Abstract
Observational spatial memory (OSM) refers to the ability of remembering food caches made by other individuals, enabling observers to find and pilfer the others’ caches. Within birds, OSM has only been demonstrated in corvids, with more social species such as Mexican jays (Aphelocoma ultramarine) showing a higher accuracy of finding conspecific’ caches than less social species such as Clark’s nutcrackers (Nucifraga columbiana). However, socially dynamic corvids such as ravens (Corvus corax) are capable of sophisticated pilfering manoeuvres based on OSM. We here compared the performance of ravens and jackdaws (Corvus monedula) in a short-term OSM task. In contrast to ravens, jackdaws are socially cohesive but hardly cache and compete over food caches. Birds had to recover food pieces after watching a human experimenter hiding them in 2, 4 or 6 out of 10 possible locations. Results showed that for tests with two, four and six caches, ravens performed more accurately than expected by chance whereas jackdaws did not. Moreover, ravens made fewer re-visits to already inspected cache sites than jackdaws. These findings suggest that the development of observational spatial memory skills is linked with the species’ reliance on food caches rather than with a social life style per se.
Keywords: Corvid, Observational spatial memory, Raven, Jackdaw
Introduction
Food caching has evolved in a wide range of mammalian and avian taxa, probably as an adaptation to an environment with high variability in food abundance (Vander Wall 1990). Among passerine birds, parids and corvids are famous for hoarding food in many different caches for a few weeks or even up to several months (e.g. Balda et al. 1987; Krebs 1990). Birds of both taxonomic groups show excellent spatial memory for the location of caches, enabling them to retrieve food more efficiently than they would do by chance (e.g. Kamil and Balda 1985; Brodin 1994). Within each taxonomic group, however, pronounced species differences can be found in spatial memory (review in de Kort et al.2006), which tend to be correlated with hippocampal size (Healy and Krebs 1992, 1996; Brodin and Lundborg 2003; Lucas et al. 2004) and with the species’ dependence upon cached food (Balda and Kamil 1989; Clayton and Krebs 1994a, b). For instance, assiduous storers such as pinyon jays (Gymnorhinus cyanocephalus) and Clark’s nutcrackers (Nucifraga columbiana) perform better in long-term spatial memory tests than Mexican jays (Aphelocoma ultramarina) and scrub jays (Aphelocoma coerulescens) (Balda et al. 1997).
Through observational spatial memory, individuals are able to remember caches they have seen others make and, consequently, to pilfer those caches in the others’ physical absence. An important difference between spatial memory and observational spatial memory is that for the former, birds are actively caching food during acquisition whereas for the latter birds are just acting as bystanders at caching. Observational spatial memory might thus be seen as a form of social learning, featuring aspects of delayed local enhancement (i.e. attraction to specific locations by a conspecific, Galef 1988) and object permanence (i.e. memory for items that are temporarily out of view, Piaget 1954). Interestingly, observational spatial memory has been experimentally demonstrated for several corvid species (Bednekoff and Balda 1996a, b; Heinrich and Pepper 1998) whereas, to our knowledge, it has not been shown for parids (Baker et al. 1988). Furthermore, comparative studies on North American corvids indicate that highly social species like Mexican jays perform better in observational spatial memory tasks than less social species such as territorial living Clark’s nutcrackers (Bednekoff and Balda 1996a).
In this study, we compared the performance of two European corvid species, ravens (Corvus corax) and jackdaws (Corvus monedula), in respect to their observational spatial memory skills. The social life of ravens is characterised by a high degree of fission–fusion dynamics (Aureli et al. 2008), with non-breeders regularly forming foraging flocks when scavenging on carcasses of larger animals (Heinrich 1989; Marzluff and Heinrich 1991). Crowd-foraging ravens hardly feed at the food source but carry off consecutive loads of food to cache privately and outside of the view of others (Heinrich and Pepper 1998). They also heavily compete over caches with conspecifics, building on their ability to remember caches they have seen others make (Bugnyar and Kotrschal 2002; Bugnyar and Heinrich 2005, 2006). Jackdaws feed mainly on insects, regularly forage in flocks of varying size, and breed in colonies (Goodwin 1976). Hence, they are considered to be more permanently social than ravens. Concerning caching, however, jackdaws perform the behaviour at low frequency (Lorenz 1932; Strauss 1938). They are also reported to lack some of the behavioural elements of caching (Schwab et al., unpublished data), notably covering of items with substrate, after inserting it into a gap (Bugnyar et al 2007). In more recent papers, they are even listed as non-storers (de Kort and Clayton 2006; Lucas et al. 2004). When tested for spatial memory abilities, jackdaws appeared to use different cues than typical long-term storers such as European jays (Garrulus glandarius) (Clayton and Krebs 1994a, b). However, jackdaws are able social learners (Röell 1978) and do possess excellent object permanence skills (Ujfallusy et al., unpublished data), suggesting that they may use foraging individuals to localise food resources and would have the potential to delay exploiting these resources because they can remember items that are temporarily outside view.
We tested subadult ravens and jackdaws for their short-term observational spatial memory skills in an experimental array with ten possible cache locations (stones or wooden blocks, known to the bird from pre-experimental trials). Birds could first witness a human experimenter hiding food in two, four, or six out of the ten possible cache sites and then were allowed to search for the caches in an immediately following pilfering trial. Previous studies showed that both ravens (e.g. Bugnyar et al 2007) and jackdaws (Ujfalussy et al., unpublished data) are sensitive to the actions of humans and readily retrieve food hidden by an experimenter. According to differences in foraging behaviour between the two species, we predicted that the frequently caching and pilfering ravens should perform more accurately in our experiment, i.e. they should make fewer errors in searching for hidden food and fewer revisits of already inspected sites, than jackdaws. Alternatively, if observational spatial memory skills evolved as an adaptation of sociality, e.g. reflecting a prerequisite for social learning, then the jackdaws should perform at least as well as the ravens.
Methods
Study site and animals
Ravens
The ravens were housed at the Cumberland Game Park in the northern part of the Austrian Alps. Their enclosure consisted of an external aviary (200 m2), and an internal experimental compartment (30 m2). The external aviary was equipped with tree trunks, natural vegetation and rocks. The internal compartment was divided in several experimental rooms, where they had perches at their disposal. They had ad libitum access to water and were fed twice daily, with beef, milk products, fruits and bread.
The birds hatched in spring 2004 and were hand-raised at the Konrad Lorenz Research Station in Grünau. They had been in contact with humans since their nestling period and were used to participate in different kinds of experiments, some of which involved humans as experimenters providing information about the location of food (Schloegl et al. 2008a, b). All birds had thus ample opportunity to associate certain behaviours of humans with the possibility of getting food. During the study period, the group was composed of nine birds, out of which eight (four males, four females) participated in the current study. The individuals were identified with coloured leg bands.
Jackdaws
The jackdaws were also housed in an outdoor aviary at the Konrad Lorenz Research Station. Their aviary was divided into two parts: a large external aviary (90 m2) and an experimental area (25 m2), which was composed of several compartments. The aviary was equipped with tree trunks, perches and vegetation. The birds were fed three times a day with a mixture of worms, milk products and meat; occasionally they received live insects, rice, potatoes and fruits.
The jackdaws were born in spring 2005 and were hand-raised at the Konrad Lorenz Research Station. Like the ravens, they were totally habituated to human presence and were trained to individually take part in experiments. At the time of this study, all subjects had already participated in an experiment involving a human experimenter hiding food (Ujfalussy et al., unpublished data) and, during daily life, they had ample opportunities to associate certain human behaviours with feeding opportunities. The group was composed of 20 individuals, out of which eight birds (six males, two females) participated in the current study. Individuals were identified with coloured leg bands.
Experimental set-up
The study was conducted in October and November 2005. Birds were tested in the morning or afternoon, approximately two hours after feeding. Thus, neither jackdaws nor ravens were food deprived on testing days. The tests took place in the experimental rooms of the aviaries. The food utilised for the tests consisted of small pieces of emmental cheese (1 cm3) for ravens and cat food (croquettes) for jackdaws. The objects used to hide the food were stones for ravens and wooden blocks for the jackdaws. These objects had a size and a weight so that the birds could move it easily (ca. 100 g for ravens, 50 g for jackdaws). Ten cache sites (i.e. stones or wooden blocks) were disposed on the gravel floor of the experimental room, so that the total area was evenly used (distance between cache sites ca. 0.8 m for ravens, 0.5 m for jackdaws). The gravelled floor allowed the food to be buried without causing the occluding object to be raised higher than those stones/wooden blocks that did not cover food. The position of cache sites in the experimental room was modified for each test, so that the birds could not use long-term memory for previous sites to find the food. All birds were habituated to this set-up and participated in other experiments in which these objects were used.
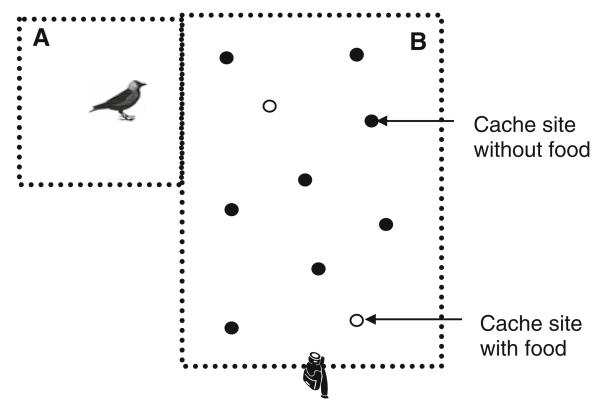
For testing, one bird was isolated from the group in the small experimental room A (Fig. 1) where it could see the total area of the large experimental room B through a wire mesh. The experimenter (CS) hid the pieces of food randomly under 2, 4 or 6 covers. While she was caching the food, the experimenter made sure that the bird was attentive by calling its name and waiting until the bird had oriented in the appropriate direction. This procedure has been done in order to reduce possible effects of attentiveness on the performance in the task (Scheid et al. 2007). One minute after the caching, the door to room A was opened by the experimenter and the bird was allowed to enter the experimental room B.
Fig. 1.
Schema of the experimental setup, showing a bird isolated in the room A, the ten cache sites (two of them being baited) placed in room B, and the position of the video camera
The retrieval phase started when the bird entered the room B and it finished either with the bird having recovered all food caches or, in some cases, when the bird had stopped searching for 120 s and was sitting on a perch or resting. “Searching” was defined by the bird walking on the floor from cache site to cache site, visually inspecting sites and moving the occluding objects with the beak. All birds received five trials per condition (two, four, six caches) in a randomised order and were tested only once a day. The birds’ behaviour during the retrieval phase was video-taped for later analysis. Video recordings were analysed by CS. A subset of 20 test trials (10 for ravens and 10 for jackdaws) were also analysed by a naïve observer. The inter observer reliability for the frequencies of visits to unbaited sites, of revisits and of spatial location of errors was 94%.
Previous studies on ravens (Heinrich and Pepper 1998; Bugnyar and Kotrschal 2002) and pilot studies on jackdaws (Ujfalussy et al., unpublished data) indicated that neither of the two species made use of olfactory cues for locating hidden food. Nevertheless, all ten covers were brought in contact to food prior to the experimental trials to control for possible effects of smell. Although chances were low that birds would rely on olfaction, a possible confound in our set-up could be seen in the different food types used: potentially, the birds could be more sensitive to the smell of the food type used for ravens (cheese) than the one used for jackdaws (cat food). We thus checked post hoc if jackdaws would perform differentially when searching for finding hidden cheese and cat food. Six birds (three males and three females) that did not participate in the original experiment were allowed to choose between two cups of which only one contained food (cheese or cat food). Both cups were covered with a paper lid perforated with tiny holes to prevent birds from seeing but not from smelling the reward. Each jackdaw performed 20 trials (10 with cheese, 10 with cat food, presented in randomised order); the probability to find the food by chance was 50%.
Analysis
For each bird and condition, we determined the number of unbaited sites visited before recovering all food caches (note that this number does not incorporate repeated visits to the same site). The maximum number of unbaited sites a bird can visit was 8 in the condition with two food caches, 6 in the condition with four food caches and 4 in the condition with six food caches. Additionally, we calculated the number of revisits, i.e. the number of times that a bird came back to check a cache (by lifting its cover) that it had already inspected before (= number of revisits). Finally, for the condition with two food caches, we recorded the frequency of visits to the unbaited site that was the closest to a baited site, in order to see if the errors made by the birds were random or if the birds did remember the approximate area where the food was hidden. In the condition with two food caches, there were two closest unbaited sites (one for each food cache) and therefore, the probability of visiting one of these unbaited sites by chance is 2/8 = 0.25.
To test if the birds performed above chance level, we evaluated for each condition, the expected frequencies for visits of unbaited sites before recovering all food caches, calculating conditional probabilities for the non-replacement sampling case with the formula: where r is the number of visits to unbaited sites, n the total number of sites and k the number of baited sites. We then compared the expected frequencies with the observed frequencies of unbaited sites visited with a Chi-square goodness-of-fit test. Similarly, we calculated the expected number of unbaited sites visited before recovering all food caches with the formula: and compared it with the observed number. In either case, the analysis was restricted to those cases in which the birds retrieved all food caches per trial.
A binomial test was used to test if the birds of each species did visit the unbaited site that was the closest from a baited site more often than expected by chance. We also used a binomial test to see if birds performed above chance before recovering the first food cache. We used a Mann–Whitney U test to compare the number of revisits between species as well as the number of errors made relative to the time spent searching between species. Finally, to test if the performance of jackdaws was influenced by the type of food, we compared the birds’ success ratio with each type of food using a Wilcoxon-signed-ranks-test.
Statistics were calculated by hand and performed according to Siegel and Castellan (1988). All tests were two-tailed, with α = 0.05. Probabilities were corrected for multiple comparisons according to Benjamini and Yekutieli (2001) with the corrected significance criterion α = 0.027. For the condition with six caches, we excluded one jackdaw from the analysis because this bird made very few searches and stopped before recovering any food.
Results
Probability of finding observed caches
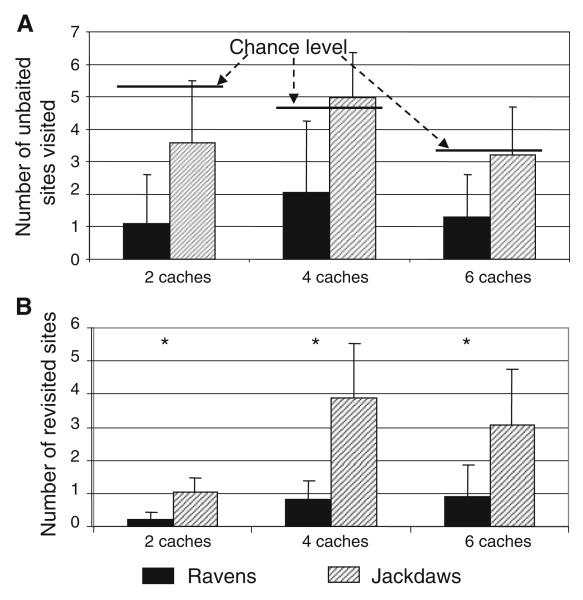
If birds had performed at chance level in searching for the baited caches, the mean number of unbaited sites visited before finding the food would have been 5.3 for the two-cache condition, 4.8 for the four-cache condition and 3.4 for the six-cache condition. The mean number of unbaited sites visited by ravens was 1.1 ± 1.5, 2.0 ± 2.1 and 1.3 ± 1.3 for conditions with two, four and six food caches, respectively (Fig. 2a). We, thus, found a significant difference between observed and expected frequencies in all three conditions (two caches: X2 = 196.2, df = 5, P = 0.001; four caches: X2 = 94.9, df = 3, P = 0.001; six caches: X2 = 116.1, df = 2, P = 0.001). In jackdaws, the number of unbaited sites visited was 3.6 ± 1.9, 4.9 ± 1.4, 3.2 ± 1.5 for conditions with two, four and six food caches, respectively (Fig. 2a). Contrary to ravens, the frequencies observed in jackdaws did not differ significantly from chance level (two caches: X2 = 2.13, df = 5, P = 0.9; four caches: X2 = 0.52, df = 3, P = 0.9; six caches: X2 = 0.14, df = 2, P = 0.9).
Fig. 2.
Number of unbaited sites visited (a) and number of revisited sites (b) for ravens and jackdaws, and for test conditions with two, four and six food caches. Diagrams represent mean values and standard deviations of number unbaited sites visited and number of revisited sites. Chance levels for visits to unbaited sites are indicated as solid lines above diagrams. Asterisks indicate significant results of Mann–Whitney tests
Both, ravens and jackdaws, searched fewer unbaited sites than would be expected by chance before recovering the first food cache in the two-cache condition (Binomial test, P = 0.001, 0.008, respectively). However, whereas ravens continued to perform better than expected by chance when finding the first cache in the four and six-cache condition (Binomial test, P = 0.001, for four and for six caches), the jackdaws did not (Binomial test, P = 0.387, for four and six caches).
Search pattern, types of errors and efficiency
Unbaited sites that were visited by ravens were more frequently next to a baited cache than would be expected by chance (P = 0.012, Binomial test), indicating that the ravens’ direction of search was not random, at least in the two-cache condition. No such effect was found in jackdaws (P = 0.4, Binomial test). Likewise, the two species differed in their propensity to revisit previously searched sites (Fig. 2b). The mean number of revisited caches was significantly lower for the ravens than for the jackdaws in all three conditions (two caches: m = 8, n = 8, Cl = 40, P = 0.0018; four caches: m = 8, n = 8, Cl = 36, P = 0.0002; six caches: m = 8, n = 8, Cl = 41, P = 0.003, Mann–Whitney U test).
These differences in searching behaviour were not caused by a lack of motivation on side of the jackdaws because the time spent searching for caches did not differ significantly from that of ravens in any of the conditions (two caches: 32.9 ± 14.7 s vs. 28.9 ± 14.9 s: Cl = 63, P = 0.64; four caches: 71.9 ± 20.3 s vs. 61.2 ± 25.0 s, Cl = 60, P = 0.44; six caches 110.3 ± 58.6 s vs. 58.8 ± 24.3 s: m = 8, n = 8, Cl = 50, P = 0.07, Mann–Whitney U test). Since the jackdaws generally made more visits to unbaited sites and re-visits to already checked sites than the ravens (Fig. 2a, b), they were also less efficient than the ravens in finding the hidden food relative to the time spent searching (two caches: Cl = 36, P = 0.002; four caches: Cl = 44, P = 0.01; six caches: Cl = 38, P = 0.006, Mann–Whitney U test).
Post hoc control for olfactory cues
The jackdaws’ success ratio in choosing the baited cup did not differ significantly between the trials with hidden cheese and hidden cat food (N = 6, T = 13.5, P = 0.34, Wilcoxon test). For either food type, the birds’ performance did not differ significantly from chance level (cheese: P = 0.45; cat food: P = 0.26, Binomial test).
Discussion
Taken together, the results of this study were clear-cut: ravens made fewer visits to unbaited cache sites than expected by chance whereas jackdaws did not. Ravens also made fewer re-visits to previously checked cache sites than jackdaws and, relative to the time spent searching, they were more efficient in finding food hidden by the experimenter than jackdaws were. Hence, the socially dynamic species which regularly stores and pilfers caches performed generally better in this short-term observational spatial memory task than the socially cohesive species which rarely caches and occasionally pilfers caches from other species.
In fact, the results for jackdaws provide little support for the use of advanced observational spatial memory skills, since the birds performed above chance only with the first cache in the relatively simple two-cache condition. Accordingly, jackdaws would be capable of remembering one cache they have seen being made, which corresponds to the findings that they are highly attracted to places where they have seen others manipulating food (Röell 1978; Schwab et al., unpublished data) and that they can track complicated displacements of an occluded item in object permanence tasks (Ujfalussy et al., unpublished data). However, when faced with more than one cache, they searched at random. Ravens, in contrast, had little problem in coping with a (limited) number of observed hiding events, this fits the results of previous studies (Heinrich and Pepper 1998; Bugnyar et al. 2007) and supports the idea that ravens utilise observational spatial memory for pilfering others’ food caches (Bugnyar and Kotrschal 2002).
Taken together, the results are in line with the assumption that food-storing birds possess advanced memory skills compared to non-storing species (Balda and Kamil 1989) and that remembering the caches of others reflects one aspect of this general pattern in corvids. Therefore, the better performance of ravens might simply be a result of their better overall memory skills in comparison to jackdaws. Still, there are a number of arguments why a different outcome could have been expected. From a phylogenetic point of view, it is likely that jackdaws have derived from a caching ancestor (de Kort et al. 2006), raising the possibility that they might have retained spatial memory capacities, originally shaped by caching but presently used predominantly in the social domain. Furthermore, the ability to remember observed caches has been discussed as a special form of memory, closely linked to social foraging and the opportunity for intra- and/or interspecific cache pilfering (Bednekoff and Balda 1996a). For instance, territorial living Clark’s nutcrackers, that are renowned for outperforming other species in spatial memory tasks (Bednekoff et al 1997; Olson 1991) proved to be inferior to highly social species such as Mexican jays in respect to their memory for observed caches (Bednekoff and Balda 1996a, b). Even though jackdaws hardly cache themselves, they regularly forage together with caching species such as rooks (Madge and Burn 1994) which, due to their body size, are capable of defending their caches but would be vulnerable to pilfering when absent. Anecdotal observations that jackdaws indeed pilfer food caches of other corvids (Clayton pers communication) are, to some extent, supported by the fact that in our experiment, birds were able to find one out of two observed caches above chance levels. However, this could be achieved by mechanisms such as delayed enhancement and/or Stage-6 competence in object permanence. Hence, our results speak against specific memory skills for locating observed caches in jackdaws, but support the assumption that these corvids possess a relatively poorly developed spatial memory in general (de Kort et al. 2006).
In their study on one-trial associative memory, Clayton and Krebs (1994a) also found that the non-storing jackdaws made more errors than frequently storing jays in discriminating between seeded and unseeded sites. Unfortunately, the set-up did not allow the authors to distinguish whether the difference was due to a greater accuracy or duration of spatial memory. Since in our study, the retrieval phase followed immediately after the observation of caching, the difference in observational spatial memory between jackdaws and ravens is likely to reflect a greater accuracy rather than longer duration of memory in ravens. The higher number of revisits to previously inspected sites made by jackdaws reflects another difference in memory mechanism between the two species. A previous comparative study (Gould-Beierle 2000) showed that unlike caching species, jackdaws tended to return to places where they had previously found food as opposed to switching to other locations when the food was depleted. Thus, it might be that jackdaws possess spatial memory skills but that they use the information differently.
Despite of the obvious differences between ravens and jackdaws in this experiment, results have to be treated with caution with respect to generalisation: searching for hidden food in a limited number of pre-determined cache locations may allow birds to use different behavioural tactics than when searching for caches under more natural conditions. Possibly, ravens could cope better with this particular situation than jackdaws (compare Kamil 1988; but note that the same jackdaws performed equally well as the ravens in other tasks using a similar set-up, e.g. Ujfalussy et al., unpublished data.). Further studies, using a more realistic scenario in terms of utilising big aviary compartments with natural vegetation and an almost unlimited number of potential cache sites, would be needed to clarify this point. In addition, switching to a more naturalistic foraging scenario would likely increase the costs of searching randomly and thus provide us with a more accurate estimate of how many of others’ caches birds are capable to remember for a short time period. Bednekoff and Balda (1997) suggested that, in laboratory experiments, many errors might not be due to forgetting where the caches are placed, but due to a change in motivation (e.g. exploration) when costs are relatively low.
Another factor that may be of importance in respect to observational spatial memory is the amount of attention birds pay to food-storing individuals. Indeed, in a recent study we found striking differences between ravens and jackdaws in when, what and whom they watched in a foraging task (Scheid et al 2007). This is why we took special care to attract the birds’ attention by calling their names and conspicuously showing the food item before making a cache, assuring that all individuals attended to the experimenter during caching. Unfortunately, our set-up does not allow us to exclude possible effects of olfactory cues; nevertheless, previous findings (Vander Wall 1982; Heinrich and Pepper 1998; Bugnyar and Kotrschal 2002) and our post hoc control suggest that the smell of hidden items is of limited importance for the birds’ likelihood of finding caches. Finally, because we proportionally matched the size of the test species with that of the experimental setup, the cache sites for jackdaws were closer together than the cache sites for ravens. The cache sites used for the jackdaws might therefore be harder to distinguish, and posed a greater memory challenge, than those used for ravens.
According to Kamil et al. (1994), spatial information processing includes perception, attention, encoding, retention, decoding and use of information. The next steps will be to evaluate the relative importance of each of these elements for observational spatial memory and to examine whether the differences observed between ravens and jackdaws relate to particular skills for spatial information processing or to a set of skills that may, in part, also be expressed in other domains.
Acknowledgments
This work has received funding from the European Community’s Sixth Framework Program (NEST 012929) and from the Austrian Science Fund (FWF projects R31-B03, P16939-B03). Research at KLF is permanently supported by the ‘Cumberland Stiftung’ and by the ‘Verein der Förderer’. C. Scheid was supported by a studentship of the French Ministry of Research. We are grateful to F. and M. Bertrand and to B. Voelkl for their help concerning statistics; K. Kotrschal and R. Noë for their advices; A. Wilkinson, C. Schloegl, C. Schwab and D. Ujfalussy for their help and three anonymous reviewers for their valuable comments. Nestlings were donated by the zoos München, Stralsund and Wuppertal. Permission to take nestlings from the wild was granted by the Ministerium für Landwirtschaft, Umweltschutz und Raumordnung des Landes Brandenburg.
Contributor Information
Christelle Scheid, IPHC-DEPE, ULP, CNRS, 23 rue Becquerel, 67087 Strasbourg, France.
Thomas Bugnyar, Konrad Lorenz Forschungsstelle Grünau and Deptartment of Neurobiology and Cognition, University of Vienna, Vienna, Austria.
References
- Aureli F, Schaffner CM, Boesch C, Bearder SK, Call J, Chapmann CA, Connor R, Di Fiore A, Dunbar RIM, Henzi SP, Holekamp K, Korstjens AH, Layton R, Lee P, Lehmann J, Manson JH, Ramos-Fernandez G, Strier KB, van Schaik CP. Fission–fusion dynamics: new research frameworks. Curr Anthropol. 2008 (in press) [Google Scholar]
- Baker MC, Stone E, Baker AEM, Shelden RJ, Skillicorn P, Mantych MD. Evidence against observational learning in storage and recovery of seeds by black-capped chickadees. Auk. 1988;105:492–495. [Google Scholar]
- Balda RP, Kamil AC. A comparative study of cache recovery by three corvid species. Anim Behav. 1989;38:486–495. [Google Scholar]
- Balda RP, Bunch KG, Kamil AC, Sherry DF, Tomback DF. Cache site-memory in birds. In: Kamil AC, Krebs JR, Pulliam HR, editors. Foraging behaviour. Plenum Press; New York: 1987. pp. 645–666. [Google Scholar]
- Balda RP, Kamil AC, Bednekoff PA. Predicting cognitive capacities from natural histories: examples from four Corvid species. Curr Ornithol. 1997;13:33–66. [Google Scholar]
- Bednekoff PA, Balda RP, Kamil AC, Hile AG. Long-term spatial memory in four seed-caching corvid species. Anim Behav. 1997;53:335–341. [Google Scholar]
- Bednekoff PA, Balda RP. Observational spatial memory in Clark’s nutcrackers and Mexican jays. Anim Behav. 1996a;52:833–839. [Google Scholar]
- Bednekoff PA, Balda RP. Social caching and observational spatial memory in pinyons jays. Behaviour. 1996b;133:807–826. [Google Scholar]
- Bednekoff PA, Balda RP. Clark’s nutcrackers spatial memory: many errors might not be due to forgetting. Anim Behav. 1997;54:691–698. doi: 10.1006/anbe.1997.0473. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]
- Brodin A. Separation of caches between individuals willow tits hoarding under natural conditions. Anim Behav. 1994;47:1031–1035. [Google Scholar]
- Brodin A, Lundborg K. Is hippocampus volume affected by specialization for food hoarding in birds? Proc R Soc Lond B. 2003;270:1555–1563. doi: 10.1098/rspb.2003.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnyar T, Kotrschal K. Observational learning and the raiding of food caches in ravens, Corvus corax: is it ‘tactical’ deception? Anim Behav. 2002;64:185–195. [Google Scholar]
- Bugnyar T, Heinrich B. Ravens, (Corvus corax) differentiate between knowledgeable and ignorant conspecifics. Proc R Soc B. 2005;272:1641–1646. doi: 10.1098/rspb.2005.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnyar T, Heinrich B. Pilfering ravens, Corvus corax, adjust their behaviour to social context and identity of competitors. Anim Cogn. 2006;9:369–376. doi: 10.1007/s10071-006-0035-6. [DOI] [PubMed] [Google Scholar]
- Bugnyar T, Stöwe M, Heinrich B. The ontogeny of caching in ravens, Corvus corax. Anim Behav. 2007;74:757–767. [Google Scholar]
- Clayton NS, Krebs JR. One-trial associative memory: comparison of food storing and nonstoring species of birds. Anim Learn Behav. 1994a;22:366–372. [Google Scholar]
- Clayton NS, Krebs JR. Memory for spatial and object-specific cues in food-storing and non-storing birds. J Comp Physiol A. 1994b;174:371–379. [Google Scholar]
- De Kort SR, Clayton NS. An evolutionary perspective on caching by corvids. Proc R Soc. 2006;273:417–423. doi: 10.1098/rspb.2005.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kort SR, Tebbich S, Dally JM, Emery NJ, Clayton NS. The comparative cognition of caching. In: Zentall TR, Wasserman E, editors. Comparative cognition. Oxford University Press; New York: 2006. pp. 602–618. [Google Scholar]
- Galef BG. Imitation in animals: History, definitions, and interpretation of data from the psychological laboratory. In: Zentall TR, Galef BG, editors. Social learning: psychological and biological perspectives. Erlbaum; Hillsdale: 1988. pp. 3–28. [Google Scholar]
- Goodwin D. Crows of the world. British Museum of Natural History Publications; London: 1976. [Google Scholar]
- Gould-Beierle K. A comparison of four corvid species in a working and reference memory task using a radial maze. J Comp Psychol. 2000;114:347–356. doi: 10.1037/0735-7036.114.4.347. [DOI] [PubMed] [Google Scholar]
- Healy SD, Krebs JR. Food storing and the hippocampus in Corvids: amount and volume are correlated. Proc R Soc Lond B. 1992;248:241–245. [Google Scholar]
- Healy SD, Krebs JR. Food storing and the hippocampus in Paridae. Brain Behav Evol. 1996;47:195–199. doi: 10.1159/000113239. [DOI] [PubMed] [Google Scholar]
- Heinrich B, Pepper JW. Influence of competitors on caching behaviour in the common raven, Corvus corax. Anim Behav. 1998;56:1083–1090. doi: 10.1006/anbe.1998.0906. [DOI] [PubMed] [Google Scholar]
- Heinrich B. Ravens in winter. Simon and Schuster; New York: 1989. [Google Scholar]
- Kamil AC. A synthetic approach to the study of animal intelligence. In: Leger DW, editor. Comparative perspectives in modern psychology: Nebraska symposium on motivation. Vol. 35. University of Nebraska Press; Lincoln: 1988. pp. 230–257. [PubMed] [Google Scholar]
- Kamil AC, Balda RP, Olson DJ. Performance of four seed caching corvid species in the radial maze analog. J Comp Psychol. 1994;108:385–393. doi: 10.1037/0735-7036.108.4.385. [DOI] [PubMed] [Google Scholar]
- Kamil AC, Balda RP. Cache recovery and spatial memory in Clark’s nutcracker. J Exp Psychol Anim Behav Process. 1985;11:95–111. [Google Scholar]
- Krebs JR. Food storing birds: adaptive specialization in brain and behaviour? Phil Trans R Soc B. 1990;329:55–62. doi: 10.1098/rstb.1990.0160. [DOI] [PubMed] [Google Scholar]
- Lorenz K. Betrachtungen über das Erkennen der arteigenen Triebhandlungen der Vögel. J Orn. 1932;80:51–98. [Google Scholar]
- Lucas JR, Brodin A, de Kort SR, Clayton NS. Does hippocampal size correlate with the degree of caching specialization? Proc R Soc B. 2004;271:2423–2429. doi: 10.1098/rspb.2004.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madge S, Burn H. Crows and Jays. C Helm; London: 1994. [Google Scholar]
- Marzluff JM, Heinrich B. Foraging by common ravens in the presence and absence of territory holders: an experimental analysis of social foraging. Anim Behav. 1991;42:755–770. [Google Scholar]
- Olson DJ. Species differences in spatial memory among Clark’s nutcrackers, scrub jays and pigeons. J Exp Psychol Anim Behav Process. 1991;17:363–376. doi: 10.1037//0097-7403.17.4.363. [DOI] [PubMed] [Google Scholar]
- Piaget J. Construction of reality in the child. Norton; New York: 1954. [Google Scholar]
- Röell A. Social behaviour of the jackdaw, Corvus monedula, in relation to its niche. Behavior. 1978;64:1–124. [Google Scholar]
- Scheid C, Range F, Bugnyar T. When, what and whom to watch? Quantifying attention in ravens (Corvus corax) and jackdaws (Corvus monedula) J Comp Psy. 2007;121:380–386. doi: 10.1037/0735-7036.121.4.380. [DOI] [PubMed] [Google Scholar]
- Schloegl C, Kotrschal K, Bugnyar T. Do common ravens (Corvus corax) rely on human or conspecific gaze cues to detect hidden food? Anim Cogn. 2008a doi: 10.1007/s10071-007-0105-4. (in press) [DOI] [PubMed] [Google Scholar]
- Schloegl C, Kotrschal K, Bugnyar T. Modifying the object-choice task: is the way you look important for ravens? Behav Proc. 2008b;77:61–65. doi: 10.1016/j.beproc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ. Nonparametric statistics for the behavioural sciences. McGraw-Hill; Boston: 1988. [Google Scholar]
- Strauss E. Vergleichende Beobachtungen über Verhaltensweisen von Rabenvögeln. Z Tierpsychol. 1938;2:145–172. [Google Scholar]
- Vander Wall SB. Food hoarding in animals. University of Chicago Press; Chicago: 1990. [Google Scholar]
- Vander Wall SB. An experimental analysis of cache recovery in Clark’s nutcracker. Anim Behav. 1982;30:84–94. [Google Scholar]