Abstract
It is thought that the low incidence of central nervous system (CNS) involvement in acute myeloid leukemia (AML) does not justify routine CNS prophylaxis, as high-dose cytarabine eliminates CNS disease. To test whether chemotherapy that does not include high-dose cytarabine increases the risk of CNS involvement, the medical records of 1,412 newly diagnosed AML patients were reviewed. In 1,370 patients, lumbar puncture (LP) was performed only if clinically indicated, and CNS disease was detected in 45 (3.3%) patients. Another 42 patients underwent routine LP as part of an investigational protocol, and in 8 (19%) CNS disease was detected (P<0.0001). Risk factors included high LDH, African-American ethnicity, and young age. Patients receiving high-dose cytarabine and those that did not had similar rates of CNS involvement. Disease free survival (DFS) and overall survival were shorter in patients with CNS involvement. It remains to be determined whether routine CNS prophylaxis would improve DFS.
Keywords: Acute Myeloid Leukemia, Central Nervous System, Risk Factors, cytarabine, Lumber puncture
Introduction
The exact incidence of central nervous system (CNS) leukemia in adult patients with acute myeloid leukemia (AML) is unknown. CNS disease was found to be relatively common in AML patients with elevated lactate dehydrogenase (LDH), hyperleukocytosis, or a prominent monocytic component[1]. Nevertheless, CNS involvement is thought to be less common in adult AML than in adult acute lymphoblastic leukemia (ALL)[2-6]. Therefore, unlike in ALL, routine lumbar puncture (LP) is rarely performed in adult patients with newly diagnosed AML.
Standard induction and post-remission chemotherapy for AML includes medium- to high-dose cytarabine, which penetrates the blood-brain barrier[7] and is thought to prevent dissemination of leukemia cells into the CNS and to eliminate CNS disease present at diagnosis. However, recent induction chemotherapy regimens, designed primarily for elderly patients with AML, do not include high-dose cytarabine. Whether eliminating cytarabine from induction protocols results in an increased risk for CNS disease is unknown.
In this study, we retrospectively assessed the cumulative incidence, risk factors, and the outcome of CNS involvement in a large cohort of AML patients treated with cytarabine-based and non-cytarabine-based induction chemotherapy.
Subjects and Methods
All patients included in this study provided written informed consent for treatment in various clinical trials, all of which have been approved by the institutional review board. In addition, separate institutional review board approval was obtained to retrospectively collect data from electronic medical records and from the department of leukemia database. We reviewed the medical records of 1,412 patients who were diagnosed with AML and who received induction chemotherapy between January 2000 and December 2012 (144 months) at The University of Texas MD Anderson Cancer Center. All subtypes of AML except for acute promyelocytic leukemia were included. The majority of AML patients (1,370/1,412, 97%) underwent diagnostic LP only if signs or symptoms suggestive of CNS disease were noted. Forty-two young AML patients were treated with an investigational protocol that required diagnostic LP followed by intrathecal administration of cytarabine at the time of diagnosis. The incidence of CNS involvement and treatment outcome in this subset of patients was analyzed separately. CNS leukemia was defined as the presence of leukemic blasts in cytospun cerebrospinal fluid. The combination of signs or symptoms of CNS involvement and the demonstration of blasts in the CSF was required for a definite diagnosis of leptomeningeal disease. Patients with blasts in the CSF together with high numbers of red blood cells (>5) were not considered to have CNS disease.
Routine workup of all AML patients included a diagnostic bone marrow (BM) aspiration and biopsy. Of the 1,370 patients, in 927 the disease was classified according to the French-American-British (FAB) system and in 1,361 patients, routine cytogenetics (G-banding) analysis was available. Mutation analysis of the Nuclophosmins-1 was performed in 598 (44%) patients and typing of the internal tandem duplicate mutations of the fms-related kinase3 (FLT3) in 598 patients (44%).
Patient characteristics were summarized using frequencies (percentages) for categorical and median and range for continuous variables. Overall survival (OS) was defined as the time from AML diagnosis to death. DFS was defined as the time from complete remission (CR) until the first event (relapse or death) or the time of the last follow-up. To compare patients on the basis of categorical variables, we used the χ2 test and applied the logistic regression model to estimate the odds ratio and the 95% confidence interval (CI) around it, applied receiver operator characteristic (ROC) analysis to determine the optimal cutoff, and applied this cutoff in a logistic regression model to separate patients with and without CNS involvement. We used the Student t-test with Levene's test statistics to test for equality of variance and applied the Welch correction when needed. Medians were compared using the Mann-Whitney test. To detect variables that predict the occurrence of CNS disease, we used multivariable logistic regression with forward selection based on the χ2 test of the change in residual deviance and used a cutoff of P < 0.05 for adding new variables. The log-rank test was used to compare survival in patients with and without CNS disease. Statistical analyses were performed using the SPSS software (version 21, SPSS Inc., Chicago, IL).
Results
Incidence of CNS disease in AML
Among the 1,370 newly diagnosed AML patients that received treatment at MDACC between 2000 and 2012, 151 (11%) underwent LP during the follow-up period. Overt CNS involvement was documented in 45 patients (3.3%). Among the 42 young AML patients (median age, 41 years; range, 14-49 years) who underwent LP at the time of diagnosis as part of a clinical protocol, CNS leukemia cells were detected by cytocentrifugation in 8 patients (19%). This was significantly higher than the incidence rate in the main AML cohort (P < .0001).
The incidence of CNS involvement was similar across most FAB subtypes, including acute myelomonocytic leukemia (FAB-M4), and ranged between 3.1% and 3.6%. Among 131 patients with acute monoblastic leukemia (FAB-M5), 10 patients (7.6%) had CNS involvement, a significantly higher rate than that of other FAB subtypes (P = .004). Notably, the incidence rate of CNS involvement in the 154 patients with documented prior myelodysplastic syndrome was particularly low (1/154, 0.6%; P = .05).
Patient characteristics and clinical course
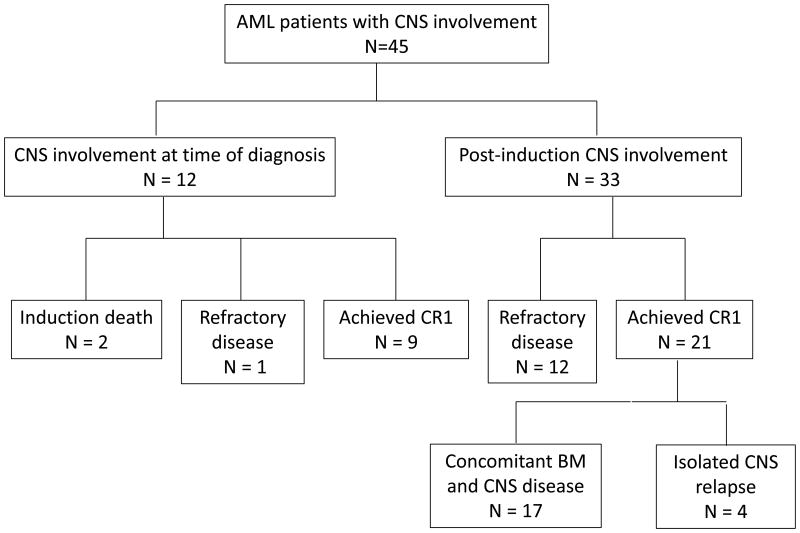
Twelve patients (0.9%) had CNS involvement at the time of diagnosis, and 33 patients (2.4%) developed CNS disease after induction chemotherapy was completed. The median time from the diagnosis of AML to the diagnosis of CNS disease was 4 months (range, 0 to 120 months). Of the 12 patients with CNS disease at diagnosis, 9 (75%) achieved CR following treatment and 2 (17%) died during induction chemotherapy, similar to the CR (65.5%) and induction death (9.1%) rates of the entire cohort.
Of the 33 patients, who developed CNS disease after induction chemotherapy, 12 never achieved CR and 21 achieved CR. In those patients who achieved CR, the median time from confirmation of CR to diagnosis of CNS disease was 10 months (range, 1 to 45 months). Only 4 had isolated CNS relapse; the remaining 17 patients had evidence of BM disease when diagnosed with CNS involvement (Fig 1). The median age and sex distribution did not significantly differ between patients with or without CNS involvement (Table I). Frequent symptoms of CNS disease included altered mental status (29%), headaches (18%), and parasthesia/numbness (16%) (Table II).
Figure 1.
Outcome of patients with CNS involvement. BM, bone marrow; CR1, first complete remission. Induction death, death within 28 days after initiation of treatment.
Table I. Baseline characteristics of the study population.
| AML patients without CNS disease (N = 1325) |
AML patients with CNS disease (N = 45) |
P value | |
|---|---|---|---|
| Median age (range) | 63 (17-89) | 60 (17-79) | 0.09 |
| Sex n (%) | |||
| Male | 732 (55) | 22 (49) | 0.4 |
| Female | 593 (45) | 23 (51) | |
| Ethnicity, n (%) | |||
| Caucasian | 1,048 (98) | 25 (2) | |
| Hispanic | 117 (94) | 7 (6) | |
| African American | 100 (91) | 10 (9) | |
| Asian | 26 (90) | 3 (10) | |
| Unknown/other | 34 (100) | 0 (0) | |
| Laboratory values, median (range) | |||
| Hemoglobin, g/dL | 8.8 (3 to 15) | 9.1 (3 to 15) | 0.8 |
| Platelets, ×109/L | 47 (2 to 787) | 47 (12 to 202) | 0.4 |
| White blood cell, ×109/L | 4.9 (0.2 to 433) | 6.5 (2 to 92) | 0.6 |
| LDH median, IU/L | 747 (200 to 20,701) | 1165 (383 to 12,405) | 0.02 |
| Albumin, g/dl | 3.5 (0.7 to 5.3) | 3.2 (1.9 to 4.9) | 0.06 |
| Percent bone marrow blasts, mean (s.d.) | 24 (27) | 36 (31) | 0.01 |
| Karyotype | 0.1 | ||
| Normal karyotype | 612 (97) | 19 (3) | |
| Trisomy 8 | 98 (95) | 5 (5) | |
| Inversion(16) | 71 (97) | 19 (3) | |
| 11q23 abnormalities | 66 (93) | 5 (7) | |
| trisomy(8:21) | 62 (95) | 3 (5) | |
| Miscellaneous | 418 (30) | 10 (22) |
s.d., standard deviation.
Table II. Neurological signs and symptoms at time of presentation of CNS involvement.
| Sign/symptom | No. (%) |
|---|---|
| Altered mental status | 13 (29) |
| Headache | 8 (18) |
| Parasthesias/numbness | 7 (16) |
| Seizure | 6 (13) |
| Blurry vision/papilledema | 4 (9) |
| Diabetes insipidus | 2 (4) |
| Facial nerve paralysis | 2 (4) |
| Cerebellar signs | 2 (4) |
| Other | 3 (7) |
Other = hearing loss, memory loss, and cognitive deterioration
All 45 patients with CNS involvement had undergone computed tomography and/or magnetic resonance imaging of the head. In all patients except one, abnormal radiological findings were reported. In most patients (43/45), BM results from the time of CNS disease diagnosis were available; in 33 patients (70%), concomitant BM disease and CNS disease were documented. Patients with CNS leukemia blasts received between 1 and 24 intrathecal injections of cytarabine alternating with methotrexate (median, 4), and 13 patients (13/45, 29%) also were treated with brain irradiation. LP was done only once in 9 patients (19%).
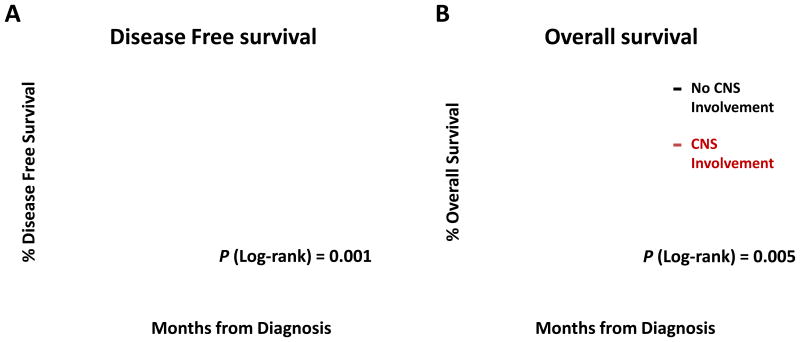
In patients who achieved CR after induction chemotherapy, the median DFS and OS were significantly shorter if CNS disease was detected: The median DFS was 6 months (95% CI, 2 to 8 months) in patients with CNS involvement and 15 months (95% CI, 10 to 16 months) in patients without CNS involvement, and the median OS was 13 months (95% CI 10 to 17 months) in patients with CNS involvement and 27 months (95% CI, 23 to 31 months) in patients without CNS involvement (Fig 2).
Figure 2.
Overall survival (A) and disease-free survival (B) in patients who achieved CR after induction treatment. CR, complete remission.
Effect of cytarabine on the occurrence of CNS disease
Induction chemotherapy included high- or medium-dose cytarabine in 788 patients (56%) and low-dose cytarabine or no cytarabine in 186 (14%) and 396 (29%) patients, respectively. Similar rates of CNS disease were found in patients who were treated with induction regimens that did not include high-dose cytarabine and in patients who were treated with high-dose cytarabine-based regimens: of the 33 patients (2.4%) who developed CNS disease after induction chemotherapy, 17 received high-dose cytarabine-based therapy (17/788, 2.2%), and 16 received induction regimens that did not include high-dose cytarabine (16/582, 2.7%).
Predictive factors for CNS involvement
Patient and disease characteristics were analyzed for potential association with CNS involvement. These baseline characteristics included age at time of diagnosis; sex; ethnicity; hemoglobin level, platelet count, and total white blood cell count; serum levels of LDH and albumin; the percentage of BM blasts; and FAB subtype. Because previous studies suggested that several cytogenetic features such as a prominent monocytic component, inv(16), or chromosome 11 abnormalities are associated with CNS involvement,[8] we also looked for a potential association with molecular abnormalities, specifically Nuclophosmins1or FLT3 mutations. In a univariate analysis, high levels of LDH, a high percentage of BM blasts, African American or Hispanic ethnicity, monoblastic FAB subtype (M5), and FLT3-ITD were predictive for CNS involvement (Table III). In the multivariate analysis, which excluded the Nuclophosmins1 (NPM1) and FLT 3 mutation status because these data were not available for more than 50% of the patients, only high levels of LDH at the time of diagnosis African-American ethnicity and young age remained predictive for developing CNS involvement. Using ROC analysis, we found that 80% of patients with CNS involvement had LDH levels of above 700 IU/L. With this cutoff, the odds ratio for CNS involvement in patients with high LDH levels was 3.0 (95% CI, 1.4 to 6.9, P = 0.003). Compared to Caucasian patients, African-American patients had an odds ratio for CNS involvement of 4.4 (95% CI, 1.9 to 9.8, P = 0.004).
Table III. Univariate analysis of factors predicting CNS involvement in patients with AML.
| Factor | Odds ratio* (95% CI) | P value |
|---|---|---|
| LDH >700 IU/L | 2.6 (1.4 to 6.9) | <0.003 |
| Percent bone marrow blasts | 2.7 (1.2 to 6.5) | 0.002 |
| Monoblastic subtype (FAB-M5) | 2.8 (1.4-5.8) | 0.004 |
| Mutations | ||
| Flt3 | 2.2 (1.1-4.7) | 0.03 |
| Nuclophosmins1 | 1.7 (0.6-4.8) | 0.3 |
| Age <50 | 2.0 (1.0 to 2.7) | 0.03 |
| Albumin | NA | 0.06 |
| Sex | NA | 0.4 |
| Complete blood cell counts | ||
| Hemoglobin | NA | 0.8 |
| White blood cell | NA | 0.1 |
| Platelets | NA | 0.5 |
Odds ratio for categorical variables is based on the exp(β) of the logistic regression model. CI, confidence interval; NA ,not applicable.
Discussion
Nearly 40 years have passed since the first report of the incidence of CNS disease in adult patients with acute leukemia treated at the MD Anderson Cancer Center.[9] CNS disease was found in 13% of those patients, with incidences ranging between 7% for patients with AML to 41% for patients with ALL. The incidence of CNS disease in patients with AML was significantly higher at autopsy, however—39%. This study led to the practice of CNS prophylaxis in all patients with acute leukemia, including patients with AML. This practice was supported by results of a subsequent study that suggested that CNS prophylaxis in adult patients with AML is beneficial.[10] With the introduction of cytarabine-based induction chemotherapy during the 1980s, however, the routine practice of CNS prophylaxis for all AML patients was abandoned by most centers, including ours.
The estimated incidence of CNS involvement is largely dependent on who is screened for this complication. On the basis of the combination of overt neurological symptoms and leukemia blasts in the CSF, CNS involvement is considered a rare event [11,12]. Similar to previous reports [2,4-6,8,13,14], we found that 3.3% of patients with AML had documented CNS involvement at least once during the course of their disease. This is probably an underestimation of the true incidence of CNS involvement and evidence from last decade suggest that using flow cytometry will significantly increase the detection rates of CNS involvement [15].
Of the 45 patients diagnosed with CNS leukemia, only 12had CNS leukemia at initial presentation. However, when LP was performed as part of an investigational protocol in all newly diagnosed patients, the incidence of CNS involvement was significantly higher. Among 42 patients who underwent LP at diagnosis, 19% (n = 8) had leukemic blasts in the CSF. LP is routinely performed in all newly diagnosed pediatric patients with AML, and the incidence of CNS disease at time of diagnosis varies from 6% to 29% in different series[16-22]. Arguably, occult CNS involvement is effectively treated with high-dose cytarabine that penetrates the blood brain barrier[7] and eliminates CNS leukemia. A report of an extremely low incidence of meningeal relapse in patients with AML treated with cytarabine-containing regimens supports this claim.[23] However, a recent report from the Fred Hutchinson Cancer Research Center suggests that systemic cytarabine-based chemotherapy may not be sufficient. Although that study included a larger percentage of patients with poor prognosis, the authors reported an 11% incidence of positive CSF cytology in post-induction patients who underwent routine diagnostic LP prior to stem cell transplantation[24]. Similarly, in a series of patients with AML who underwent hematopoietic stem cell transplantation, Bommer et al. found that 15% of patients had CNS involvement throughout the treatment period[25].
We have found no difference in post-induction rates of CNS disease in AML patients who received high-dose cytarabine-based induction chemotherapy and those who were treated with either low-dose cytarabine or with induction regimens that did not include cytarabine. Therefore the CNS prophylaxis policy for newly diagnosed AML should be considered for all patients, regardless of the induction chemotherapy regimen.
Patients with CNS disease had a significantly shorter median DFS, and most CNS relapses occurred with concomitant BM relapse. Whether CNS disease simply reflects the bulk of the disease, as is suggested by the correlation with the percentage of BM blasts, or whether the CNS serves as a sanctuary for leukemia blasts from which they can be re-seeded systemically is not known. However, in patients with ALL, CNS prophylaxis remains the single best approach for preventing CNS and systemic recurrence.[26]
Identifying newly diagnosed AML patients who are at risk for having CNS disease either at diagnosis or during the course of the disease will enable us to restrict the use of CNS prophylaxis to those patients who are most likely to benefit. For example, 20% (n = 7) of patients with LDH > 5000 IU/L at time of diagnosis developed CNS disease during the course of their disease. However, if CNS prophylaxis is restricted to patients with high LDH, most patients who eventually developed CNS disease would have been missed. Furthermore, because the sensitivity of CSF cytology may be as low as 50% [15], even patients with negative CSF may still carry undetectable CNS disease and probably benefit from intrathecal treatment.
CNS dissemination of leukemia cells has been associated with monocytic blast morphology, elevated white blood cell count, and specific cytogenetics abnormalities, in particular inversion(16), trisomy(8) and 11q23[3,8]. Remarkably, none were independent predictors. Elevated LDH at time of diagnosis was an independent prognostic indicator, probably because it reflected disease burden. As a continuous variable, LDH had minimal predictive power. Whether the cutoff of 700 IU/L, which we found using ROC analysis, is a good predictor disease, should be determined in a separate validation cohort. A higher frequency of CNS involvement in patients with the FAB-M5 subtype and a lower incidence in patients with myelodysplastic syndrome-related AML (Table I) are fully accounted for by the higher LDH in patients with FAB-M5 and lower LDH in patients with myelodysplastic syndrome-related AML (compared to LDH in the entire cohort). CNS involvement was more common in young patients and among African-American patients. This finding may reflect racial differences in the clinical and biologic characteristics of AML. Such a finding was previously reported. For example, higher incidence of CNS involvement in African-Americans was also found in a retrospective analysis of 2,570 patients from 7 Cancer and Leukemia Group B studies[27].
Differences in patient selection may explain inconsistencies in different reports regarding the significance of cytogenetics findings in predicting CNS involvement. For example, Shihadeh et al. referred to patients with cranial MRI abnormalities and normal CSF as having CNS leukemia[8]. Although MRI has been reported to be a highly sensitive tool for the detection of meningeal pathology, its specificity is low as it detects clinically nonspecific abnormalities[14,28]. Therefore we did not include patients with abnormal MRI and normal CSF in our analysis.
In conclusion, although CNS leukemia is detected in less than 10% of adult patients with ALL at the time of diagnosis,[2,4-6,14] intrathecal prophylaxis chemotherapy is routinely administered and has been proven to be beneficial[26]. In contrast, intrathecal prophylaxis chemotherapy is not routinely administered to adult patients with AML.
According to our analysis the incidence of CNS leukemia in patients with newly diagnosed AML is higher than currently appreciated. If LP was performed in all AML patients at diagnosis, the incidence of CNS disease would have approached approximately 20%. These findings support the need for prospective studies to determine whether CNS prophylaxis should be administered as part of induction chemotherapy in all patients with newly diagnosed AML.
Acknowledgments
We thank Luanne Jorewicz for reviewing our manuscript.
Grant Support: This work was supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant P30 CA016672.
Footnotes
Authorship: Conception: ZE, UR, MO.
Data collection and assembly of data: UR, MO, SP.
Provision of study patients: FR, GGM, SF, JC, HK, ZE.
Manuscript writing: UR, ZE.
Final approval of manuscript: All authors.
Competing interests: The authors have no competing interests.
References
- 1.Schiffer CA. Involvement of the central nervous system with acute myeloid leukemia. UpToDate; MA: Waltham: 2013. [Google Scholar]
- 2.Cortes J, O'Brien SM, Pierce S, Keating MJ, Freireich EJ, Kantarjian HM. The value of high-dose systemic chemotherapy and intrathecal therapy for central nervous system prophylaxis in different risk groups of adult acute lymphoblastic leukemia. Blood. 1995;86:2091–2097. [PubMed] [Google Scholar]
- 3.Holmes R, Keating MJ, Cork A, et al. A unique pattern of central nervous system leukemia in acute myelomonocytic leukemia associated with inv(16)(p13q22) Blood. 1985;65:1071–1078. [PubMed] [Google Scholar]
- 4.Kantarjian HM, O'Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 5.Lazarus HM, Richards SM, Chopra R, et al. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis: results from the international ALL trial MRC UKALL XII/ECOG E2993. Blood. 2006;108:465–472. doi: 10.1182/blood-2005-11-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersdorf SH, Kopecky KJ, Head DR, et al. Comparison of the L10M consolidation regimen to an alternative regimen including escalating methotrexate/L-asparaginase for adult acute lymphoblastic leukemia: a Southwest Oncology Group Study. Leukemia. 2001;15:208–216. doi: 10.1038/sj.leu.2402006. [DOI] [PubMed] [Google Scholar]
- 7.Slevin ML, Piall EM, Aherne GW, Harvey VJ, Johnston A, Lister TA. Effect of dose and schedule on pharmacokinetics of high-dose cytosine arabinoside in plasma and cerebrospinal fluid. J Clin Oncol. 1983;1:546–551. doi: 10.1200/JCO.1983.1.9.546. [DOI] [PubMed] [Google Scholar]
- 8.Shihadeh F, Reed V, Faderl S, et al. Cytogenetic profile of patients with acute myeloid leukemia and central nervous system disease. Cancer. 2012;118:112–117. doi: 10.1002/cncr.26253. [DOI] [PubMed] [Google Scholar]
- 9.Wolk RW, Masse SR, Conklin R, Freireich EJ. The incidence of central nervous system leukemia in adults with acute leukemia. Cancer. 1974;33:863–869. doi: 10.1002/1097-0142(197403)33:3<863::aid-cncr2820330336>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Stewart DJ, Keating MJ, McCredie KB, et al. Natural history of central nervous system acute leukemia in adults. Cancer. 1981;47:184–196. doi: 10.1002/1097-0142(19810101)47:1<184::aid-cncr2820470130>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Castagnola C, Nozza A, Corso A, Bernasconi C. The value of combination therapy in adult acute myeloid leukemia with central nervous system involvement. Haematologica. 1997;82:577–580. [PubMed] [Google Scholar]
- 12.Peterson BA, Brunning RD, Bloomfield CD, et al. Central nervous system involvement in acute nonlymphocytic leukemia. A prospective study of adults in remission. Am J Med. 1987;83:464–470. doi: 10.1016/0002-9343(87)90756-x. [DOI] [PubMed] [Google Scholar]
- 13.Rees JK, Gray RG, Swirsky D, Hayhoe FG. Principal results of the Medical Research Council's 8th acute myeloid leukaemia trial. Lancet. 1986;2:1236–1241. doi: 10.1016/s0140-6736(86)92674-7. [DOI] [PubMed] [Google Scholar]
- 14.Pui CH, Thiel E. Central nervous system disease in hematologic malignancies: historical perspective and practical applications. Semin Oncol. 2009;36:S2–S16. doi: 10.1053/j.seminoncol.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crespo-Solis E, Lopez-Karpovitch X, Higuera J, Vega-Ramos B. Diagnosis of acute leukemia in cerebrospinal fluid (CSF-acute leukemia) Curr Oncol Rep. 2012;14:369–378. doi: 10.1007/s11912-012-0248-6. [DOI] [PubMed] [Google Scholar]
- 16.Abbott BL, Rubnitz JE, Tong X, et al. Clinical significance of central nervous system involvement at diagnosis of pediatric acute myeloid leukemia: a single institution's experience. Leukemia. 2003;17:2090–2096. doi: 10.1038/sj.leu.2403131. [DOI] [PubMed] [Google Scholar]
- 17.Bisschop MM, Revesz T, Bierings M, et al. Extramedullary infiltrates at diagnosis have no prognostic significance in children with acute myeloid leukaemia. Leukemia. 2001;15:46–49. doi: 10.1038/sj.leu.2401971. [DOI] [PubMed] [Google Scholar]
- 18.Creutzig U, Zimmermann M, Ritter J, et al. Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia. 2005;19:2030–2042. doi: 10.1038/sj.leu.2403920. [DOI] [PubMed] [Google Scholar]
- 19.Gibson BE, Wheatley K, Hann IM, et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19:2130–2138. doi: 10.1038/sj.leu.2403924. [DOI] [PubMed] [Google Scholar]
- 20.Johnston DL, Alonzo TA, Gerbing RB, Lange BJ, Woods WG. The presence of central nervous system disease at diagnosis in pediatric acute myeloid leukemia does not affect survival: a Children's Oncology Group study. Pediatr Blood Cancer. 2010;55:414–420. doi: 10.1002/pbc.22511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pui CH, Dahl GV, Kalwinsky DK, et al. Central nervous system leukemia in children with acute nonlymphoblastic leukemia. Blood. 1985;66:1062–1067. [PubMed] [Google Scholar]
- 22.Webb DK, Harrison G, Stevens RF, et al. Relationships between age at diagnosis, clinical features, and outcome of therapy in children treated in the Medical Research Council AML 10 and 12 trials for acute myeloid leukemia. Blood. 2001;98:1714–1720. doi: 10.1182/blood.v98.6.1714. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Cuadron D, Montesinos P, Perez-Sirvent M, et al. Central nervous system involvement at first relapse in patients with acute myeloid leukemia. Haematologica. 2011;96:1375–1379. doi: 10.3324/haematol.2011.042960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayadev JS, Douglas JG, Storer BE, Appelbaum FR, Storb R. Impact of cranial irradiation added to intrathecal conditioning in hematopoietic cell transplantation in adult acute myeloid leukemia with central nervous system involvement. Int J Radiat Oncol Biol Phys. 2011;80:193–198. doi: 10.1016/j.ijrobp.2010.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bommer M, von Harsdorf S, Dohner H, Bunjes D, Ringhoffer M. Neoplastic meningitis in patients with acute myeloid leukemia scheduled for allogeneic hematopoietic stem cell transplantation. Haematologica. 2010;95:1969–1972. doi: 10.3324/haematol.2010.025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surapaneni UR, Cortes JE, Thomas D, et al. Central nervous system relapse in adults with acute lymphoblastic leukemia. Cancer. 2002;94:773–779. doi: 10.1002/cncr.10265. [DOI] [PubMed] [Google Scholar]
- 27.Sekeres MA, Peterson B, Dodge RK, et al. Differences in prognostic factors and outcomes in African Americans and whites with acute myeloid leukemia. Blood. 2004;103:4036–4042. doi: 10.1182/blood-2003-09-3118. [DOI] [PubMed] [Google Scholar]
- 28.Phillips ME, Ryals TJ, Kambhu SA, Yuh WT. Neoplastic vs inflammatory meningeal enhancement with Gd-DTPA. J Comput Assist Tomogr. 1990;14:536–541. doi: 10.1097/00004728-199007000-00007. [DOI] [PubMed] [Google Scholar]