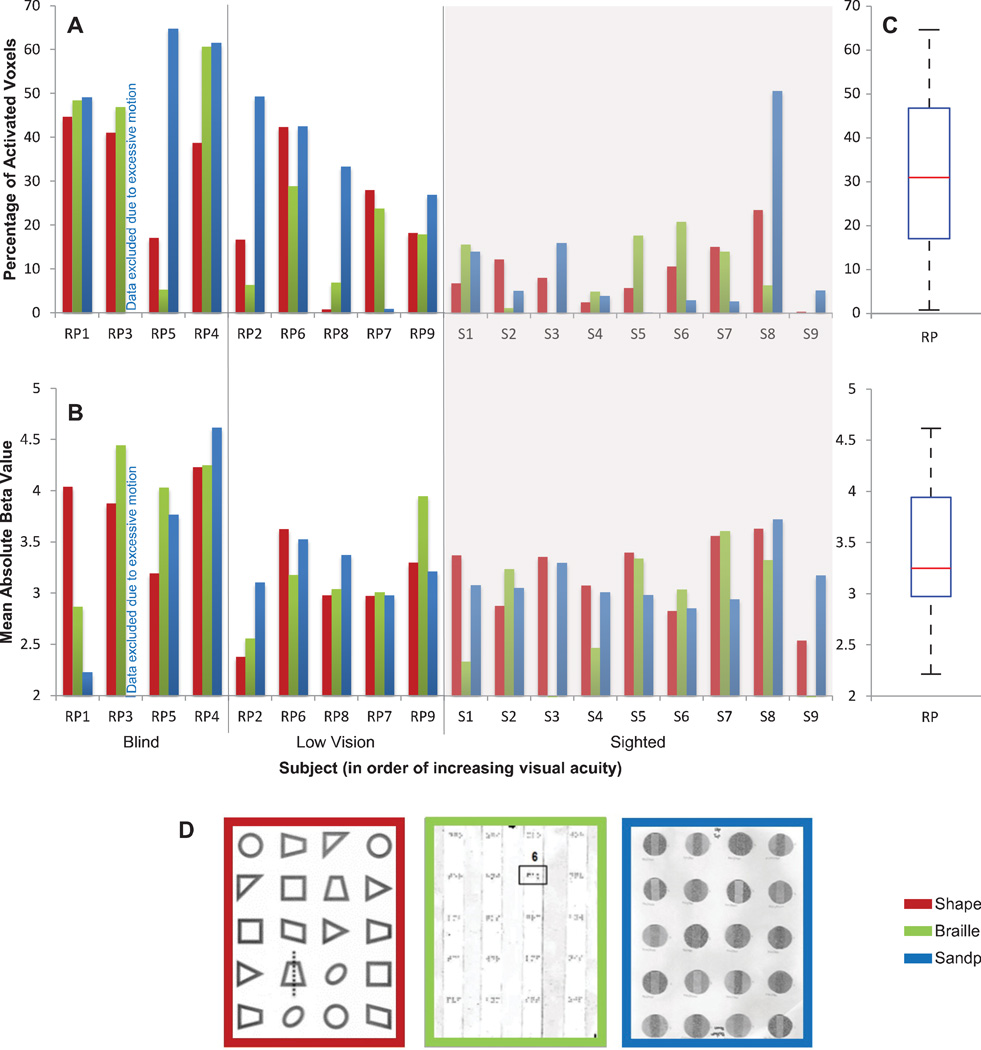
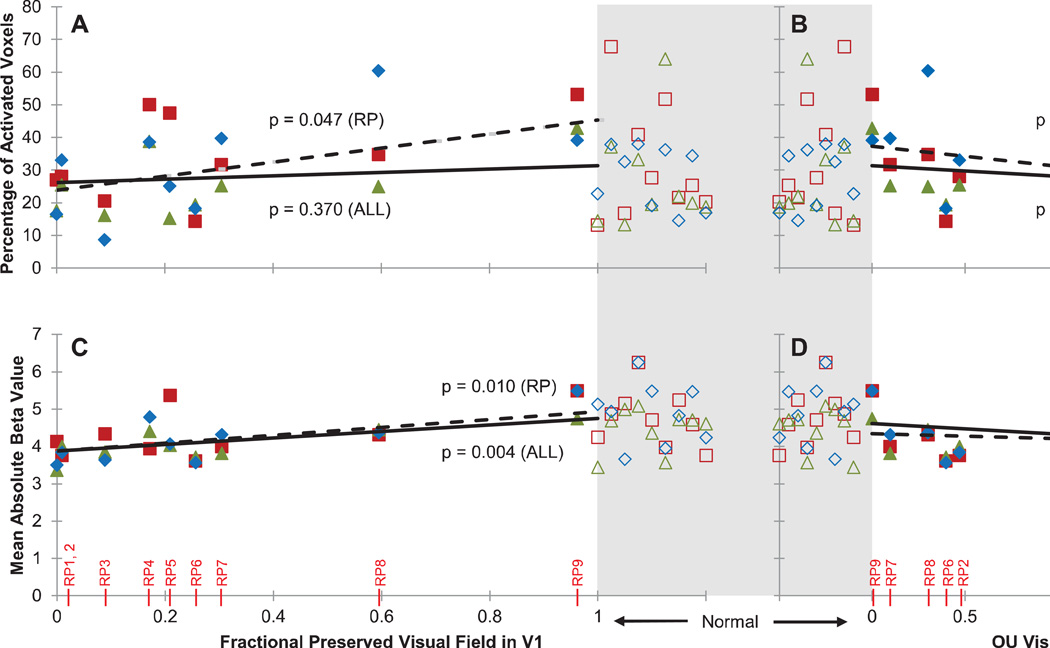
Figure 2. Extent and strength of tactile-evoked responses in V1.
A: The extent of tactile-evoked BOLD responses in V1, measured in terms of the percentage of modulated voxels (FDR < 0.05) in V1 for each subject and each task. RP subjects are ranked along the x-axis in descending order of severity of visual field loss. Sighted controls are grouped against a gray background. B: The strength of tactile-evoked BOLD responses in V1, measured in terms of mean absolute beta value of the significantly modulated V1 voxels for each subject and each task. C: Boxplots illustrating the distributions of the areal percentage (top) and mean absolute beta value (bottom) of modulated V1 voxels in RP and sighted control groups across all tasks. The red line indicates the median within each group, the edges of the boxes indicate the 25th and 75th percentiles, and the whiskers illustrate the extreme datapoints, excluding outliers (red data points). D: Example stimuli of the three tactile tasks, with shapes in red, Braille in green, and sandpaper in blue.
The tasks were performed in a block design paradigm, in which individuals scanned a column during active blocks and rested their fingers in the empty space between columns during rest blocks. Each run was composed of four 20s active blocks (one active block per column) and five 20s rest blocks. These blocks were interleaved, with the run starting and ending on a rest block. Two runs were completed for each task, with each run lasting a total of 180s. Subjects were given 4s per tactile element (for a total of 20s per active block/column) for determining symmetry, number of dots, or relative roughness and were instructed to either explore the tactile elements or rest between columns. We chose to use these simple tasks due to challenges associated with test subjects learning difficult tasks.
Subjects wore headphones and auditory instructions were given under computer control using a text-to-speech function. These instructions also indicated when subjects should move from one tactile element to another in a column; the auditory instructions were presented during both rest and active blocks. Participants did not report their answers during scanning. All subjects were asked to keep their eyes open while wearing a light-excluding eye mask (made of black molded cell foam and nylon interlock fabric with a contoured rim) throughout the task. This “eyes-open-in-darkness” condition was found to minimize visual cortex activity due to imagination and multisensory activity (Marx et al., 2004). Both the scanner and scanner room lights were turned off. All completed a training session prior to entering the scanner and completed a verbal survey about their performance following the scans to ensure that the task was completed properly.
2.3 Image Acquisition
MR images were acquired in a 3 Tesla Siemens scanner, MAGNETOM Trio with TIM, using a 12-channel Matrix head array coil. Anatomical images were obtained using a T1-weighted sequence (MPRAGE) with TR/TE/flip angle/slice thickness = 1.95s/2.26ms/9°/1.2mm for sighted subjects, and TR/TE/flip angle/slice thickness = 2.3s/2.98ms/9°/1.0mm for RP subjects. Functional images with blood oxygenation level-dependent (BOLD) contrast were acquired using a T2* weighted echo-planar imaging (EPI) sequence with TR/TE/flip angle = 2s/25ms/60° and Prospective Acquisition Correction (PACE). 36 slices with isotropic voxels of 3×3×3 mm3 were axially oriented and covered the entire cerebral cortex except for the tip of the temporal lobe for some subjects. Subjects laid head first and supine in the scanner. Foam padding was placed around the head to minimize movement during scanning, while earplugs and sound-attenuating headphones were provided to dampen scanner noise.
2.4 Characterization of Vision Loss
Two measures were used to define each subject’s level of vision loss: visual acuity and preserved visual field in V1.
Visual acuities were measured using a Snellen eye chart, where subjects were asked to stand 20 feet away from the chart and read each line with both eyes open and without any corrective lenses. The smallest best read line was considered to be their OU visual acuity. This fractional Snellen value was then converted to logMAR for analysis purposes.
Preserved visual field was quantified in terms of the fractional areal size of a subject’s preserved visual field as projected to V1 cortex and is referred to as the “preserved visual field in V1”. Since we were unable to perform functional retinotopic mapping with our RP subjects, the amount of preserved visual field in V1 was estimated by mapping subjects’ visual field to a commonly used model of V1 based on macaque monkey data (Daniel and Whitteridge, 1961; Schira et al., 2010). The interspecies difference is largely irrelevant for our purpose. Goldmann visual field maps were determined based on subject responses to a 15 dB, 64 mm2 light stimulus. The maps were transferred to ImageJ, where each image pixel within the sighted regions of the visual field was isolated (see Figure 1 for illustrations of Goldmann visual field results). We then found the eccentricity value for each pixel and its corresponding areal cortical magnification factor, according to the following equation described by Motter et al., 2009:
| (1) |
where W is the eccentricity in degrees and magnification (Ma) is in square millimeters of cortex per square degree of visual field. Application of Ma according to Equation 2 yielded each pixel’s V1 cortical area:
| (2) |
where Ac is the cortical area for a single pixel and AP is the area of a single pixel as determined in ImageJ. The cortical area of all pixels within a sighted region were then summed to give the total V1 cortical area corresponding to the sighted region of each subject’s visual field (one areal value was obtained for each eye). To minimize interspecies difference, the total area derived from the macaque model was divided by the maximum of 2171.3 mm2 such that a subject with normal visual field corresponds to a fractional V1 area of 1.0 (the maximum area was derived from the typical spatial extent of a nominal human visual field, as described by Walker et al., 1990). The total fractional V1 area of preserved visual field reported here are averages of values for both eyes for each subject.
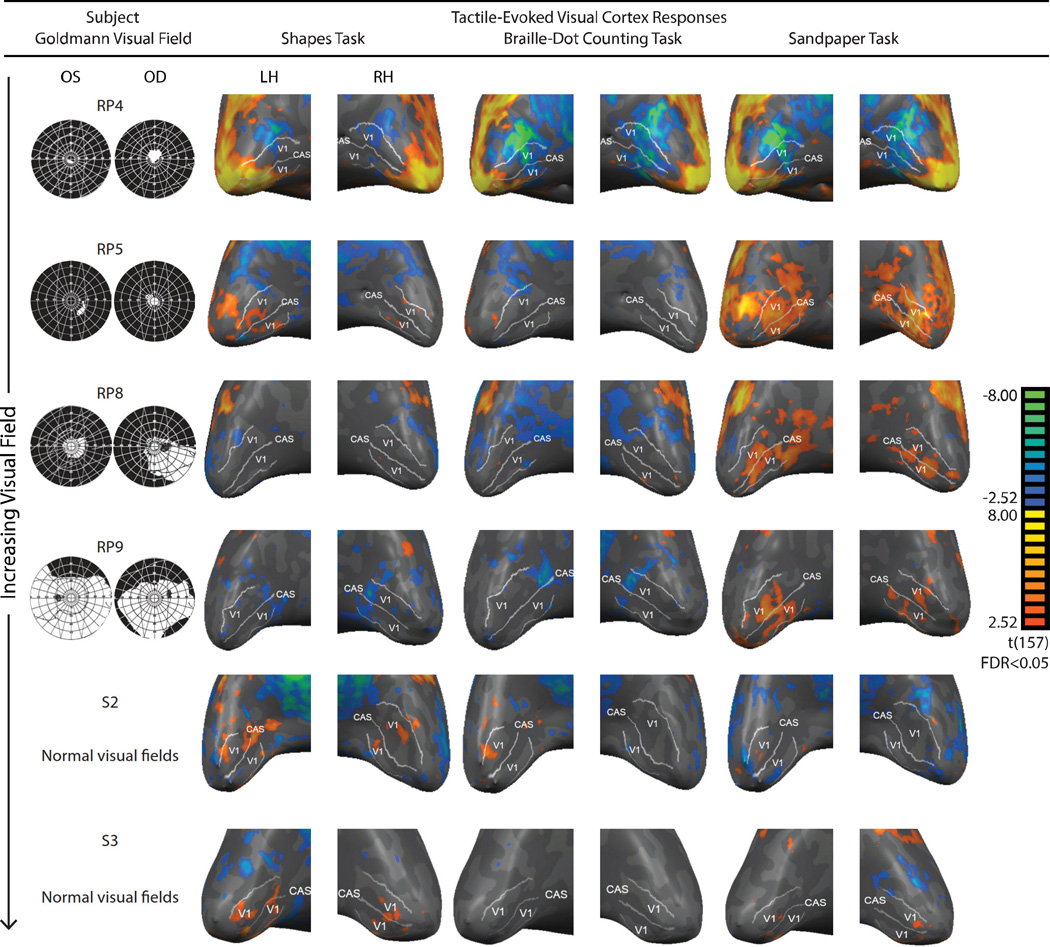
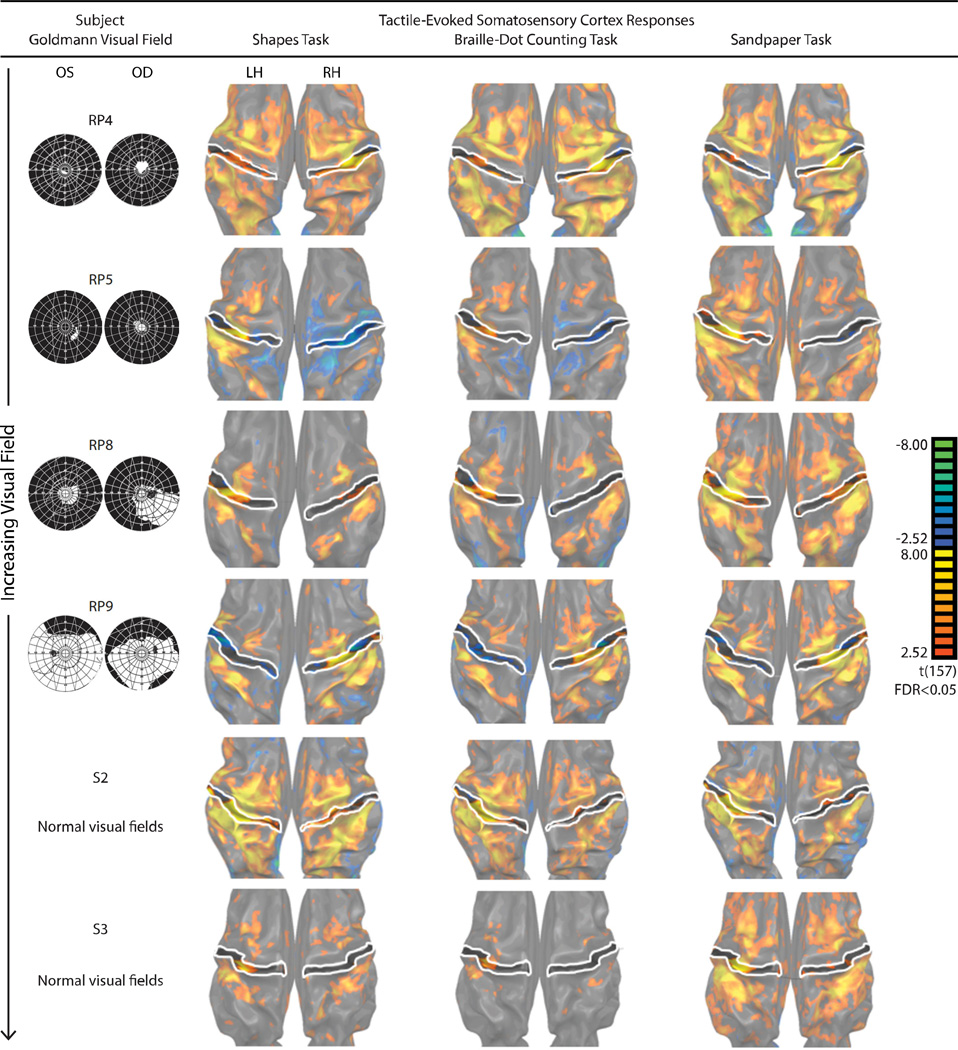
Figure 1. V1 BOLD responses to the three tactile tasks in four representative RP subjects and two sighted control subjects.
Significant responses (FDR < 0.05) were color-coded, with warm colors denoting increases in BOLD responses relative to rest. For each subject, the response patterns were projected onto an inflated representation of the occipital lobe; the outer white line represents the assumed V1/V2 boundary while the center white line represents the calcarine sulcus (CAS). Goldmann visual field results for both eyes (right eye on right) are presented in the first column and represent the subject’s visual field loss (black) and preserved field (white).
2.5 fMRI Data Analysis
Image data was analyzed using BrainVoyager QX (Goebel et al., 2006) in subjects’ native space (as opposed to normalizing to a standard space). Anatomical data underwent inhomogeneity correction and were reoriented via rigid-body rotation and translation to place the origin at the Anterior Commissure and the Posterior Commissure on the y-axis. All functional data was preprocessed with 3D motion correction (PACE and post hoc), slice timing correction, and temporal filtering. In cases of excessive head movement, which occurred in 3 RP subjects and 1 sighted subject, volumes in which a subject exhibited movement greater than 0.6 mm/degree of motion (based on online PACE estimation) and the corresponding entries in the design matrix were excluded from the analysis. Spatial smoothing was not applied to the functional data.
Whole-brain voxel-wise BOLD modulation was obtained by estimating the signal level during the active blocks with respect to that during the resting blocks using a general linear model (GLM), with head-motion parameters as covariates. For each subject, individual functional data sets of each run were concatenated after normalization (z-transform). Significant voxel-wise activations were identified at false discovery rates (FDR) less than 0.05 with a cluster threshold of 25mm2. The activation maps displayed below for each subject were constructed by projecting the GLM contrast (t statistics) obtained from voxels on the cortex onto the reconstructed and inflated cortical surface meshes of the subject.
Putative primary visual cortex (V1) was identified anatomically for each subject, consisting of both banks of the calcarine fissure, the parietal-occipital fissure, and the posterior end of the calcarine sulcus (Hinds et al., 2009). We calculated, for each subject, two complimentary measures (extent and strength) of the unsigned cross-modal response. The areal extent of cross-modal activation in the primary visual cortex was defined as the percentage of significantly modulated voxels on the cortex within the V1 ROI, while the strength of the response was calculated as the mean absolute parameter estimate (beta value) of the responding voxels within the V1 ROI. Mean absolute beta value was used in order to include all instances of cross-modal response, as tactile stimulation was found to evoke both negative and positive activities. These measures jointly provide a comprehensive characterization of the tactile-evoked BOLD response, including in subjects whose responses were spatially extensive but weakly modulated and those with both strong positive and negative modulations. ANOVA, multiple regression, and a linear mixed effects model (described in Section 2.6) were used to identify statistically significant relationships (α = 0.05) between these dependent measures and the two measures of a subject’s visual function (acuity and preserved visual field area in V1) across the three tasks.
Putative primary somatosensory cortex (S1) was also identified anatomically for each subject, extending from the middle of the central sulcus to the peak of the postcentral gyrus, and extending from the medial longitudinal fissure to the lateral sulcus. The percentage of modulated voxels and mean absolute beta value of those voxels were similarly calculated within the S1 ROI.
2.6 Statistical Analysis
A linear mixed effects model was used to analyze the relationship between vision loss and V1 tactile-evoked responses across tasks (dependent variable: extent or strength; covariate: fractional preserved visual field in V1 or visual acuity; repeated variable / factor: Task – Shapes, Braille, and Sandpaper). The factor and covariate were entered into the model as fixed effects without any interaction term. We assumed an unstructured covariance pattern. The same linear mixed effects model was also used to determine the relationship between visual function and tactile-evoked responses in S1.
To determine the relationship between vision loss and the V1 response when controlling for the influence of S1, a combined linear regression and linear mixed effects analysis was used to compute a pseudo-partial correlation. Specifically, S1 responses were controlled as a contributing factor in order to study the relationship between vision loss and V1 responses, while appropriately combining the effects across the three tasks that were run for each subject. For example, the following steps were used to study the between-subjects relationship between strength of the response in V1 and visual acuity, while controlling for strength and extent of the response in S1 (“partialling out” the S1 responses). First, for each task, separate linear regression models were fitted to the subjects’ response strength in V1 and visual acuity with response strength and extent in S1 as the regressors. This was done in order to estimate the residuals of the V1 response and visual acuity after accounting for variation in S1 responses across subjects. For a given task, the correlation between these residuals would thus be the partial correlation between V1 response strength and visual acuity across subjects, controlling for S1 responses (strength and extent). A mixed effect model was then used to combine these partial correlations across tasks, resulting in a pseudo-partial correlation. Specifically, to calculate the pseudo-partial correlation between V1 response strength and visual acuity while controlling for S1 responses, the residuals from the aforementioned linear regression model for each task were modeled together using a mixed effects linear regression of the V1 residuals on the visual acuity residuals. The subject variable was modeled as a random intercept and the task variable was modeled as a fixed effect on slope, with the assumption that the covariance structure between the subject and task follows a compound symmetry structure (exchangeable).
3 Results
3.1 Extent and strength of V1 BOLD responses to tactile stimulation in RP and sighted subjects
A summary of responses elicited with tactile stimulation is presented on an inflated cortical surface of V1 for four representative RP subjects and two representative sighted subjects (Figure 1). Individuals exhibited a range of vision loss (Table 1), which allowed us to partially account for the inter-subject variability of V1 responses to the tactile tasks.
For each subject, V1 BOLD responses were quantified in terms of cortical extent, which was calculated as the percentage of voxels in V1 significantly modulated by the tactile stimuli during each task. A large degree of variability was found in the extent of tactile-evoked V1 responses among RP subjects [M = 31.63%, SD = 19.25%] (Figure 2). A repeated measures ANOVA (between-subjects factor: Visual Ability – Blind, Low Vision, and Sighted; within-subject factor: Task – Shapes, Braille, Sandpaper) revealed a highly significant effect of visual ability, coarsely categorized into the three levels, on extent of the tactile-evoked BOLD response in V1 [F(2,14) = 16.758, p < 0.0001]. The spatial spread of the modulated voxels increased significantly with the severity of vision loss (Figure 1). The effect size was 0.705 (partial Eta Squared), and post hoc analyses (Tukey) showed that this effect of vision ability on extent of the BOLD response was present between the Blind and Sighted groups [p < 0.0001], Blind and Low Vision groups [p = 0.017], and Low Vision and Sighted groups [p = 0.047]. An effect of task was also found on the extent of V1 BOLD activity [F(2,28) = 4.116, p = 0.027], where the Sandpaper task seemed to elicit the strongest response; interactions between visual ability and task were not significant [p = 0.189]. Levene’s test for equality of variances demonstrated equal variance between the Blind and Low Vision groups for all tasks.
Tactile-evoked V1 BOLD responses were further quantified in terms of the strength of the response, measured using the mean absolute beta value for each subject during each task (Figure 2). The absolute beta value was used in order to include all instances of cross-modal response, as tactile stimulation was found to evoke both negative and positive activities (for 1 sighted and 5 RP subjects, at least 50% of the V1 voxels were significantly suppressed during tactile stimulation). A large degree of variability was found in the strength of V1 BOLD responses among RP subjects (in units of parameter estimate) [M= 3.41, SD = 0.63] (Figure 2). However, a repeated measures ANOVA did not reveal any significant effect of visual ability (Blind, Low Vision, and Sighted) on strength of the tactile-evoked BOLD response in V1 (p = 0.093, Figure 1). Similarly, no significant effect of task was found [p = 0.547] and interactions between vision level and task were not significant [p = 0.562].
Several studies have suggested that early blind individuals use the occipital cortex for language processing, resulting in visual cortex responses to reading Braille (Amedi et al., 2003; Bedny et al., 2011; Watkins et al., 2012). We asked if our late-blind RP subjects’ ability to read Braille had a significant effect on V1 responses during the Braille task. For RP subjects only, repeated measures ANOVA (between-subjects factor: Braille and non-Braille reader; within-subjects factor: Task – Shapes, Braille, and Sandpaper) revealed a significant effect [F(1,6) = 26.988, p = 0.002] of an ability to read Braille on extent of the V1 response (but not strength, p = 0.291). However, there was no interaction between the ability to read Braille and task [p > 0.10]. In other words, the effect of Braille reading ability was not more pronounced during the Braille task.
3.2 Relationship between cross-modal V1 BOLD responses and degree of preserved visual functions
The effect of vision loss on the BOLD response to tactile stimulation was characterized in greater detail by comparing activity in V1 to each subject’s preserved visual functions, quantified in terms of acuity and the fractional area of V1 cortex that corresponded to the preserved visual field (as described in Section 2.4). A linear mixed effects model was used to analyze the relationship between a measure of visual function (visual acuity or preserved visual field in V1) and a measure of V1 tactile-evoked responses (extent or strength), with task as a random effect (see Section 2.6). Visual inspection of residual plots for RP subjects alone did not reveal any obvious deviations from homoscedasticity and a Shapiro-Wilk test confirmed normality of the data [p’s > 0.10]. The modeled effects of visual function on V1 response are shown for RP subjects alone (Figure 3 bold lines), as well as for RP and sighted subjects combined (Figure 3 dashed lines).
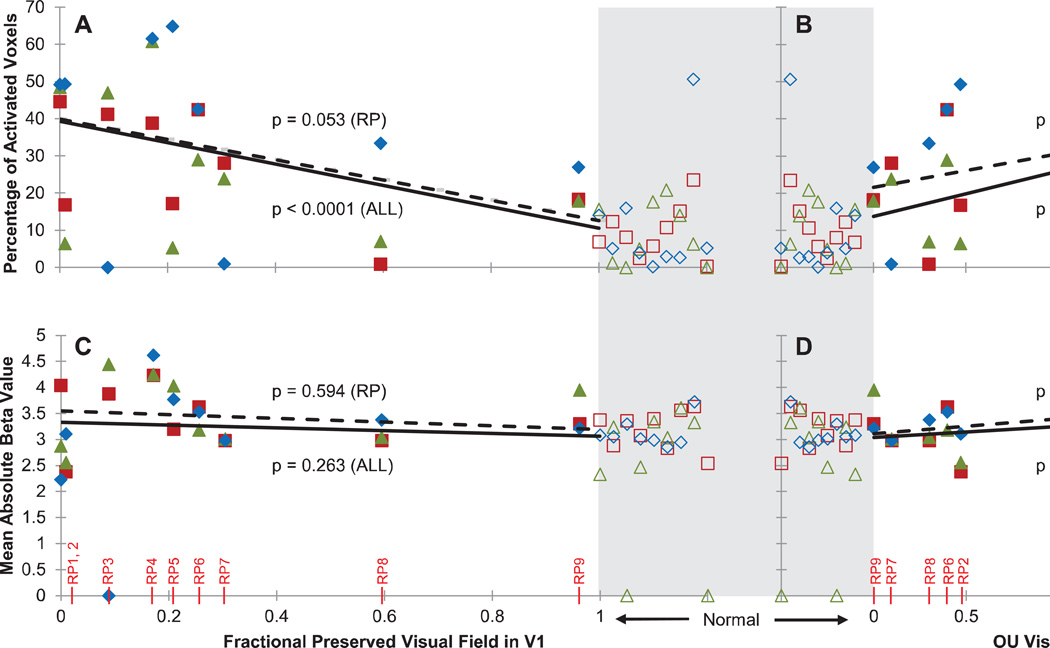
Figure 3. Predictive margins from a linear mixed effects model relating visual function to tactile-evoked responses in V1.
For each plot, the lines indicate the marginalized predicted (fixed) effect of preserved visual field (left panels) or visual acuity (right panels) by the linear mixed effects model. The observed data (for each subject and task) are overlaid. The dashed lines illustrate marginalized effects among RP subjects only (“RP”), while solid lines illustrate marginalized effects among RP and sighted subjects combined (“AH”). Statistics (p-values) above and below the lines correspond to dashed and solid lines, respectively, and indicate significance of the effect of visual function on the V1 response based on the mixed effects model. Sighted control subjects have by definition a fractional preserved visual field in V1 of 1 and average acuity of 0 logMAR, but are plotted separately (open symbols) from patient data, highlighted in gray and spread out horizontally for visualization. A: Percentage of tactile-modulated voxels in V1 (extent) versus fractional preserved visual field in V1. Fractional preserved visual field in V1 was determined by calculating the areal cortical magnification factor for spared regions of the visual field. This value was then normalized by the total V1 area. B: Percentage of modulated voxels in V1 versus visual acuity (logMAR) (subjects RP1 and RP3 were assigned a visual acuity of logMAR = 3 for analysis purposes, as these subjects’ had minimal light perception only and their acuity could not be measured). C: Mean absolute tactile-evoked BOLD modulation amplitude (strength) of modulated V1 voxels versus fractional preserved visual field in V1. D: Mean absolute BOLD amplitude of modulated V1 voxels versus visual acuity.
When the analysis was restricted to just the nine RP subjects, we found that visual acuity significantly affected the extent of V1 responses ([Parameter estimate β(d.f. = 8.085) = 8.992, SE = ±2.983, p = 0.017], Figure 3B). A trending effect of preserved visual field in V1 was found on the cortical extent of tactile-evoked responses in V1 ([β(d.f. = 6.930) = −27.710, SE = ±11.904, p = 0.053], Figure 3A). No effect of visual acuity or fractional preserved visual field in V1 was found on the strength of V1 responses [p’s > 0.07] (Figure 3C and 3D).
When sighted subjects were included in the model, visual acuity and preserved visual field in V1 had a highly significant effect on extent of the V1 response (visual acuity: [β(d.f. = 17.269) = 12.154, SE = ±2.349, p < 0.0001], Figure 3B; preserved visual field: [β(d.f. = 16.339) = −28.720, SE = ± 5.421, p < 0.0001], Figure 3A). The effect of visual acuity on the strength of the response was also significant ([β(d.f. = 17.575) = 0.209, SE = ± 0.091, p = 0.034], Figure 3D), while the effect of preserved visual field in V1 on strength of the response remained insignificant [p = 0.263, Figure 3C].
A previous tactile study by Merabet et al. (2008) found a correlation between the duration of vision deprivation and cross-modal responses in the occipital cortex. However, a linear mixed effects model (using years since onset of blindness as a covariate) revealed no significant effect of duration of blindness on either the extent or strength of the cross-modal V1 BOLD response in RP subjects [p’s > 0.70].
3.3 Comparison of tactile-evoked BOLD responses in V1 and S1
The observation that V1 responded to tactile stimulation prompted the question of whether S1 activity as a function of vision loss, including variability due to a change in motor strategy or tactile sensitivity, could be a source of the observed relationship between V1 responses and vision loss. With early blind individuals, repetitive TMS stimulation to S1 activated V1 (Wittenberg et al., 2004). Similarly, an effective connectivity study by Fujii et al. (2009) suggests that an indirect cortico-cortical feedback pathway from S1 to V1 exists that is modulated by vision loss, resulting in an expansion of tactile processing into visual areas.
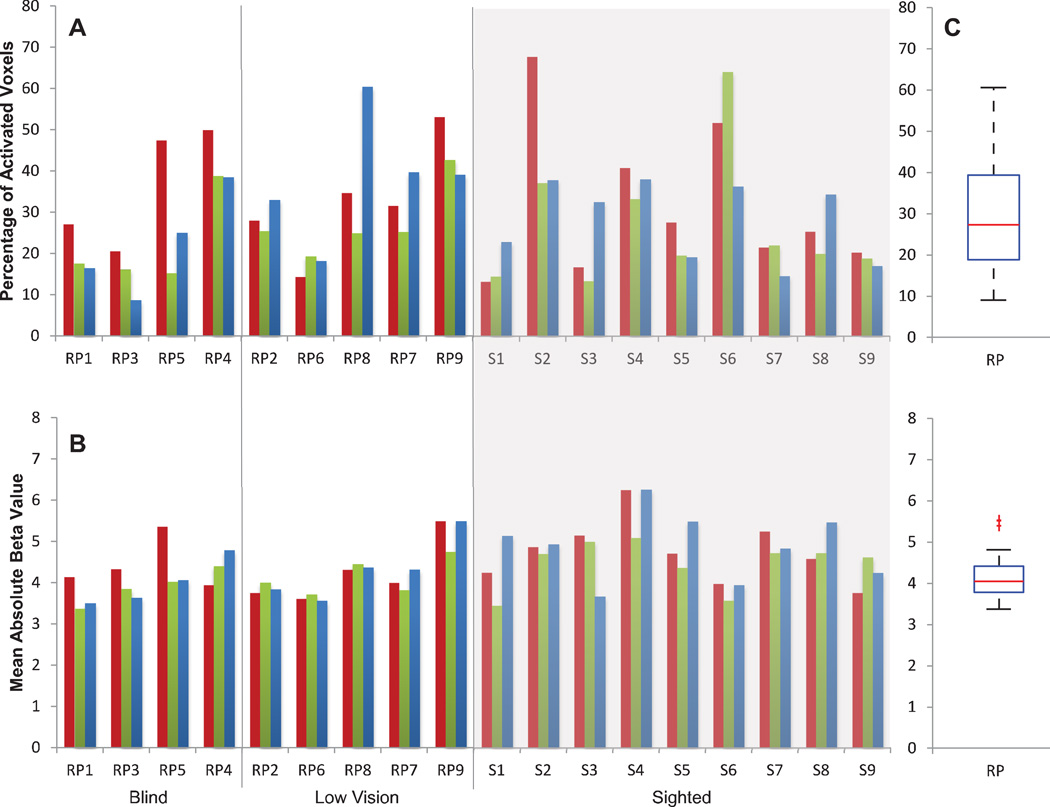
We first, determined the effect of vision loss on tactile-evoked BOLD responses in S1, similar to what we did with responses in V1: a repeated measures ANOVA (between-subjects factor: Visual Ability – Blind, Low Vision, and Sighted; within-subject factor: Task – Shapes, Braille, Sandpaper) revealed no significant effect of visual ability on either the extent or the strength of tactile-evoked S1 BOLD responses [p’s > 0.10] and no significant effect of task [p’s > 0.20] (Figure 4). However, when visual ability was further quantified in terms of preserved visual field and visual acuity, a significant relationship was found between tactile-evoked responses in S1 and vision loss. Among RP subjects only, a linear mixed effects model found a significant effect of preserved visual field in V1 on the extent and strength of the S1 response (extent: [β(d.f. = 7.000) = 21.430, SE = ±8.881, p = 0.047], Figure 5A; strength: [β(d.f. = 7.000) = 1.056, SE = ± 0.304, p = 0.010], Figure 5C). A significant effect of visual acuity was also found on the extent of the S1 response [β(d.f. = 7.000) = −6.145, SE = ± 2.374, p = 0.036, Figure 5B]. No effect of visual acuity was found on S1 response strength [p = 0.256, Figure 5D].
Figure 4. Extent and strength of tactile-evoked responses in S1.
A: The extent of tactile-evoked BOLD responses in S1, measured in terms of the percentage of modulated voxels (FDR < 0.05) in S1 for each subject and each task. RP subjects are ranked along the x-axis in descending order of severity of visual field loss. Sighted controls were grouped against a gray background. B: The strength of tactile-evoked BOLD responses in S1, measured in terms of mean absolute beta value of the significantly modulated S1 voxels for each subject and each task. C: Boxplot illustrating the distributions of the areal percentage (top) and mean absolute beta value (bottom) of activated S1 voxels in RP and sighted control groups across all tasks. The red line indicates the median within each group, the edges of the boxes indicate the 25th and 75th percentiles, and the whiskers illustrate the extreme datapoints, excluding outliers (red data points).
Figure 5. Predictive margins from a linear mixed effects model relating visual function to tactile-evoked responses in S1.
For each plot, the lines indicate the marginalized predicted (fixed) effect of preserved visual field (left panels) or visual acuity (right panels) by the linear mixed effects model. The observed data (for each subject and task) are overlaid. The dashed lines illustrate marginalized effects among RP subjects only (“RP”), while solid lines illustrate marginalized effects among RP and sighted subjects combined (“AH”). Statistics (p-values) above and below the lines correspond to dashed and solid lines, respectively, and indicate significance of the effect of visual function on the S1 response based on the mixed effects model. Sighted control subjects have by definition a fractional preserved visual field in S1 of 1 and average acuity of 0 logMAR, but are plotted separately (open symbols) from patient data and highlighted in gray for comparison. A: Percentage of tactile-modulated voxels in S1 (extent) versus fractional preserved visual field in V1. B: Percentage of modulated voxels in S1 versus visual acuity (logMAR) (subjects RP1 and RP3 were assigned a visual acuity of logMAR = 3 for analysis purposes, as these subjects’ had minimal light perception only and their acuity could not be measured). C: Mean absolute tactile-evoked BOLD modulation amplitude (strength) of modulated S1 voxels versus fractional preserved visual field in V1. D: Mean absolute BOLD amplitude in modulated S1 voxels versus visual acuity.
When sighted subjects were included in the mixed effects analysis, the effect of visual acuity and preserved visual field in V1 on the strength of S1 response was significant [p’s < 0.03], while the effect of visual acuity and visual field on the extent of the S1 response did not reach significance [p’s > 0.06]. This difference in the effect of vision loss on S1 responses when compared to RP subjects alone may be attributed to variability in S1 responses among the sighted subjects (Figure 4). It is worth noting that vision loss was found to result in an increased tactile-evoked response in V1 but a decrease in S1 response. This effect in S1 may suggest that individuals with greater vision loss did not press as hard on the tactile elements when compared to their sighted counterparts, possibly as a result of increased tactile sensitivity with blindness (Goldreich et al., 2006). Given that different individuals may have employed diverse tactile strategies when completing the tasks, it is conceivable that these strategic differences could account for variation in the V1 BOLD response. The tactile-evoked responses in S1 can be seen as a proxy for tactile strategy differences across individuals. However, the opposite effects of vision loss on the responses of V1 and S1 suggest that the tactile-evoked activity in V1 is not a direct consequence of S1 activity. Nevertheless, the two may still be related in a way resembling the finding that reduced auditory cortex activation was associated with increased auditory activity in the occipital lobe in early blindness (Watkins et al., 2013).
We examined the effect of S1 activity on the relationship between vision loss and the observed cross-modal responses in V1. When we controlled for task-evoked responses (i.e. extent and strength) in S1 across RP and sighted subjects, the (pseudo-partial) correlations between visual function (indexed by either the preserved visual field in V1 or visual acuity) and the extent or strength of tactile-evoked responses in V1 became stronger (Table 2A) when compared to the effects of visual function on V1 responses found without controlling for responses in S1. Among RP subjects only, controlling for the extent and strength of the S1 responses also resulted in a more significant pseudo-partial correlation between visual acuity and the extent and strength of the V1 BOLD response when compared to results not controlling for the S1 responses (Table 2B). These findings suggest that S1 activity could not explain the observed relationship between cross-modal responses in V1 and vision loss.
Table 2.
The effect of visual function (preserved visual field in V1 or visual acuity) on V1 BOLD responses (extent or strength) with and without controlling for S1 BOLD responses (both extent and strength of the tactile-evoked response in S1). The marginalized effect column was duplicated from the linear mixed effects model results shown in Figure 3. The column showing the effect when controlling for S1 responses represents the results of the pseudo-partial correlation between visual function and V1 BOLD responses, excluding the influence of S1 responses (see Section 2.6).
| A. | ||
|---|---|---|
| Effect of Visual Function on V1 Responses (Sighted and RP subjects) |
Marginalized Effect |
Effect controlling for S1 responses |
| V1 Extent vs. Preserved Visual Field | p< 0.0001* | p< 0.0001* |
| V1 Extent vs. Visual Acuity | p< 0.0001* | p< 0.0001* |
| V1 Strength vs. Preserved Visual Field | p = 0.263 | p = 0.015* |
| V1 Strength vs. Visual Acuity | p = 0.034* | p = 0.003* |
| B. | ||
|---|---|---|
| Effect of Visual Function on V1 Responses (RP subjects only) |
Marginalized Effect |
Effect controlling for S1 responses |
| V1 Extent vs. Preserved Visual Field | p = 0.053 | p = 0.072 |
| V1 Extent vs. Visual Acuity | p = 0.017* | p< 0.0001* |
| V1 Strength vs. Preserved Visual Field | p = 0.594 | p = 0.317 |
| V1 Strength vs. Visual Acuity | p = 0.072 | p = 0.008* |
indicates significant relationships (p > 0.05).
A: Results including both sighted and RP groups. B: Results including RP subjects only.
4 Discussion
Individuals with retinitis pigmentosa are the target population for recent sight restoration technologies, including retinal implants and gene therapies. We focused solely on RP patients in the current study in order to build a foundation for future studies that investigate the cortical effects of sight-restoration treatments in RP patients. Previous studies, using a diverse subject population, have demonstrated that the visual cortex becomes responsive to tactile input with vision loss (Amedi et al., 2003; Buchel et al., 1998; Burton 2003; Cheung et al., 2009; Merabet et al., 2006, 2008; Ptito et al., 2005; Sadato et al. 1996, 2004; Sathian 2005). Here, we sought to expand upon these findings by determining if a relationship exists between severity of vision loss and the extent and strength of tactile-evoked V1 responses in late-blind individuals with RP. Our results indicate that a significant correlation indeed exists between the degree of vision loss and amount of cross-modal modulation: as visual acuity and preserved visual field decrease, V1 becomes more responsive to tactile stimulation.
We found that the pseudo-partial correlation between tactile-evoked responses in V1 and visual function (preserved visual field in V1 and visual acuity) across subjects became even stronger after we had controlled for tactile-evoked responses in S1. This suggests that the relationship between V1 tactile-evoked responses and vision loss is partially masked by variations in S1 activity. Several factors may result in a variable S1 response, including differences in subject tactile exploration strategies and subject differences in sensitivity to tactile elements; these tactile strategy and sensitivity differences may be especially pronounced between sighted and blind subjects. Removing these variances thus increases the association between vision loss and V1 cross-modal activity.
Several theories exist as to the cause of these cross-modal activations. Merabet et al. (2008) suggest that preexisting multisensory pathways remain suppressed in sighted individuals and are “unmasked” with vision deprivation. Others have endorsed the notion that vision deprivation results in the creation of new neural networks and sensory associations that support cross-modal responses (Burton 2003), while others suggest that the occipital cortex may act as an operator of a function based on the best-suited input available (Pascual-Leone et al., 2001). However, recent studies on humans and primates have questioned these functional claims and instead suggest that the observed activations may be unmasked feedback signals driven by task demands that are otherwise suppressed in the presence of visual inputs (Smirnakis et al., 2007; Wandell and Smirnakis, 2009). If tactile-evoked responses in V1 are due to “unmasking” of otherwise suppressed connections from S1, it is expected that the relationship between task-evoked responses in S1 and V1 would be stronger for individuals with more severe vision loss. We did not observe this simple version of unmasking.
Overall, the observed cross-modal modulation is highly variable across late-blind individuals. The degree of preserved visual functions, particularly when expressed in terms of the fractional areal size of the V1 cortex that corresponds to the preserved visual field, explains some of this variance. Other factors may include preserved visual functions beyond the ones we quantified, variations in functional connectivity between visual and other cortical areas (Fujii et al., 2009; Leo et al., 2012), differences in tactile sensitivity, and unspecific individual differences.
No significant effect of years since onset of blindness was found on tactile-evoked responses in V1. This is counter to a finding of Merabet et al. (2008), which suggests that visual cortex responses to tactile stimulation become increasingly stronger over time after the onset of vision deprivation. The number of subjects presented in this study may not be sufficient to observe this effect. Alternatively, week-long vision deprivation may not be generalizable to blindness. For RP subjects, whose photoreceptor layer deteriorates at varying rates in different individuals, it may be the amount of vision loss that has occurred over time—and not the duration of vision deprivation—that affects cross-modal changes. Since the pace of vision deterioration varies significantly among RP patients, years since onset of blindness may be insufficient to infer the degree of cross-modal modulation that has occurred.
5 Conclusions
In summary, vision loss was found to have a significant effect on tactile-evoked V1 BOLD responses in late-blind individuals. Our findings indicate that while tactile-evoked V1 responses are variable among late-blind individuals and partially depend upon the type of tactile task being performed, the correlation between tactile responses in V1 and vision loss is reliable across subjects, particularly after controlling for tactile-evoked S1 responses. Cross-modal modulation may be a useful biomarker for assessing progress and identifying bottlenecks in different visual areas following sight restoration treatments. In particular, if pre-treatment cross-modal responses are found to correlate with an individual’s ability to adapt to sight restoration treatments, such a biomarker—relating vision loss to tactile-evoked responses—could be used to predict how a late-blind RP patient will respond to treatment given their severity of blindness.
Tactile-evoked response in V1 correlates with vision loss in late blind subjects.
Preserved visual field is negatively correlated with the extent of V1 response.
Visual acuity is positively correlated with extent and strength of V1 response.
These correlations strengthen after controlling for somatosensory cortex response.
Acknowledgments
This material is based on work supported by the National Science Foundation under Grant No. EEC-0310723, the National Eye Institute under Grant No. R01 EY017707, Research to Prevent Blindness (RPB), the USC Dana and David Dornsife Cognitive Neuroscience Imaging Center, and the NSF Graduate Research Fellowship Program. We thank Nick Jackson for consultations on statistics and in particular for suggesting the mixed effects analyses. Some results in this study have been reported previously in conference paper form [Cunningham, et al. (2011). IEEE Engineering in Medicine and Biology Conference (EMBC), Boston, MA].
Appendix A: Additional Figure
Figure A1. S1 BOLD responses to the three tactile tasks in four representative RP subjects and two sighted control subjects.
Significant activations (FDR < 0.05) were color-coded, with warm colors denoting increase in BOLD responses relative to rest. For each subject, the response patterns were projected onto a partially-inflated representation of the cortex; the white-bolder regions represent S1, extending from the middle of the central sulcus to the peak of the postcentral gyrus. Goldmann visual field results for both eyes (right eye on right) are presented in the first column and represent the subject’s visual field loss (black) and sighted field (white). Subject handedness is given in parentheses in the first column. All subjects used their dominant hand to complete the tasks (subject RP4, who is ambidextrous, used his left hand to complete the tasks).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Interests or conflicts of interest: The authors declare no competing financial interests.
References
- Amedi A, Raz N, Pianka P, et al. Early ‘visual’ cortex activation correlates with superior verbal memory performance in the blind. Nature Neurosci. 2003;6(7):758–766. doi: 10.1038/nn1072. [DOI] [PubMed] [Google Scholar]
- Amedi A, Raz N, Azulay H, et al. Cortical activity during tactile exploration of objects in blind and sighted humans. Restor Neurol Neurosci. 2010;28(2):143–156. doi: 10.3233/RNN-2010-0503. [DOI] [PubMed] [Google Scholar]
- Bedny M, Pascual-Leone A, Dravida S, et al. A sensitive period for language in the visual cortex: Distinct patterns of plasticity. Brain and Language. 2011;122:162–170. doi: 10.1016/j.bandl.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson NC, Buff OH, Datta R, et al. The retinotopic organization of striate cortex is well predicted by surface topology. Curr Biol. 2012;22:1–5. doi: 10.1016/j.cub.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Price C, Frackowiak RSJ, Friston K. Different activation patterns in the visual cortex of late and congenitally blind subjects. Brain. 1998;121:409–419. doi: 10.1093/brain/121.3.409. [DOI] [PubMed] [Google Scholar]
- Burton H. Visual cortex activity in early and late blind people. J Neurosci. 2003;23(10):4005–4011. doi: 10.1523/JNEUROSCI.23-10-04005.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung S-H, Fang F, He S, Legge GE. Retinotopically-specific reorganization of visual cortex for tactile pattern recognition. Curr Biol. 2009;19:596–601. doi: 10.1016/j.cub.2009.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR, Snell EJ. On test statistics calculated from residuals. Biometrika. 1971;58.3:589–594. [Google Scholar]
- Daniel PM, Whitteridge D. The representation of the visual field on the cerebral cortex in monkeys. J Physiol. 1961;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Tanabe HC, Kochiyama T, Sadato N. An investigation of cross-modal plasticity of effective connectivity in the blind by dynamic causal modeling of functional MRI data. J Neurosci Res. 2009;65:175–186. doi: 10.1016/j.neures.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with BrainVoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldreich D, Kanics IM. Performance of blind and sighted humans on a tactile grating detection task. Percept Psychophys. 2006;68(8):1363–1371. doi: 10.3758/bf03193735. [DOI] [PubMed] [Google Scholar]
- Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis. 2006;1(40) doi: 10.1186/1750-1172-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson L, Karvonen J, Salminen-Vaparanta N, et al. Retinotopic Maps, Spatial Tuning, and Locations of Human Visual Areas in Surface Coordinates Characterized with Multifocal and Blocked fMRI Designs. PLoS One. 2012;7(5):e36859. doi: 10.1371/journal.pone.0036859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds O, Polimeni JR, Rajendran N, et al. Locating the functional and anatomical boundaries of human primary visual cortex. Neurolmage. 2009;46:915–922. doi: 10.1016/j.neuroimage.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humayun MS, Dorn JD, da Cruz L, Daagnelie G. Interim results from the international trial of second sight’s visual prosthesis. Ophthalmology. 2012 doi: 10.1016/j.ophtha.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Lee JS, Oh SH, et al. Cross-modal plasticity and cochlear implants. Nature. 2001;409:149–150. doi: 10.1038/35051653. [DOI] [PubMed] [Google Scholar]
- Leo A, Bernardi G, Handjaras G, et al. Increased BOLD variability in the parietal cortex and enhanced parieto-occipital connectivity during tactile perception in congenitally blind individuals. Neural Plast. 2012 doi: 10.1155/2012/720278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx E, Deutschlander A, Stephan T, et al. Eyes open and eyes closed at rest conditions: impact on brain activation patterns. Neurolmage. 2004;21:1818–1824. doi: 10.1016/j.neuroimage.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Merabet LB, Swisher JD, McMains SA, et al. Combined activation and deactivation of visual cortex during tactile sensory processing. J Neurophysiol. 2006;97:1633–1641. doi: 10.1152/jn.00806.2006. [DOI] [PubMed] [Google Scholar]
- Merabet LB, Hamilton R, Schlaug G, et al. Rapid and reversible recruitment of early visual cortex for touch. PLoS ONE. 2008;8:1–12. doi: 10.1371/journal.pone.0003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter BC. Central V4 receptive fields are scaled by the V1 cortical magnification and correspond to a constant-sized sampling of the V1 surface. J Neurosci. 2009;29(18):5749–5757. doi: 10.1523/JNEUROSCI.4496-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leon A, Hamilton R. The metamodal organization of the brain. Prog Brain Res. 2001;134:427–445. doi: 10.1016/s0079-6123(01)34028-1. [DOI] [PubMed] [Google Scholar]
- Ptito M, Moesgaard SM, Gjedde A, Kupers R. Cross-modal plasticity revealed by electrotactile stimulation of the tongue in the congenitally blind. Brain. 2005;128:606–614. doi: 10.1093/brain/awh380. [DOI] [PubMed] [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, Ibanez V. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380(11):526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- Sadato N, Okada T, Kubota K, Yonekura Y. Tactile discrimination activates the visual cortex of the recently blind naive to Braille: a functional magnetic resonance imaging study in humans. Neurosci Lett. 2004;359:49–52. doi: 10.1016/j.neulet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Sathian K. Visual cortical activity during tactile perception in the sighted and the visually deprived. Dev Psychobiol. 2005;46:279–286. doi: 10.1002/dev.20056. [DOI] [PubMed] [Google Scholar]
- Schira MM, Tyler CW, Spehar B, et al. Modeling Magnification and Anisotropy in the Primate Foveal Confluence. PLoS Comput. Biol. 2010;6(1) doi: 10.1371/journal.pcbi.1000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnakis SM, Schmid MC, Weber B, et al. Spatial specificity of BOLD versus cerebral blood volume fMRI for mapping cortical organization. J Cereb Blood Flow Metab. 2007;27(6):1248–1261. doi: 10.1038/sj.jcbfm.9600434. [DOI] [PubMed] [Google Scholar]
- Walker HK, Hall WD, Hurst JW. Clinical Methods: The History, Physical, and Laboratory Examinations. “Visual Fields.”. 3rd ed. Boston: Butterworths; 1990. [PubMed] [Google Scholar]
- Wandell BA, Smirnakis SM. Plasticity and stability of visual field maps in adult primary visual cortex. Nat Rev Neurosci. 2009;10(12):873–884. doi: 10.1038/nrn2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Shakespeare TJ, O’Donoghue MC, et al. Early Auditory Processing in Area V5/MT of the Congenitally Blind Brain. J Neurosci. 2013;33(46):18242–18246. doi: 10.1523/JNEUROSCI.2546-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Cowey A, Alexander I, et al. Language networks in anophthalmia: maintained hierarchy of processing in “visual” cortex. Brain: A Journal of Neurology. 2012;135(Pt. 5):1566–1577. doi: 10.1093/brain/aws067. [DOI] [PubMed] [Google Scholar]
- Wittenberg GF, Werhahn KJ, Wassermann EM, et al. Functional connectivity between somatosensory and visual cortex in early blind humans. Euro J Neurosci. 2004;20:1923–1927. doi: 10.1111/j.1460-9568.2004.03630.x. [DOI] [PubMed] [Google Scholar]