Abstract
Averse effects of social stress may be buffered by the presence of social allies, which mainly has been demonstrated in mammals and recently also in birds. However, effects of socio-positive behavior prior to fledging in relation to corticosterone excretion in altricial birds have not been investigated yet. We here monitored corticosterone excretion patterns in three groups of hand raised juvenile ravens (n=5, 6 and 11) in the nest, post-fledging (May–July) and when ravens would be independent from their parents (September–November). We related these corticosterone excretion patterns to socio-positive behavior. Behavioral data were collected via focal sampling in each developmental period considered. We analyzed amounts of excreted immunoreactive corticosterone metabolites (CM) using enzyme immuno assays. We collected fecal samples in each developmental period considered and evaluated the most appropriate assay via an isolation stress experiment. Basal CM was significantly higher during the nestling period than post-fledging or when birds were independent. The time nestlings spent allopreening correlated negatively with mean CM. Post-fledging, individuals with higher CM levels sat close to (distance <50 cm) conspecifics more frequently and tended to preen them longer. When birds were independent and a stable rank hierarchy was established, dominant individuals were preened significantly longer than subordinates. These patterns observed in ravens parallel those described for primates, which could indicate that animal species living in a complex social environment may deal with social problems in a similar way that is not restricted to mammals or primates.
Keywords: Ravens, Corvus corax, Corticosterone, Affiliative relationships, Enzyme immunoassay
Introduction
Social challenges and changes in the social environment are one of the most potent stressors (Sapolsky, 1992; DeVries et al., 2003). On the other hand, the presence of social allies may buffer averse effects of social stress (e.g., Creel, 2001; Mendl, 2001; Sgoifo et al., 2001), which has mainly been demonstrated in mammals (e.g., Levine, 1993; Sachser et al., 1998; DeVries et al., 2003; Hennessy et al., 2006) and recently also has been observed in birds (greylag geese, Anser anser, Frigerio et al., 2003; Scheiber et al., 2005a). These calming effects are even more pronounced when socio-positive behaviors, such as allopreening/grooming are exchanged (e.g., Boccia et al., 1989; Boccia, 1989). Thus, social context may modulate individuals’ hormonal stress response to challenges.
While short-time elevations of corticosterone enhance adaptive behavioral and physiological responses to environmental and social challenges (e.g., Wingfield and Ramenofsky, 1999; Goymann and Wingfield, 2004), chronically elevated corticosterone may have detrimental consequences on growth, immune defence and body condition (e.g., Sapolsky, 2002; Korte et al., 2004). Hence, stress management and buffered stress responses may crucially affect fitness.
In nestlings short-term elevated corticosterone may be associated with begging activity (Kitaysky et al., 2001), but chronically high levels impair growth as well as cognitive abilities later on (Kitaysky et al., 2003). According to the developmental hypothesis altricial bird nestlings may show a limited hypothalamic–pituitary–adrenal (HPA)-axis reactivity compared to adult individuals, due to their high degree of dependence on their parents’ care and their limited capacities to overcome perturbations. Precocial birds, in contrast, would show a fully developed HPA-axis reactivity responding to a challenge with an increase in fluctuating corticosterone right after hatching (Starck and Ricklefs, 1998). Experimental evidence supports this hypothesis (Blas et al., 2006 and references therein).
Individual differences in both baseline and stress corticosterone are modulated by factors such as genetic heritability, maternal effects, via androgen content of the egg yolk and laying order (Schwabl, 1999; Love et al., 2003; Hayward and Wingfield, 2004; Groothuis et al., 2005; Freire et al., 2006) and early socialization (Kaiser et al., 2007 and references therein).
Until to date, only a few studies focused on CM over development in altricial birds (Proeve, 1983; Blas et al., 2006 and references therein). To our knowledge none of them related nestling CM to socio-positive behavior among nestlings. In this context ravens (Corvus corax) are an interesting model, since there is no aggression among nestlings (own observation, M.S.) and agonistic interactions among siblings over food start only in the 2nd month post-fledging (Bugnyar et al., 2007). Young fledge at 5–7 weeks of age and remain with their parents for 1–3 months. Thereafter, they join non-breeder groups until they pair bond, become territorial and start breeding with approximately 3 to 4 years of age (Boarman and Heinrich, 1999). Especially after independence from their parents the presence of conspecifics and siblings in particular, may facilitate object exploration in juvenile ravens (Stöwe and Kotrschal, 2007; Stöwe et al., 2006a,b). Moreover, siblings actively support each other in fights (Bugnyar, personal communication). Ravens are scavengers on ephemeral food. Pairs breed once per year and may raise 3–4 young (Glutz von Boltzheim and Bauer, 1993; Ratcliffe, 1997).
In mammals parenting style and handling affects cognitive abilities (Bredy et al., 2004) and HPA-axis reactivity and thus, corticosteroid excretion patterns not only in the pups but also later on in life (Levine, 2001; Macrì and Würbel, 2006). In altricial birds, the parents search for food most of the time. Thus, the time they spend at the nest to feed the young is rather short. The nestlings sit in close contact all the time becoming more active and manipulative the older they get. Hence, in altricial birds sibling interactions such as allopreening could have an affect on corticosteroid excretion patterns and thus on fitness. Post-hatch corticosterone levels not only affect growth rates and nestling body condition, but have life-long effects on HPA axis reactivity, cognitive abilities (Kitaysky et al., 2001, 2003), on success when competing for resources and on behavioral traits such as neophobia (Spencer and Verhulst, 2007). While hormone excretion in relation to sibling aggression in altricial birds has received some attention (e.g., Tarlow et al., 2001), effects of affiliative behaviors have not been studied so far.
We monitored corticosterone excretion patterns in three groups of hand-raised juvenile ravens (n=5, 6 and 11) in the nest, post-fledging (May–July) and when they would be independent from their parents (September–November) and related these patterns to socio-positive behavior. Behavioral data were collected via focal sampling in each developmental period considered. The method of measuring corticosterone excretion in feces is well established (e.g., Hirschenhauser et al., 2005; Möstl et al., 2005; Touma and Palme, 2005). It allows regular sampling without handling and stressing the animal. Because separation usually causes a considerable stress response (e.g., Levine, 1993; Canoine et al., 2002; Müller and Schrader, 2005), we chose social isolation as a stressor to evaluate the most appropriate assay to analyze CM in raven fecal samples.
Considering that altricial nestlings especially towards the end of the nestling period do show hormonal stress responses (e.g.,Love et al., 2003) and social support affects corticosterone excretion post-fledging (Frigerio et al., 2003; Scheiber et al., 2005a), we predicted CM levels to be inversely related to affiliative behaviors already in nestlings as well as post-fledging.
Materials and methods
Animals and housing
In spring 2001, we hand raised a group of five ravens (4 males, 1 female, KLF1 ravens) in one nest at the Konrad Lorenz Research Station (KLF), Grünau, Austria. Three birds were taken from the wild with permission, two nestlings were zoo bred. Four nestlings (2 birds from the wild, 2 zoo-bred nestlings) were 2 weeks old when we got them, one of the birds taken from the wild hatched in the breeder and was hand reared from the beginning. The aviary consisted of an indoor room, where the birds were raised and an outdoor compartment (23.3 m2 indoor, 121.8 m2 outdoor). After fledging birds could freely move between in- and outdoor compartment.
In spring 2002, we hand raised a group of six ravens (4 males, 2 females, UVM ravens) at the University of Vermont (UVM), Burlington, USA. The birds were taken from the wild with permission, with 2 weeks (4 siblings) and 10 days of age (2 siblings). Birds originated from two different nests (4 birds from one nest, 2 birds from another nest) and were raised in a single nest. Once fledged, we kept them in a social group together with one adult male (4 years of age) in order to contribute to appropriate socialization of the juveniles. The aviary was situated in a wood close to Burlington. It was divided into three sections that were separated by wire mesh and doors (30 m2, 100 m2, 64 m2, Bugnyar et al., 2004).
In spring 2004, we hand-raised another group of thirteen ravens (7 males, 6 females, KLF2 ravens) at the KLF. Ravens from two nests (containing 4 and 3 ravens respectively) were zoo bred, birds from the two other nests (containing 3 ravens each, one nest of 3 siblings, one nest with 3 non-siblings) were taken from the wild with permission. The four zoo-bred siblings were almost fledged (6 weeks of age) when we got them, the other three zoo-bred birds were 3 weeks of age. The birds taken from the wild were 2 and 3 weeks of age. Before fledging, raven nestlings generally habituated quickly to human foster parents and after half a day even those nestlings taken from their parents at the end of the nestling period stopped showing any fear response towards the hand raisers. We kept the birds in nests together (see above) in an indoor aviary. Just before fledging we transferred all birds into one big nest in an outdoor aviary in the Cumberland game park, Austria, where they remained thereafter. Once fledged, we kept them together in one group with two previously raised adult males (9 and 4 years of age). The aviary was divided into three sections separated by wire mesh (80 m2, 35 m2, 80 m2) and in addition had three experimental rooms, separated by wooden walls and opaque doors. One female nestling fell ill and received medical treatment. We excluded data of this birds from analysis. In late summer this bird was transferred into another aviary together with one male sibling. Hence, in data analysis the number of birds varies between developmental periods. KLF1 ravens were released in September of the same year and joined wild ravens in the valley. UVM and KLF2 ravens remained in the aviary for further observations.
The equipment of all aviaries consisted of trees, branches, stones, tree trunks, shallow pools for bathing, natural shadow, wooden breeding niches and natural grass ground cover. The floor of the experimental room (KLF2 ravens) was covered with gravel. For individual identification nestlings were marked with colored cotton leg bands which were substituted by colored metal leg rings before fledging. Nestlings were fed every 2 h from 7.00 a.m. to 7.00 p.m. and every 3 h after fledging. Feedings were further reduced to 3–4 per day. Two months post-fledging and thereafter we fed the ravens twice per day (in the mornings and afternoons after the experiments). Food consisted of meat (minced for nestlings), milk products, cereals, kitchen leftovers and vitamins (drops). During the nestling period, until the 2nd month post-fledging we also added milled snail shells and cuttlebone for calcium supply. Nestling food was moistened with water, which we also distributed with spoons. Later on water was provided ad libitum.
Data collection
Focal observations
UVM ravens
T. B. carried out observation-sessions during the nestling period (mid-April to the beginning of May: X̄±SD=42±10 focal observations per bird), post-fledging (May–August: X̄±SD=52±3 focal observations per bird) and when birds were independent, during 5 weeks distributed from September to November (X̄±SD=15±2 focal observations per bird). Each session consisted of six 5-min observations (one per bird). Thus, three birds were observed before and the remaining three after feeding. The order of birds varied randomly between sessions with the restriction that those subjects that were recorded before feeding in one session, were recorded during feeding in the next session of that day and vice-versa. Since activity bouts of ravens were relatively short during the nestling period, we started with three observation-sessions per day (morning, noon, afternoon), for 5 days a week. Post-fledging and when birds were independent, we conducted two sessions per day (morning, afternoon), for 4 days a week. Observations were carried out from beside the nest and later from outside the aviary (1–2 m away from the wire partition). A second person (M.S., B.H.) was feeding the birds. During the first 2 weeks, birds were fed by hand. Afterwards the food was delivered on a standard location in the aviary.
We did not conduct focal observations of KLF1 nestlings
KLF2 ravens were observed by C.S. (parallel analysis of video sequences: inter-observer reliability test, T.B. and C.S.: Cohen’s kappa: 0.82) following the procedure of UVM ravens. Six birds were randomly chosen as focal individual in the morning (between 8 a.m. and 9 a.m.) and six individuals in the evening (between 6 p.m. and 8 p.m.). Three birds were observed before and three after the regular morning/evening feedings. Observations lasted for 5 min per bird. During the nestling period C.S. conducted X̄±SD=4±1 focal observations per bird, post-fledging X̄±SD=30±4 and when birds were independent X̄±SD=28±4. Due to the aviary construction (opaque walls separating aviary compartments, structures in the aviary such as stones and tree trunks), observations were conducted from inside the aviary to allow C.S. to observe the focal bird continuously. During observations in UVM and KLF2 ravens, all birds were together and were free to use the entire aviary complex. We measured the duration (s) and frequency of autopreening, manipulation of familiar objects (such as nest material and aviary equipment), frequency and duration (s) of allopreening and sitting close to another bird (distance b50 cm). We also noted the number of approach-retreat interactions and fights (=agonistic interactions with body contact, i.e., pecking).
Fecal sample collection over development
We collected fecal samples from each individual in the nestling period, starting 2 days after we got the birds until 2 days before fledging; KLF1 ravens: X̄±SD=6± 3 days, X̄±SD=27±9 samples per bird, UVM ravens: X̄±SD=9±2 days, X̄±SD=33±7 samples per bird, KLF2 ravens: X̄±SD=6±3 days, X̄±SD=11±3 samples per bird. In the KLF2 ravens three birds were almost fledged at the time we got them, hence we could not collect fecal samples during the nestling period of these birds. Post-fledging we collected X̄±SD=26±7 days, X̄±SD=40±16 samples per bird (KLF1 ravens), X̄±SD=9±3 days, X̄±SD=12±3 samples per bird (UVM ravens), X̄±SD=6±2 days, X̄±SD=6±2 samples per bird (KLF2 ravens) and when birds were independent X̄±SD=8±1 days, X̄±SD=11±3 samples per bird (UVM ravens), X̄±SD=6±1 days, X̄±SD=8±1 samples per bird (KLF2 ravens). At that time KLF1 ravens were already released and were free flying.
Isolation in a novel environment
Two months post-fledging, we once isolated each raven of the UVM group in a novel aviary for 2 h between 9 a.m. and 12 a.m. In addition to the juvenile ravens, we tested two adult birds (both 4 years of age, 1 male, 1 female), which were hand raised by B.H. previously. They had been kept in the same aviary as the juveniles, but were tested and released before the juveniles fledged. The novel aviary (12 m2) was situated in a distance of approximately 50 m to the home aviary. Visual and acoustical contact to conspecifics was possible. The ground was covered with plastic foil to facilitate fecal sample collection. We provided food and water, branches to sit on and shade. Since the birds were all hand raised and tame, M.S. could take the subject to be tested by hand and transfer it in a transport-box to the novel aviary. M.S. subsequently stayed with the isolated bird in the new aviary the entire 2 h of the experiment to collect each fecal sample. These were stored on ice during the test, and frozen at −20 °C immediately after the end of the test. We videotaped the fist 60 min of the isolation and analyzed the birds’ behavior for 1 min every fifth minute. We recorded how often birds changed place (hops >50-cm distance), feeding duration (s), preening duration (s) and the duration (s) of object manipulation (objects were part of the aviary equipment, such as twigs, stones, branches). When stressed or nervous, ravens increase their locomotory activity, they frequently hop and fly between perches (own observation). Hence, we counted the number of hops as a behavioral parameter indicating stress.
Before the onset of these experiments we once added silver-colored glitter to food of one raven in order to estimate gut passage time. Most material was excreted between the first and the second hour post-feeding. However, hormonal stress response might be detectable earlier, since fecal samples also contain urine, and in addition to metabolization in the liver and excretion via gut and kidneys, corticosterone may diffuse through the gut walls. We terminated the isolation after 2 h to minimize the time the birds were exposed to this isolation stress.
We adhered to the NIH standards (DHEW Publication 80-23, Revised 1985) and to the laws of the countries where the research was conducted.
Analysis
We calculated individual means per developmental period, both of behavioral parameters and CM values. We compared both behavior and CM of the ravens in three developmental periods: as nestlings, post-fledging (mid-May until the end of July) and when birds were independent (September, October, November). Post-fledging, free-ranging ravens would still be with and dependent on their parents, whereas in autumn they would be independent. To compare allopreening activity among nests, we assessed the mean allopreen activity per nest by dividing the total daily allopreen duration per nest by the number of individuals in the nest. In the KLF2 ravens we excluded the nest of the almost fledged young in this comparison, since we could not collect plenty of data from all the nestlings. We calculated dominance rank on the basis of approach–retreat interactions observed during the focal protocols using the software package MatMan (1998). Post-fledging rank hierarchy was not stable yet: the probability that the observed linearity h′=0.24 resulted from a random process was p=0.37. When birds were independent, all but one male outranked all females. The probability that the observed linearity h′=0.79 resulted from a random process was p<0.001. Data were analyzed using the software package SPSS (2001) and by hand according to Siegel and Castellan (1988, Friedman two-way analysis of variance by ranks, Wilcoxon signed ranks test for n<13, Spearman rank order correlation, Kruskal–Wallis test). Only non-parametric tests were used. Test results are given two-tailed. 0.1>α>0.05 were considered as trends. Because the usefulness of alpha corrections in case of a low sample size seems debated (Nakagawa, 2004), we present the original p-values. We mention the maximum p-value possible in each case to still reach a significance level of α<0.05 after Bonferroni correction (Table 1).
Table 1.
Corticosterone excretion patterns and behavior during the 2-h isolation period, using four different assays for fecal sample analysis
| Assay | A corticosterone-3-carboxymethyloxim |
B cortisone |
C tetrahydro-corticosterone |
D 5β-androstane-3α-ol, 11,17-dione-17-CMO |
||||
|---|---|---|---|---|---|---|---|---|
| CM range | mean CM | CM range | mean CM | CM range | mean CM | CM range | mean CM | |
| n Droppings | rs=−0.66, p=0.076 | rs=−0.70, p=0.056 | rs=−0.26, p=0.53 | rs=−0.43, p=0.29 | rs=−0.71 p=0.05 | rs=−0.78, p=0.023 | rs=−0.61, p=0.11 | rs=−0.74, p=0.035 |
| n Hops, distance > 50 cm | rs=0.41, p=0.32 | rs=0.048, p=0.91 | rs=0.52, p=0.18 | rs=0.26, p=0.53 | rs=0.74, p=0.034 | rs=0.48, p=0.23 | rs=0.69, p=0.058 | rs=0.52, p=0.18 |
| Feeding duration (s) | rs=−0.66, p=0.076 | rs=−0.50, p=0.20 | rs=−0.06, p=0.89 | rs=−0.10, p=0.82 | rs=−0.22, p=0.61 | rs=−0.50, p=0.20 | rs=−0.46, p=0.26 | rs=−0.66, p=0.076 |
| Duration of object manipulation (s) | rs=−0.41, p=0.32 | rs=−0.49, p=0.22 | rs=−0.08, p=0.85 | rs=0.01, p=0.98 | rs=−0.52, p=0.19 | rs=−0.40, p=0.33 | rs=−0.53, p=0.18 | rs=−0.54, p=0.17 |
| Preening duration (s) | rs=0.25, p=0.55 | rs=0.41, p=031 | rs=−0.08, p=0.85 | rs=0.08, p=0.85 | rs=−0.08, p=0.85 | rs=−0.08, p=0.85 | rs=0.25, p=0.56 | rs=0.41, p=0.31 |
| Range and median CM ng/g feces | 44.37–268.86 | 114.04 | 115.14–969.41 | 580.90 | 89.28–111.04 | 283.48 | 206.49–806.29 | 252.83 |
Significant Spearman rank-order correlations are written in bold and italics, trends are indicated in italics. P<0.0125 would remain significant after Bonferroni correction, P<0.05. Number of birds=8. We also present median CM value in ng/g feces and the CM range with each assay.
Extraction and analysis of immunoreactive corticosterone metabolites
Fecal samples were kept frozen at −20 °C until analysis. For extraction 0.25 g of wet feces were shaken in a mixture of methanol (2 ml, 96%) anddistilled water (0.25 ml) for 2 min. One ml of this extract was evaporated, afterwards dissolved in 0.5 ml Na-acetate buffer and 1 μl β-glucuonidase-aryl-sulfatase (Merck 4114, Kotrschal et al., 1998) and hydrolyzed for 18 h.
Choosing the most appropriate assay
We determined the immunoreactive corticosterone metabolites which were most abundant in raven fecal samples (collected during the isolation in a novel environment) using high-performance liquid chromatography (HPLC) separation as described by Nakagawa et al. (2003). The amounts of excreted immunoreactive corticosterone metabolites (CM) were determined with enzyme immunoassays using group-specific antibodies raised in rabbits against (A) corticosterone-3-carboxymethyloxim: bovine serum albumin, described for measuring CM in greylag geese, A. anser (Kotrschal et al., 1998; Hirschenhauser et al., 2000; Frigerio et al., 2001), (B) a cortisone assay described by Rettenbacher et al. (2004) for measuring CM in hens, Gallus gallus domesticus, (C) an assay against tetrahydrocorticosterone used by Nakagawa et al. (2003) for measuring CM in penguins, Pygoscelis adeliae and in Wilson’s storm petrels, Oceanites oceanicus (Quillfeldt and Möstl, 2003) and (D) an assay using an antibody against 5β-androstane-3α-ol, 11,17-dione-17-CMO: bovine serum albumin, which was used for measuring CM in ruminants (Möstl et al., 2002). This assay shows cross-reactions not only with C19O3 steroids but also with C21O4 metabolites that have a 3α-ol, 11-oxo structure, therefore measuring 3α,11oxo-CM.
Mean intra-assay coefficients of variation were 8.4% with assay A, 12.0% with assay B, 10.4% with assay C and 10.0% with assay D. Mean inter-assay coefficients of variation were 11.0% with assay A, 26.9% with assay B, 11.7% with assay C and 11.0% with assay D. These relatively high values are in the typical range for EIA procedures on feces, due to the number of steps involved increasing the amount of total variation.
To assess the effect of deconjugating corticosterone metabolites with β-glucuonidase-aryl-sulfatase (Merck 4114) we randomly chose 28 of all UVM raven samples collected and analyzed them both with either β-glucuonidase-aryl-sulfatase being added (see above) or without, using assay D. Since CM values after deconjugation with β-glucuonidase-aryl-sulfatase were significantly higher (Wilcoxon signed ranks test: n=28, z=−4.62, p<0.001) than without deconjugating, we deconjugated further samples for analysis.
Mean CM values differed significantly between assays (Friedman two-way analysis of variance by ranks, further referred to as Friedman test: n=8, χ2=14.63, p<0.01, post hoc test for multiple comparisons: p<0.05: assay A < assay B, assay A < assay D, assay B > assay C). We defined the difference between the minimum and the maximum CM value measured in the course of the 2-h isolation period as CM range. It varied accordingly between assays (Friedman test: n=8, χ2=14.20, p<0.01, post hoc test for multiple comparisons: p<0.05: assay A < assay B, assay A < assay D, assay B > assay C).
Individuals differed significantly in amounts of CM during the 2-h isolation period with the assays A, C, D and tended to do so with assay B (Kruskal–Wallis: n=8, df=7, assay A: KW2=35.75, p<0.001, assay C: KW2=54.39, p<0.001, assay D: KW2=39.83, p<0.001, assay B: KW2=13.06, p=0.071). CM values partly correlated with the observed behavior during the isolation test (Table 1). The observed differences among the four assays are most probably due to their cross-reactions with diverse corticosterone metabolites. Assay B detected highest amounts of CM, but correlations with behavior and among individual differences were lowest. Assay A revealed lowest CM values. The other two assays, C and D, showed similar results, however, those of assay D were stronger related to individual behavioral response patterns to the isolation stress (Table 1). Therefore, we considered assay D (3α,11oxo-CM) the most appropriate assay to determine CM in ravens.
Birds exposed to a stressor, increase their defecation frequency, which might lead to a dilution effect lowering the CM concentration per sample. In fact, both mean CM and CM range negatively correlated with the number of droppings in all four assays (Table 1). Even if three droppings per individual are sufficient to assess individual stress response to an acute stressor (Scheiber et al., 2005b), our results show instead that the number of droppings excreted after a stressor should be considered as a parameter whenever hormonal stress response to an acute stressor is measured via fecal sample analysis.
Our comparison of the four assays indicates that the assay showing highest values is not necessarily the most appropriate one to detect biological relevant changes in CM (CM levels did not relate to the behavior observed). This underlines the importance of a “bio-evaluation” combining chemical analysis with behavioral observations (Goymann, 2005; Frigerio et al., 2004), to assure that excreted corticosterone metabolites measured, are the ones corresponding to behavior.
Results
Corticosterone over development
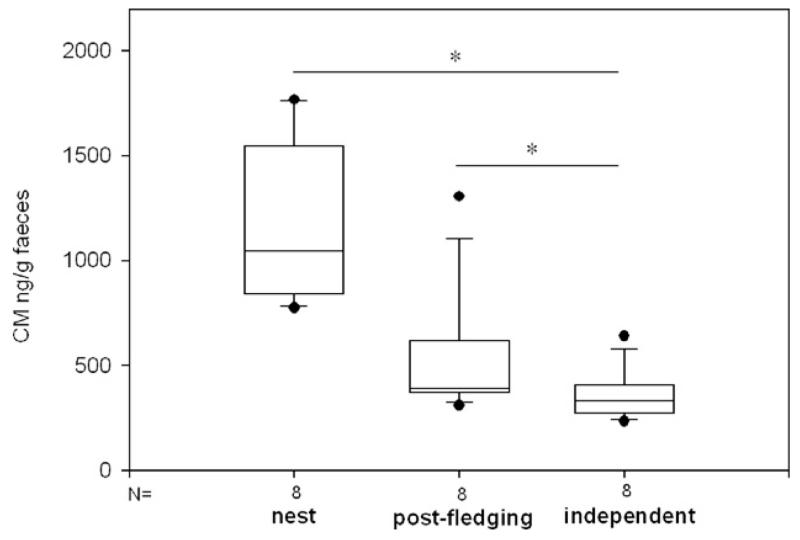
Ravens of the UVM group and KLF2 ravens excreted significantly higher amounts of immunoreactive corticosterone metabolites (CM) during the nestling period than when independent (Friedman test: UVM ravens: n=6, df=2, χ2 = 12.0, p<0.01, KLF2 ravens: n=8, χ2 = 13.0, p<0.01, post hoc test for multiple comparisons: p<0.05: UVM ravens: nest>independent, KLF2 ravens: nest>post-fledging, post-fledging>independent, Fig. 1). CM did not correlate between developmental periods (Spearman rank-order correlation, further referred to as Spearman: UVM ravens: n=6, nestling-post-fledging: rs=0.6, p>0.05, post-fledging–independent: rs=−0.14, p>0.05, KLF2 ravens: nestling–post-fledging: n=9, rs=−0.02, p>0.05, post-fledging–independent: n=11, rs=0.54, p>0.05).
Fig. 1.
Amounts of excreted corticosterone metabolites (ng/g feces) in three developmental periods in KLF2 ravens: nest, post-fledging and when birds were independent. N=number of birds, box plots show the median and the interquartile range from the 25th to the 75th percentile. Whiskers above and below the box indicate the 10th and the 90th percentiles. Asterisks mark significant between-phase differences as determined by post hoc tests for multiple comparisons for Friedman two-way analyses of variance by ranks (*p<0.05).
Similarly, KLF1 ravens tended to excrete higher amounts of CM as nestlings than post-fledging (Wilcoxon signed ranks test: n=5, T+=15, p=0.062, lowest p possible in a two-tailed test with n=5). As in the UVM and KLF2 ravens, nestling CM did not correlate with CM post-fledging (Spearman: n=5, rs=0.6, p>0.05). In all three raven groups among individual differences in CM were not pronounced (Table 2).
Table 2.
Among individual differences in CM when in the nest, post-fledging and when independent
| Corvid group | Nestling period | Post-fledging | Independent |
|---|---|---|---|
| UVM | df=5, χ2 = 5.23, p=0.39 | df=5, χ2 = 2.72, p=0.74 | df=5, χ2 = 13.17, p=0.67 |
| KLF 1 | df=4, χ2 = 11.43, p=0.022 | df=4, χ2 = 5.15, p=0.27 | |
| KLF 2 | df=8, χ2 = 1.11, p=0.11 | df=11, χ2 = 15.48, p=0.16 | df=11, χ2 = 23.47, p=0.015 |
We used the Kruskal–Wallis test to compare among individuals.
Corticosterone and behavior
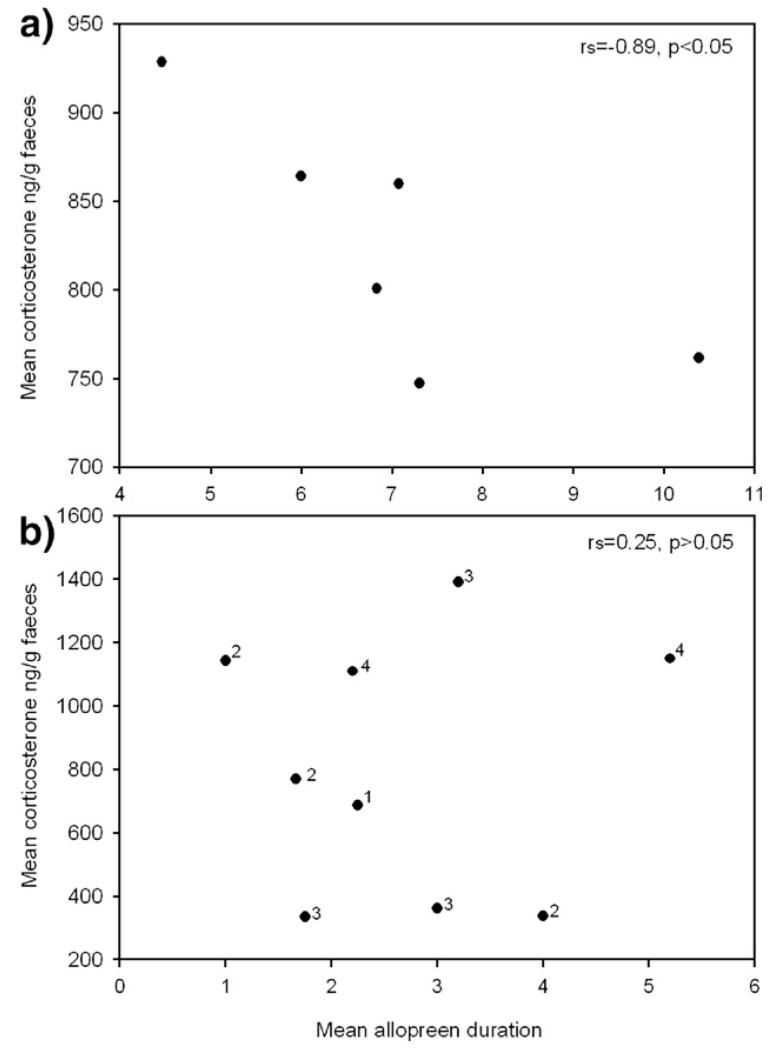
Nestling CM correlated negatively with the time birds spent allopreening in the UVM ravens but not in KLF2 ravens (Spearman: n=6, rs=−0.89, p<0.05, KLF2 ravens: n=9, rs=0.25, p>0.05, Fig. 2). In both groups the duration of allopreening was neither related to the time birds spent autopreening (Spearman: UVM ravens: n=6, rs=0.49, p>0.05, KLF2 ravens: n=9, rs=−0.12, p>0.05), nor to the time they spent manipulating nest material (Spearman: UVM ravens: n=6, rs=0.71, p<0.05, KLF2 ravens: n=9, rs=−0.54, p>0.05). Allopreening activity differed among nests and was higher in the UVM nest than in the nests of the KLF2 ravens (Kruskal–Wallis: df=3, KW=17.92, p<0.001, comparison among all nests: p<0.05: UVM nest–KLF2 nest1, UVM nest–KLF2 nest2, UVM nest–KLF2 nest3).
Fig. 2.
Time birds spent allopreening nest-mates related to amounts of excreted corticosterone metabolites (ng/g feces) in UVM nestlings (a) but not in KLF-2 nestlings (b). UVM nestlings: N=6, raised all together in one nest, KLF-2 nestlings: N=9, raised in 4 different nests, numbers in the graph indicate nest number. CM data of three nestlings of nest 1 are missing, therefore data of only one bird of this nest are included. We inserted the results of Spearman rank-order correlations.
Post-fledging, KLF2 ravens with higher CM levels sat close to conspecifics more frequently and tended to preen them longer than individuals with lower CM did (Spearman: sitting close: UVM ravens: n=6, rs=0.66, p>0.05, KLF2 ravens: n=12, rs=0.63, p<0.05, allopreening: UVM ravens: n=6, rs=−0.43, p>0.05, KLF2 ravens: n=12, rs=0.55, p<0.1). Nestling allopreening duration did neither correlate with duration of allopreening post-fledging (Spearman: UVM ravens: n=6, rs=−0.54, p>0.05, KLF2 ravens: n=9, rs=0.45, p>0.05) nor did nestling allopreening frequency relate to frequency of sitting close to conspecifics post-fledging (Spearman: UVM ravens: n = 6, rs = −0.37, p >0.05, KLF2 ravens: n = 9, rs = 0.19, p<0.05).
Socio-positive activities post-fledging did not relate to actual social rank (Spearman: sitting close: UVM ravens: n=6, rs=−0.24, p>0.05, KLF2 ravens: n=12, rs=−0.20, p>0.05, allopreening: UVM ravens: n=6, rs=0.09, p>0.05, KLF2 ravens: n=12, rs=−0.07, p>0.05). However, CM levels tended to be negatively correlated with social rank in KLF2 ravens (Spearman: UVM ravens: n=6, rs=−0.32, p>0.05, KLF2 ravens: n=12, rs=−0.56, p<0.1, highest rank=1), hence animals with a higher rank tended to have higher CM levels than lower ranking birds.
When birds were independent and rank hierarchy was stable, dominant KLF2 ravens were preened significantly longer than subordinates (Spearman: n=11, rs=−0.83, p<0.005) but in none of the groups did CM values relate to social rank (Spearman: UVM ravens: n=6, rs=0.31, p>0.05, KLF2 ravens: n=11, rs=−0.32, p>0.05) or to socio-positive behavior (Spearman: sitting close: UVM ravens: n=6, rs=0.24, p>0.05, KLF2 ravens: n=11, rs=−0.15, p>0.05, allopreening: UVM ravens: n=6, rs=−0.37, p>0.05, KLF2 ravens: n=11, rs= 0.07, p>0.05). Over development UVM ravens showed individual consistency in time spent with affiliative behaviors. The time birds spent allopreening (Spearman: n=6, rs=0.94, p<0.05) and the frequency of sitting close to conspecifics (Spearman: n=6, rs=0.93, p<0.05) correlated between post-fledging and independence. In KLF2 ravens, in contrast, neither their allopreening activity (duration of allopreening: Spearman: KLF2 ravens: post-fledging–independent: n=11, rs=0.41, p> 0.05) nor the time birds spent sitting close to conspecifics correlated (KLF2 ravens: post-fledging–independent: n=11, rs=−0.14, p>0.05) between developmental periods.
Discussion
We could indeed, show a relation of CM and socio-positive behavior already in the nestling period. The more time UVM nestlings spent preening nest-mates the lower were the preeners’ CM values. Allopreening activity did neither correlate with the time birds preened themselves, nor with duration of nest material manipulation. Thus, allopreening does not seem to be a by-product of autopreening or exploration/object manipulation, but rather seems to reflect an active choice to preen nest-mates. Our data indicate that allopreening may not only modify CM levels in individuals that are preened, but also in the allopreener itself. In contrast to UVM ravens, we did not observe a relation of CM and allopreening activity in KLF2 nestlings. Some of the KLF2 ravens were relatively old (almost fledged) when we got them and we transferred all the birds into an outdoor aviary in one big common nest in the week before fledging. Therefore, fewer behavioral and hormonal data of the nestling period were available, which might have affected these results. In addition, allopreening activity differed among the nests: the UVM nestlings showed higher mean allopreening activity as compared to the KLF2 nestlings. This indicates individual differences in allopreening activity of the nestlings. Thus nest composition seem to have an impact on affiliative behaviors and CM excretion patterns. Even if observed in only one of the two raven groups, our data show that CM may be related to affiliative behavior in nestlings.
Post-fledging, KLF2 ravens with higher CM sat close to conspecifics more frequently and tended to preen them longer. Agonistic interactions among siblings over food started in the 2nd month post-fledging (own observation, Bugnyar et al., 2007). In this period of hierarchy establishment, dominant individuals tended to have higher CM levels than subordinate individuals, which parallels observations in mammals (e.g.,Sapolsky, 1992; Creel, 2001). Challenged individuals might have searched social support via proximity and comfort in engaging in socio-positive activities (de Waal and Aureli, 1996; Seed et al., 2007, Kotrschal et al., unpublished). Also, individuals might have sought to form coalitions and/or to strengthen social alliances via socio-positive behaviors, as is common in primates (e.g., Kummer, 1978; Noë et al., 1991; Watts, 2002) and dolphins (Connor et al., 2006; Connor, 2007). Here, in the ravens for instance, the three males competing for top rank sat close to conspecifics most frequently. Our data could indicate that ravens show similar patterns in alliance formation and stress management than those observed in mammals.
In autumn, when birds were independent and rank hierarchy was stable, dominant individuals were preened significantly longer than subordinates, which is common in primates (Seyfarth, 1980, Hemelrijk, 1990, Noë and Hammerstein, 1994). We did not observe these relations in the UVM ravens. The UVM group had half the size compared to the KLF2 group. In addition, UVM ravens were raised all together in one nest like siblings, whereas KLF2 ravens were raised in four different nests. Former nest-mates support each other in fights (Bugnyar et al., unpublished). Even 1 year after fledging, siblings exchange affiliative behaviors more frequently between each other than with non-siblings (Stöwe et al., 2006a; Loretto et al., 2005). Differences in social dynamics between the raven groups might explain why we observed certain patterns in one group, which did not emerge in the other. In some cases, Spearman rank-order correlation coefficients were similar in both groups, however results reached significance level in KLF2 ravens (n=12), whereas with in UVM ravens (n=6) they did not.
Neither CM nor affiliative behavior correlated between developmental periods in KLF2 ravens. UVM ravens showed consistency in time spent with affiliative behaviors post-fledging and when independent. Considering how much social dynamics and challenges change over development (establishment of a rank hierarchy, sharing food post-fledging, then competing over resources, see above). It is not surprising that CM and quantity of affiliative behaviors expressed did not correlate between developmental periods in all groups.
Elevated baseline corticosterone shortly before fledging may lead to increased motor activity (Astheimer et al., 1992) and thus may promote fledging and dispersal, as was described for American kestrels (Heath, 1997; Sockman and Schwabl, 2001; Love et al., 2003), screech owls, Otus asio and O. kennicottii (Belthoff and Dufty, 1998) and northern mockingbirds, Mimus polyglottos (Sims and Holberton, 2000). Among species differences in onset, magnitude and duration of increased corticosterone baseline levels around fledging may reflect adaptations to ecological differences, i.e., in nest type (e.g., nests on the ground: graduate leaving of the nest, open nest, or nest in caves, Heath, 1997) and intensity of parental care before and after fledging. In addition to increased activity, moderately elevated corticosterone may promote cognitive performance and learning (Sandi and Rose, 1997; Mendl, 1999; Pfeffer et al., 2002). In precocial birds learning is important soon after hatching, during social imprinting (e.g., Bateson, 1966; Nordgreen et al., 2006) and learning about their environment (McNabb et al., 1998; Frigerio et al., 2001, Swoboda et al., unpublished). In altricial birds these learning processes become necessary around fledging (e.g., recognition of parents and siblings) and thereafter. Indeed, before fledging ravens excreted significantly more CM than post-fledging.
In contrast to the pattern observed in ravens, canaries, Serinus canaria (Schwabl, 1999) showed higher CM levels after fledging as compared to the nestling period. Our birds were hand raised to differing extents. In contrast, the canaries were reared by their parents in captivity. Behaviorally ravens habituate to the human foster parents within half a day after taking them out of their original nest (own observation). We started fecal sample collection only the third day after we got the birds to allow habituation to the novel situation. Mean CM values of 3 to 5 days after we got the nestlings did not differ significantly from CM values of days 5 to 3 before fledging. These results render the explanation unlikely that the observed higher nestling CM values compared to after fledging were due to hand raising. Moreover, we continued feeding by hand after the birds fledged, and fledglings came to sit on the hand raisers manipulating their hair, clothes etc. Hence, human foster parents were socio-positive partners not stressors for the birds. Also in American kestrels, Falco sparverius, breed in captivity, nestlings showed higher corticosterone baseline levels than 1-year-old adults (Love et al., 2003), which parallels the pattern we observed in the ravens.
Corticosteroid binding globulins may alter amounts of free corticosteroids and thus affect CM values. In birds capacity and levels of corticosteroid binding globulins change seasonally, with body condition and food availability (reviewed in Breuner and Orchinik, 2002). Wada et al. (2007) showed in white crowned sparrow nestlings (Zonotrichia leucophrys nuttalli) that corticosteroid binding globulin capacity increases with age, buffering nestlings’ hormonal stress response. In our study we measured CM, thus only free (unbound) corticosteroids, which were higher in nestlings than post-fledging.
In conclusion, the ravens’ CM excretion patterns over development parallel those described in the few previous studies on corticosterone in altricial nestlings. We here show for the first time in birds that socio-positive behavior (allopreening) may relate to CM levels already in the nest, which was so far only described for mammals (e.g., Levine, 2001; Suchecki et al., 1993). After fledging, patterns of affiliative behavior and CM in relation to social rank, parallel those observed in primates. This could indicate that several animal species living in a complex social environment may deal with social problems in a similar way, which is not restricted to mammals or even primates.
Acknowledgments
We acknowledge support by the University of Vienna, research grant for M. Stöwe, by the FWF (project: P16939 B03 and the E. Schrödinger fellow ships J2064 and R31B03 for T. Bugnyar). Permanent support was provided by the Verein der Förderer der Konrad Lorenz Forschungsstelle and the Herzog von Cumberland Stiftung. We are grateful for the help with raising the ravens and collection of fecal samples of E. Spielauer and M.-C. Loretto. A. Schöbitz’s and A. Kuchar’s laboratory assistance was indispensable. K. van Oers helped with statistical advice. We thank the zoos of Munich and Wuppertal (Germany) and P. Sömmer for the supply with raven nestlings. Raven nestlings from the wild were taken with permission from the Ministerium für Landwitschaft, Umweltschutz und Raumordnung des Landes Brandenburg, the US Federal Fish and Wildlife Permit Number MB689376-0, State of Maine Department of Inland Fisheries and Wildlife Permit 22077, and Vermont Fish and Wildlife Department Scientific Collecting Permit to B. Heinrich. We thank two anonymous referees for their constructive comments on an earlier draft of the manuscript.
References
- Astheimer LB, Buttemer WA, Wingfield JC. Interactions of corticosterone with feeding, activity, and metabolism in passerine birds. Ornis Scand. 1992;23:355–365. [Google Scholar]
- Bateson PPG. The characteristics and context of imprinting. Biol. Rev. 1966;41:177–220. doi: 10.1111/j.1469-185x.1966.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Belthoff JR, Dufty AM., Jr. Corticosterone, body condition and locomotor activity: a model for dispersal in screech-owls. Anim. Behav. 1998;55:405–415. doi: 10.1006/anbe.1997.0625. [DOI] [PubMed] [Google Scholar]
- Blas J, Baos R, Bortolotti GR, Marchant TA, Hiraldo F. Age-related variation in the adrenocortical response to stress in nestling white storks (Ciconia ciconia) supports the developmental hypothesis. Gen. Comp. Endocrinol. 2006;148:172–180. doi: 10.1016/j.ygcen.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Boarman WI, Heinrich B. Common raven. Birds N. Am. 1999;476:1–32. [Google Scholar]
- Boccia ML. Comparison of the physical characteristics of grooming in two species of macaques (Macaca nemestrine and M. radiata) J. Comp. Psychol. 1989;103:177–183. doi: 10.1037/0735-7036.103.2.177. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Reite M, Laudenslager M. On the physiology of grooming in a pigtail macaque. Physiol. Behav. 1989;45:667–670. doi: 10.1016/0031-9384(89)90089-9. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Lee AW, Meaney MJ, Brown RE. Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous California mouse (Peromyscus californicus) Horm. Behav. 2004;46:30–38. doi: 10.1016/j.yhbeh.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Orchinik M. Beyond carrier proteins: plasma binding proteins as mediators of corticosteroid action in vertebrates. J. Endocrinol. 2002;175:99–112. doi: 10.1677/joe.0.1750099. [DOI] [PubMed] [Google Scholar]
- Bugnyar T, Stöwe M, Heinrich B. Ravens, Corvus corax, follow gaze direction of humans around obstacles. Proc. R. Soc. Lond., B. 2004;271:1331–1336. doi: 10.1098/rspb.2004.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnyar T, Stöwe M, Heinrich B. The ontogeny of caching in ravens, Corvus corax. Anim. Behav. 2007;74:757–767. [Google Scholar]
- Canoine V, Hayden TJ, Rowe K, Goymann W. The stress response of European stonechats depends on the type of stressor. Behaviour. 2002;139:1303–1311. [Google Scholar]
- Connor RC. Dolphin social intelligence: complex alliance relationships in bottlenose dolphins and a consideration of selective environments for extreme brain size evolution in mammals. Philos. Trans. R. Soc., B. 2007;362:587–602. doi: 10.1098/rstb.2006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor RC, Smolker R, Bejder L. Synchrony, social behaviour and alliance affiliation in Indian Ocean bottlenose dolphins, Tursiops aduncus. Anim. Behav. 2006;72:1371–1378. [Google Scholar]
- Creel S. Social dominance and stress hormones. TREE. 2001;16:491–498. [Google Scholar]
- DeVries AC, Glasper ER, Detillion CE. Social modulation of stress responses. Physiol. Behav. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- de Waal FBM, Aureli F. Consolation, reconciliation and a possible cognitive difference between macaques and chimpanzees. In: Russon AE, Bard KA, Parker ST, editors. Reaching into thought: the minds of the Great apes. Cambridge University Press; Cambridge: 1996. pp. 80–110. [Google Scholar]
- Freire R, van Dort S, Rogers LJ. Pre- and post-hatching effects of corticosterone treatment on behavior of the domestic chick. Horm. Behav. 2006;49:157–165. doi: 10.1016/j.yhbeh.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Frigerio D, Möstl E, Kotrschal K. Excreted metabolites of gonadal steroid hormones and corticosterone in greylag geese (Anser anser) from hatching to fledging. Gen. Comp. Endocrinol. 2001;124:246–255. doi: 10.1006/gcen.2001.7706. [DOI] [PubMed] [Google Scholar]
- Frigerio D, Weiss B, Dittami J, Kotrschal K. Social allies modulate corticosterone excretion and increase success in agonistic interactions in juvenile hand-raised greylag geese (Anser anser) Can. J. Zool. 2003;81:1746–1754. [Google Scholar]
- Frigerio D, Dittami J, Möstl E, Kotrschal K. Excreted corticosterone metabolites co-vary with ambient temperature and air pressure in male Greylag geese (Anser anser) Gen. Comp. Endocrinol. 2004;137:29–36. doi: 10.1016/j.ygcen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Glutz von Boltzheim UN, Bauer KM. Handbuch der Vögel Mitteleuropas. 13/III. Aula Verlag; Wiesbaden: 1993. Passeriformes (4.Teil) [Google Scholar]
- Goymann W. Noninvasive monitoring of hormones in bird droppings: biological validations, sampling, extraction, sex differences and the influence of diet on hormone metabolite levels. Ann. N. Y. Acad. Sci. 2005;1046:35–53. doi: 10.1196/annals.1343.005. [DOI] [PubMed] [Google Scholar]
- Goymann W, Wingfield JC. Allostatic load, social status and stress hormones: the costs of social status matter. Anim. Behav. 2004;67:591–602. [Google Scholar]
- Groothuis TGG, Müller W, von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Hayward LS, Wingfield JC. Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen. Comp. Endocrinol. 2004;135:365–371. doi: 10.1016/j.ygcen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Heath JA. Corticosterone levels during nest departure of juvenile American kestrels. Condor. 1997;99:806–811. [Google Scholar]
- Hemelrijk CK. Models of, and tests for, reciprocity, unidirectionality, and other social interaction patterns at group level. Anim. Behav. 1990;39:1023–1029. [Google Scholar]
- Hennessy MB, Hornschuh G, Kaiser S, Sachser N. Cortisol responses and social buffering: a study throughout the life span. Horm. Behav. 2006;49:383–390. doi: 10.1016/j.yhbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hirschenhauser K, Möstl E, Wallner B, Dittami J, Kotrschal K. Endocrine and behavioural responses of male greylag geese (Anser anser) to pairbond challenges during the reproductive season. Ethology. 2000;106:63–77. [Google Scholar]
- Hirschenhauser K, Kotrschal K, Möstl E. Synthesis of measuring steroid metabolites in goose feces. Ann. N. Y. Acad. Sci. 2005;1046:138–153. doi: 10.1196/annals.1343.011. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Harderthauer S, Sachser N, Hennessy MB. Social housing conditions around puberty determine later changes in plasma cortisol levels and behavior. Physiol. Behav. 2007;90:405–411. doi: 10.1016/j.physbeh.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Kitaysky AS, Wingfield JC, Piatt JF. Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behav. Ecol. 2001;12:619–625. [Google Scholar]
- Kitaysky AS, Kitaiskaia EV, Piatt JF, Wingfield JC. Benefits and costs of increased levels of corticosterone in seabird chicks. Horm. Behav. 2003;43:140–149. doi: 10.1016/s0018-506x(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, Mc Ewen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 2004:1–36. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kotrschal K, Möstl E, Hirschenhauser K. The relationship between social stress and dominance is seasonal in Greylag geese. Anim. Behav. 1998;55:171–176. doi: 10.1006/anbe.1997.0597. [DOI] [PubMed] [Google Scholar]
- Kummer H. On the value of social relationships to nonhuman primates: a heuristic scheme. Soc. Sci. Inf. Stud. 1978;17:687–705. [Google Scholar]
- Levine S. The influence of social factors on the response to stress. Psychother. Psychosom. 1993;60:33–38. doi: 10.1159/000288677. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic–pituitary–adrenal axis in the rat. Physiol. Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Loretto M-C, Kotrschal K, Bugnyar T. Ontogeny of dominance relations of juvenile common ravens (Corvus corax); XXIX International Ethology Conference; Budapest, Hungary. August 20–27.2005. p. 136. [Google Scholar]
- Love OP, Bird DM, Shutt LJ. Plasma corticosterone in American kestrel siblings: effects of age, hatching order, and hatching asynchrony. Horm. Behav. 2003;43:480–488. doi: 10.1016/s0018-506x(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Macrì S, Würbel H. Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm. Behav. 2006;50:667–680. doi: 10.1016/j.yhbeh.2006.06.015. [DOI] [PubMed] [Google Scholar]
- MatMan . Noldus Information Technology, Version 1.0 for Windows. 1998. [Google Scholar]
- McNabb AFM, Scanes CG, Zeman M. Endocrine control of development. In: Starck JM, Ricklefs RE, editors. Avian growth and development: evolution within the altricial–precocial spectrum. Oxford University; New York: 1998. pp. 174–202. [Google Scholar]
- Mendl M. Performing under pressure: stress and cognitive function. Appl. Anim. Behav. Sci. 1999;65:221–244. [Google Scholar]
- Mendl M. How do animals cope with social problems? In: Broom DM, editor. Coping with challenge. Welfare in animals including humans. Dahlem University Press; 2001. pp. 211–228. [Google Scholar]
- Möstl E, Maggs JL, Schrötter G, Besenfelder U, Palme R. Measurement of cortisol metabolites in faeces of ruminants. Vet. Res. Commun. 2002;26:127–139. doi: 10.1023/a:1014095618125. [DOI] [PubMed] [Google Scholar]
- Möstl E, Rettenbacher S, Palme R. Measurement of corticosterone metabolites in birds’ droppings: an analytical approach. Ann. N.Y. Acad. Sci. 2005;1046:17–34. doi: 10.1196/annals.1343.004. [DOI] [PubMed] [Google Scholar]
- Müller R, Schrader L. Behavioural consistency during social separation and personality in dairy cows. Behaviour. 2005;142:1289–1306. [Google Scholar]
- Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav. Ecol. 2004;15:1044–1045. [Google Scholar]
- Nakagawa S, Möstl E, Waas JR. Validation of an enzyme immunoassay to measure faecal glucocorticoid metabolites from Adélie penguins (Pygoscelis adeliae): a non-invasive tool for estimating stress? Polar Biol. 2003;26:491–493. [Google Scholar]
- Noë R, Hammerstein P. Biological markets: supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol. 1994;35:1–11. [Google Scholar]
- Noë R, van Schaik CP, van Hooff JARAM. The market effect: an explanation for payoff asymmetries among collaborating animals. Ethology. 1991;87:97–118. [Google Scholar]
- Nordgreen J, Janczak AM, Bakken M. Effects of prenatal exposure to corticosterone on filial imprinting in the domestic chick, Gallus gallus domesticus. Anim. Behav. 2006;72:1217–1228. [Google Scholar]
- Pfeffer K, Fritz J, Kotrschal K. Hormonal correlates of being an innovative greylag goose, Anser anser. Anim. Behav. 2002;63:687–695. [Google Scholar]
- Proeve E. Hormonal correlates of behavioural development in male zebra finches. In: Balthazart J, Proeve E, Gilles R, editors. Hormones and behaviour in higher vertebrates. Springer Verlag; Berlin: 1983. pp. 368–374. [Google Scholar]
- Quillfeldt P, Möstl E. Resource allocation in Wilson’s storm-petrels Oceanites oceanicus determined by measurement of glucocorticoid excretion. Acta Ethol. 2003;5:115–122. [Google Scholar]
- Ratcliffe D. The Raven. Academic Press Inc.; San Diego: 1997. [Google Scholar]
- Rettenbacher S, Möstl E, Hackl R, Ghareeb K, Palme R. Measurement of corticosterone metabolites in chicken droppings. Br. Poult. Sci. 2004;45:407–711. doi: 10.1080/00071660400006156. [DOI] [PubMed] [Google Scholar]
- Sachser N, Dürschlag M, Hirzel D. Social relationships and the management of stress. Psychoneuroendocrinology. 1998;23:891–904. doi: 10.1016/s0306-4530(98)00059-6. [DOI] [PubMed] [Google Scholar]
- Sandi C, Rose SPR. Training-dependent biphasic effects of corticosterone in memory formation for a passive avoidance task in chicks. Psychopharmacology. 1997;133:152–160. doi: 10.1007/s002130050385. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Cortisol concentrations and the social significance of rank instability among wild baboons. Psychoendocrinology. 1992;17:701–709. doi: 10.1016/0306-4530(92)90029-7. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Endocrinology of the stress response. In: Becker JB, Breedlove SM, Crews D, McCarthy M, editors. Behavioural Endocrinology. 2nd edn. MIT Press; Cambridge, Massachusetts: 2002. pp. 409–450. [Google Scholar]
- Scheiber IBR, Weiß BM, Frigerio D, Kotrschal K. Active and passive social support in families of greylag geese (Anser anser) Behaviour. 2005a;142:1535–1575. doi: 10.1163/156853905774831873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiber IBR, Kralj S, Kotrschal K. Sampling effort/frequency necessary to infer individual acute stress responses from fecal analysis in greylag geese (Anser anser) Ann. N.Y. Acad. Sci. 2005b;1046:154–167. doi: 10.1196/annals.1343.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H. Developmental changes and among-sibling variation of corticosterone levels in an altricial avian species. Gen. Comp. Endocrinol. 1999;116:403–408. doi: 10.1006/gcen.1999.7379. [DOI] [PubMed] [Google Scholar]
- Seed AM, Clayton NS, Emery NJ. Postconflict third-party affiliation in rooks, Corvus frugilegus. Curr. Biol. 2007;17:1–7. doi: 10.1016/j.cub.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM. The distribution of grooming and related behaviours among adult female vervet monkeys. Anim. Behav. 1980;28:798–813. [Google Scholar]
- Sgoifo A, Koolhaas JM, Alleva E, Musso E, Parmigiani S. Social stress: acute and long term effects on physiology and behaviour. Physiol. Behav. 2001;73:253–254. doi: 10.1016/s0031-9384(01)00544-3. [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ., Jr. Nonparametric statistics for the behavioural sciences. 2nd edition. McGraw-Hill; Singapore: 1988. [Google Scholar]
- Sims CG, Holberton RL. Development of the corticosterone stress response in young northern mockingbirds (Mimus polyglottos) Gen. Comp. Endocrinol. 2000;119:193–201. doi: 10.1006/gcen.2000.7506. [DOI] [PubMed] [Google Scholar]
- Sockman KW, Schwabl H. Plasma corticosterone in nestling American kestrels: effects of age, handling stress, yolk androgens and body condition. Gen. Comp. Endocrinol. 2001;122:205–212. doi: 10.1006/gcen.2001.7626. [DOI] [PubMed] [Google Scholar]
- Spencer KA, Verhulst S. Delayed behavioral effects of post-hatch exposure to corticosterone in the zebra finch (Taeniopygia guttata) Horm. Behav. 2007;51:273–280. doi: 10.1016/j.yhbeh.2006.11.001. [DOI] [PubMed] [Google Scholar]
- SPSS . SPSS for Windows, Version 11.0.1. SPSS, Inc.; Chicago: 2001. [Google Scholar]
- Starck JM, Ricklefs RE. Avian growth and development: evolution within the altricial-precocial spectrum. Oxford University; New York: 1998. [Google Scholar]
- Stöwe M, Kotrschal K. Behavioural phenotypes may determine whether social context facilitates or delays novel object exploration in ravens (Corvus corax) J. Ornithol. 2007 doi:10.1007/s10336-007-0145-1. (published online) [Google Scholar]
- Stöwe M, Bugnyar T, Loretto M-C, Schloegl C, Range F, Kotrschal K. Novel object exploration in ravens (Corvus corax): effects of social relationships. Behav. Processes. 2006a;73:68–75. doi: 10.1016/j.beproc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Stöwe M, Bugnyar T, Heinrich B, Kotrschal K. Effects of group size on approach to novel objects in ravens (Corvus corax) Ethology. 2006b;112:1074–1088. [Google Scholar]
- Suchecki D, Mozaffarian D, Gross G, Rosenfeld P, Levine S. Effects of maternal deprivation on the ACTH stress response in the infant rat. Neuroendocrinology. 1993;57:204–232. doi: 10.1159/000126361. [DOI] [PubMed] [Google Scholar]
- Tarlow EM, Wikelski M, Anderson DJ. Hormonal correlates of siblicide in Galapagos Nazca boobies. Horm. Behav. 2001;40:14–20. doi: 10.1006/hbeh.2001.1661. [DOI] [PubMed] [Google Scholar]
- Touma C, Palme R. Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann. N. Y. Acad. Sci. 2005;1046:54–74. doi: 10.1196/annals.1343.006. [DOI] [PubMed] [Google Scholar]
- Wada H, Hahn TP, Breuner CW. Development of stress reactivity in white-crowned sparrow nestlings: total corticosterone response increases with age, while free corticosterone response remains low. Gen. Comp. Endocrinol. 2007;150:405–413. doi: 10.1016/j.ygcen.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Watts DP. Reciprocity and interchange in the social relationships of wild male chimpanzees. Behaviour. 2002;139:343–370. [Google Scholar]
- Wingfield JC, Ramenofsky M. Hormones and the behavioural ecology of stress. In: Baum PHM, editor. Stress physiology in animals. Sheffield Academic Press; Sheffield: 1999. pp. 1–51. [Google Scholar]