Abstract
It has been suggested that affiliated social relations may facilitate information transfer between individuals. We here tested this rarely examined hypothesis with juvenile and adult jackdaws (Corvus monedula) in three stimulus enhancement tasks, both in a non-food context (experiment 1) and in a food context (experiments 2 and 3). We first show that siblings and pair partners maintain stronger bonded social relations than do non-siblings and non-pair partners. We therefore tested individuals in sibling and non-sibling dyads and, later in ontogeny, in pair and non-pair dyads. Jackdaws either did not learn from any other conspecific (experiment 1), or they learned from non-affiliated individuals (non-siblings, non-pair partners in experiments 2 and 3). This may be related to two main characteristics of jackdaws’ affiliated relationships. First, affiliates share food at a high rate and may rely on their knowledgeable partners to secure food rather than learning from them. Second, affiliates spend most time in close spatial proximity to each other which increases the probability that they simultaneously experience occurrences in their environment. Hence, spatially more distant individuals, which are more likely to be non-affiliated, face different foraging situations and may therefore provide more relevant information which may lead to selective social learning.
Keywords: Affiliation, Cognition, Corvus monedula, Jackdaw, Social learning
Paying attention to conspecifics and monitoring their behaviours and skills may depend on the conspecific B’s value and function for a certain individual A (Kummer, 1978). This value may be expressed as certain qualities of B such as sex, age or skills, its tendencies towards A such as fighting against, or caring for, A and its availability for A, influenced by physical distance and presence of third parties (Kummer, 1978). Differences in paying attention to, and monitoring of, others will affect social learning strategies, as well as performance in social learning. Individuals may either copy the majority, successful or older individuals, good social learners, kin or friends (Laland, 2004). Moreover, public information, cues that are provided inadvertently by efficiently performing individuals that share similar environmental requirements (Danchin et al., 2004), potentially affects the decision of observers about when, where, what and how to forage (Galef and Giraldeau, 2001; Templeton and Giraldeau, 1995).
Strategies from whom to learn are based on social dynamics within a group which are best characterized through differences in the frequency and degree of spatial proximity that is sought and tolerated between individuals (Coussi-Korbel and Fragaszy, 1995). The less evenly social dynamics are distributed within a group, the more likely directed social learning (Coussi-Korbel and Fragaszy, 1995) or preferential learning (Hatch and Lefebvre, 1997) will occur, meaning that particular individuals are more influential models for certain individuals than are others (Coussi-Korbel and Fragaszy, 1995). Close spatial proximity may enhance the probability of social learning (Coussi-Korbel and Fragaszy, 1995; Wechsler, 1988a). Among other behaviours the time individuals spend in close spatial proximity to each other is regarded as an important parameter characterizing social bonding and affiliate relations (Bonnie and de Waal, 2006; de Kort et al., 2003; van Schaik and Aureli, 2000; Wechsler, 1988a). Using spatial proximity between individuals in non-experimental situations as an indicator for affiliate relations, we tested the hypothesis that affiliated individuals learn more readily from each other than non-affiliated individuals.
A number of variables have been shown to affect social learning. Learning performance was enhanced when the model was dominant to the observer (Nicol and Pope, 1994, 1999), of different sexes (Benskin et al., 2002; Katz and Lachlan, 2003; Mason and Reidinger, 1981), older (Choleris et al., 1997; Galef and Whiskin, 2004), kin (Hatch and Lefebvre, 1997; Valsecchi et al., 1996) or familiar (Benskin et al., 2002; Lachlan et al., 1998; Swaney et al., 2001; Ward and Hart, 2005). There are some observational studies on the enhancing effects of affiliation (Bonnie and de Waal, 2006; Russon and Galdikas, 1995) on social learning but experimental studies are still rare.
We previously showed that observers in ravens, Corvus corax, manipulated a particular object for a longer period of time than other objects when it had been handled by a sibling model beforehand. However, this was not the case when the model was not a sibling (Schwab et al., 2008). In our group of ravens, siblings showed significantly higher levels of affiliated relations than did non-siblings (Schwab et al., 2008), supporting the hypothesis that socio-positive relations between individuals may enhance social learning. In contrast, pair bonding in jackdaws, Corvus monedula, neither accelerated learning of new food producing techniques nor did pair partners learn the same food producing technique (Wechsler, 1988b). This is interesting, considering that Wechsler’s jackdaws showed strong bonds similar to our juvenile ravens (Schwab et al., 2008).
Relating to both studies, we first determined social dynamics within our group of hand-raised jackdaws via daily behavioural observations. Second, we carried out three social learning experiments. For reasons of comparability the first experiment was identical to that done in ravens, in which juvenile sibling and non-sibling dyads were tested in a non-food stimulus enhancement task. Experiment 2 was a colour discrimination task, carried out with the same juvenile birds, but now in a food context. Experiment 3 was identical to experiment 2 and the same birds were used as test subjects. However, in experiment 3, the juveniles from experiment 2 had reached adulthood and had formed pairs. Pair and non-pair dyads were tested to allow comparisons with Wechsler’s 1988 study.
Jackdaws are socially living in groups throughout their lifes. They mainly breed in colonies and forage together in flocks which vary seasonally in size (Haffer and Bauer, 1993; Röell, 1978) depending largely on food availability and quality. Social dynamics within the group are not evenly distributed between individuals (Röell, 1978) which provides the basis for directed social learning (Coussi-Korbel and Fragaszy, 1995). Jackdaws maintain within-male and within-female hierarchies, with males generally being more dominant than females (Röell, 1978; Tamm, 1977; Wechsler, 1988a). Life-long pair bonds are usually monogamous (Henderson et al., 2000) and pair partners remain together throughout the year (Röell, 1978). They spend most of the time in close spatial proximity, allopreen each other (Wechsler, 1989) and support each other in agonistic interactions (Wechsler, 1988a). Recent studies indicate that also juvenile jackdaws maintain strongly bonded relations (Schwab et al., submitted; von Bayern et al., 2007). Therefore, if social dynamics determine preferential social learning in jackdaws, the birds should learn more readily from affiliated than from non-affiliated individuals. However, another main characteristic of affiliated relationships in jackdaws is a high rate of food-sharing between strongly bonded individuals. Food-sharing was found between adult pair partners (Wechsler, 1989) as well as between juvenile affiliated birds (Schwab et al., submitted; von Bayern et al., 2007). If following a definition of scrounging as benefiting from the food discoveries of others (Giraldeau and Lefebvre, 1987), food-sharing between individuals could be considered as a special case of scrounging (tolerated theft, co-feeding) (Giraldeau and Caraco, 2000). There are conflicting results dealing with the influence of scrounging opportunities on social learning, sometimes having an inhibiting (Giraldeau and Lefebvre, 1987; Giraldeau and Templeton, 1991), sometimes a facilitating effect (Caldwell and Whiten, 2003; Fritz and Kotrschal, 1999). Taking both possible outcomes of scrounging into account, we could expect two scenarios. First, that individuals learn more readily from affiliated than from non-affiliated individuals if scrounging (in the sense of sharing) facilitates social learning. Second, individuals should learn more readily from non-affiliated than from affiliated individuals if scrounging opportunities inhibit social learning.
1. Methods
1.1. Subjects and keeping
Subjects were 20 juvenile jackdaws (C. monedula) that had been hand-raised from 13 to 20 days after hatching to fledging at the Konrad Lorenz Research Station in Gruenau, Austria in spring 2005. The 14 males and 6 females were taken out of wild nests with permission. Until fledging birds were kept in an indoor room in six separate nestboxes. Nests one and two consisted of four biological siblings each, nest three consisted of two single nestmates, nest four was composed of three single nestmates, nest five consisted of two biological sibling pairs and nest six was composed of one biological sibling pair plus one single bird. Because behavioural observations showed that nestmates maintained the same relation patterns to their conspecifics than did biological siblings we refer to all nestmates as siblings, even when the individuals are not genetically related. After fledging, the birds were transferred to an outdoor aviary and from then on housed together in one social group. The aviary consisted of one outdoor compartment (100 m2, maximum height of 5 m) which was equipped with wooden perches, breeding boxes, rocks and natural vegetation. Outdoor experimental compartments, 2.5 m high, consisted of a central room (20 m2) and two pathways (left and right, each 7 m2) which could all be divided by wire mesh doors and which were equipped with wooden perches. When not being tested, birds could move around freely in all areas. Birds had ad libitum access to water and were fed three to four times a day with a mixture of shredded meat, dry insects, cottage cheese and eggs and various kinds of fruits, grain, milk products and vegetables. Jackdaws were individually marked with coloured metal rings.
1.2. Behavioural observations
We made behavioural observations on average every second day, alternating at morning and afternoon feedings. These 30-min observations consisted of 5-min focal observations from six birds and were counterbalanced for frequency and order of observations for each individual. We recorded all social interactions between the focal individual and any other conspecific. To determine affiliate relationships, we used two parameters: the duration that birds sat within 10 cm to each other, and the frequency with which each conspecific was the focal individual’s nearest neighbour at the beginning of each 5 min focal observation. The observation period lasted from fledging of the birds, beginning of June 2005 to the end of the experimental trials, middle of April 2006 and resulted in 3.23 ± 0.3 (range: 0–7) 30 min observations per week.
To assemble dyads for the first and second experiments which were conducted in short succession, we analysed behavioural data from the beginning of June 2005 until the end of July 2005 when we started experiment 1. Experiment 2 was started at the middle of September 2005 right after experiment 1 was finished. To determine dyads for the third experiment we analysed data from the end of December 2005, when birds reached sexual maturity and pair bonding started, until the end of March 2006, when this final experiment started. Unfortunately, seven birds died between experiments 2 and 3 due to predation. Therefore, behavioural observations for experiment 3 were based on 13 birds. For analysis we first summed up durations of sitting close and frequencies for nearest neighbours for each individual separately. To obtain these sums we only used individual values which were recorded when the individual was the focal individual to avoid pseudoreplication. Second, we divided these sums through the number of actual siblings and non-siblings or pair and non-pair partners, respectively, and corrected for the number of observations for each individual to obtain one average data point per individual per condition. Because these data were not normally distributed we used Wilcoxon signed-ranks tests to investigate whether siblings and pair partners spent significantly more time sitting close to each other, and were significantly more frequently observed as the nearest neighbour at the start of each focal observation, than non-siblings and non-pair partners. Tests were calculated by hand according to Siegel and Castellan (1988) when the number of individuals was <16, i.e. in adult birds. Test results are given two-tailed and considered significant when P < 0.05.
1.3. Composition of experimental model–observer dyads
We assembled nine sibling dyads, consisting of 12 males and 6 females. From the two nests with an uneven number of nestmates (three individuals each) we excluded one bird per nest to obtain one dyad out of each nest for testing. Whenever possible we tested biological siblings in a dyad, but three out of the nine tested dyads were in fact nestmates. For composing non-sibling dyads we at random chose individuals which were non-nestmates. Due to increasing shyness of two sibling birds we tested only 16 individuals (11 males, 5 females) in experiment 2 but otherwise dyads were composed as in experiment 1. The sex of most juvenile birds was unknown when they were tested but dyads turned out to result in 7 and 5 same sex dyads in the sibling and non-sibling conditions, respectively, in experiments 1 and 2. At the end of December 2005 individuals entered sexual maturity and pair bonding started being six pairs which were tested in experiment 3. For composing non-pair dyads we again chose individuals at random out of those 12 which were non-pair partners and of different sexes.
1.4. General experimental procedure
Dyads were tested in physical and visual separation from the rest of the group in the experimental compartments. The order of conditions was semi-randomized, interspersing sibling and non-sibling trials and pair and non-pair trials, respectively. As a principle, birds are never caught or grabbed, therefore, the experimenter waited for the test subjects to fly into the corresponding experimental compartments and then closed them off via wooden doors or wooden windows to the rest of the group. Birds were well habituated to this procedure and initially rewarded for flying into compartments and being separated from other individuals. If an individual chose not to participate in experiments on a given day, it was simply tested the next day. The experimental dyads themselves were physically separated from each other by wire mesh, but were in visual contact. Each pair was used twice, with model and observer roles reversed. Experimental trials consisted of a demonstration phase and a test phase.
During the demonstration phase the model was in the central experimental room, while the observing bird was able to watch the model through the wire mesh door either from the left or the right pathway. The model was allowed to handle one object (target object—experiment 1) or to eat mealworms from a coloured filmbox (rewarded colour—experiments 2 and 3). Handling was defined as manipulation of objects by the bird with its beak and/or feet. There was no time restriction to the handling time of the model in the demonstration phase, but if the model had not touched the object for more than 20 s or had eaten five times from the coloured box it was removed from the experimental compartment.
After removing the model bird, the experimenter (C.S.) temporarily blocked the view of the observer bird with her body while collecting the item. In experiment 1 (juveniles, non-food context) she then arranged all five objects of a certain set, including the target object, on the floor of the experimental room. She then touched all objects again in reverse order to avoid enhancement effects by the human. In experiment 2 (juveniles, food context) and 3 (adults, food context) she placed both boxes (rewarded and unrewarded colour) of a certain set simultaneously on the ground. Then, in all three experiments, she opened the separating wire mesh door and the observer bird was allowed to enter the central experimental room which started the test phase. As in the demonstration phase there was no time restriction for observers to manipulate objects or boxes. Trials were terminated 2 min after the last touch of any object or box by the bird. If a bird did not handle any of the objects or boxes at all, the trial was finished after 5 min. All trials were video-taped (Sony DCR-TRV14E, Digital Video Camera Recorder).
1.5. Experiment 1: juveniles, non-food context
Experiment 1 was carried out from end of July 2005 until beginning of September 2005. For reasons of comparability C.S. conducted the experiment in exactly the same way as she did with the ravens (Schwab et al., 2008). In the demonstration phase the model bird was allowed to handle one object (target object) out of a set of five. We used 20 different plastic objects (four sets of five objects each), 2–3 cm in diameter, which were novel to the birds but small enough not to provoke a neophobic reaction. Objects were differently coloured but arranged into sets for categorial similarity (size, shape). Sets of objects were equally distributed between observers and each set was used only once with each tested dyad. In case the model handled the target object less than 5 s the trial was terminated and started once again on another day. For the test phase objects were placed 30 cm apart, all at the same distance (1 m) to the door separating experimental room and pathway. Locations of the target objects were equally balanced between trials.
We first compared models’ handling times of the target objects in the sibling and in the non-sibling condition. Second, we measured observers’ handling time of objects and compared the handling times of the target object and any other object in both the sibling and the non-sibling condition. To obtain average values for those objects that had not been presented and handled by the models in the demonstration phase (average object), we calculated the observers’ handling time for all objects minus the handling time for the target object and divided this result by four. Because data were not normally distibuted we used Wilcoxon signed-ranks tests to compare conditions. Results of tests are given two-tailed and considered significant when P < 0.05.
1.6. Experiment 2: juveniles, food context, and experiment 3: adults, food context
In the demonstration phase, the model bird was allowed to eat two mealworms five times out of a coloured filmbox (rewarded colour) attached to a similarly coloured wooden block. After baiting, the box was put onto the ground and the model bird was free to approach. The box was covered with its lid upside-down so the lid could be easily pushed away or lifted by the birds. Only the model bird was allowed to watch the baiting process of the box. We used eight differently coloured boxes randomly combined into four pairs (yellow-blue, grey-violet, green-red, brown-white). To overcome the jackdaws’ neophobia we habituated the birds to the boxes by leaving the boxes firmly closed in the aviary for 1 week before starting the tests but otherwise the birds did not have any experience nor did they receive any training with the boxes. We controlled for smell by keeping mealworms in each box before starting the tests. In all demonstration phases the model birds readily ate the mealworms. For the test phase, both boxes (rewarded and unrewarded colour) of the particular pair were placed simultaneously on the ground, 1 m apart and at the same distance to the separating door between central experimental room and pathway. Boxes were covered with upside-down lids. We avoided giving spatial cues, by placing the rewarded box in the demonstration phase somewhere else than the boxes in the test phase. Furthermore, both the rewarded and unrewarded box in the test were not baited with mealworms to avoid affecting the handling time by the observer bird through contents of the boxes. Each pair of boxes was used only once with each tested dyad. Model birds that had fed from a certain coloured box in experiment 2 were tested with the same pair of boxes in experiment 3 when used as observers. But in experiment 3 the previously non-rewarded colour of the pair became the rewarded one to control for the possibility that the birds remembered their experiences from experiment 2 with regard to feeding from a certain coloured box. We controlled for possible colour preferences by counterbalancing the rewarded colour of each pair of boxes between trials. We furthermore controlled for possible side effects by counterbalancing the placement of the rewarded box (left or right with regard to the entering bird) between trials and within individuals.
We measured the observers’ handling time of rewarded and unrewarded box and the number of visits to each of the boxes. A visit was counted either when a bird was manipulating a box or approaching a box within 10 cm and looking inside the already open box with either one or two eyes. Both parameters could be determined without ambiguity and the latter would additionally express a checking behaviour of the individual to reconfirm about the content of an already open box. Because data were not normally distributed we used Wilcoxon signed-ranks tests to compare handling times of and visits to boxes in the sibling and non-sibling and in the pair and non-pair conditions. Tests were calculated by hand according to Siegel and Castellan (1988) when the number of individuals was <16, i.e. in adult birds. Test results are given two-tailed and considered significant when P < 0.05.
Experiment 2 (juveniles, food context) was carried out from middle of September 2005 until end of October 2005. To the end of the experiment daily behavioural observations showed that the birds manipulated objects (like leafs, twigs, stones or plastic toys which were provided for behavioural enrichment) in the aviary on average for 12.89 ± 0.38 s per manipulation bout. In the test we used two differently coloured boxes. Therefore, we repeated the average manipulation time for objects and added a few seconds for the bird to switch position between objects. This calculation results in 30 s. Hence, we analysed the first 30 s of each trial starting with the observer’s first touch of any object. Experiment 3 (adults, food context) was carried out from end of March 2006 until middle of April 2006 after pairs had formed. At that time the birds were starting to build nests and became easily distracted when separated from their pair partners in the non-pair condition. Therefore, we did not leave the partners with the rest of the group but brought them in the second pathway where their vision was blocked by opaque curtains. Nevertheless, the tested birds often hesitated to manipulate the boxes. Therefore, in experiment 3 we analysed the entire time until the bird did not touch any box anymore for 2 min (mean duration was 95.38 ± 15.53 s).
2. Control trials
In October 2006 we carried out control trials with 12 juvenile birds being naïve to the task to check for colour preferences of the birds. Those birds were hand-raised in spring 2006 and were kept in the same way as the tested birds. These seven males and five females out of five sibling groups were housed together with four more juveniles and the tested birds in one social group since fledging in June 2006. Each bird was tested alone in four trials, physically and visually separated from the rest of the group. In each of the four trials a different colour set of boxes was presented with upside-down lids for easy removal. The experimenter placed both boxes simultaneously at locations in the central experimental room were they had been placed in the experimental trials while the bird was in one of the pathways. Then the experimenter opened the wire mesh door and the bird was free to enter and to manipulate. As in the experimental trials there was no time restriction to the control trials but the trial was terminated 2 min after any of the boxes had been touched by the bird. If no box was handled, the trial was finished after 5 min. Placing of boxes was counterbalanced with regard to left–right location of colours between trials. For habituation to the boxes they were left in the aviary with firmly closed lids for 1 week before starting the control trials.
Although control birds were well habituated to the test situations and to the experimental compartments, they hardly manipulated any of the boxes. In only 5 out of 48 control trials did a bird manipulate at least one of the boxes (three individuals). In six more trials a bird visited a box without touching it (three individuals). Six individuals neither manipulated nor visited any of the boxes in any of the trials. In 37 control trials there was neither a manipulation nor a visit by any of the birds, resulting in insufficient data for statistical analysis. In the experimental trials we controlled for the same number of left and right locations of rewarded boxes within individuals and trials and also controlled for counterbalancing the colour of the rewarded box to left and right location between trials. This enabled us to calculate possible colour or side preferences out of the experimental trials.
3. Results
3.1. Behavioural observations on affiliation
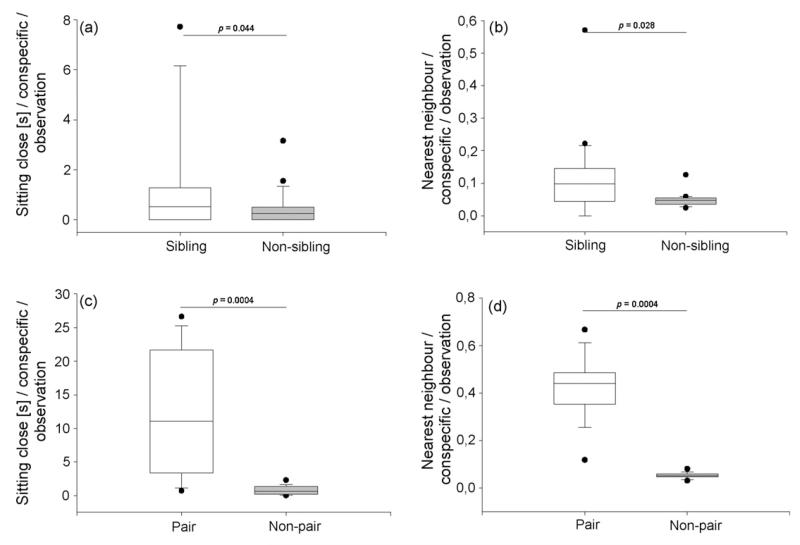
Juvenile jackdaws showed higher affiliate relations between siblings than between non-siblings. Siblings sat within 10 cm to each other for significantly longer than non-siblings (N = 17, Z = −2.012, p = 0.044, Fig. 1a, sitting close). Also, the nearest neighbour of the focal individual at the beginning of each observation was significantly more often a sibling than it was a non-sibling (N = 20, Z = −2.203, p = 0.028, Fig. 1b). Adult jackdaws showed similar results but with their pair partners. Pair partners sat within 10 cm to each other for significantly longer than non-pair partners (N = 12, T+ = 78, p = 0.0004, Fig. 1c, sitting close) and the nearest neighbour of the focal individual at the beginning of each observation was significantly more often the pair partner than a non-pair partner (N = 12, T+ = 78, p = 0.0004, Fig. 1d).
Fig. 1.
Behavioural observations of social interactions of jackdaws in their social group. Duration (s) birds sit close to each other in (a) juvenile and (c) adult jackdaws. Frequency of nearest neighbours at the beginning of each observation in (b) juvenile and (d) adult jackdaws. (a) and (b) show comparisons between siblings and non-siblings and (c) and (d) show comparisons between pair and non-pair partners. All graphs are corrected for the number of actual siblings and non-siblings and pair and non-pair partners and for the number of observations for each individual. Boxes represent mean durations and frequencies of 20 juvenile and 13 adult birds. Boxes indicate median, 25th and 75th percentiles, whiskers indicate 10th and 90th percentiles and dots indicate outliers. Wilcoxon signed-ranks tests.
3.2. Experiment 1: juveniles, non-food context
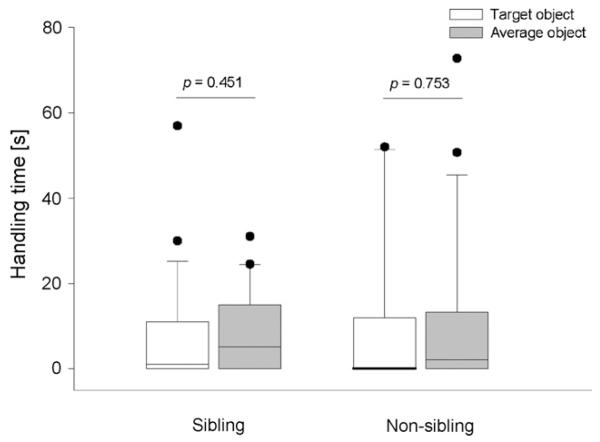
Following the social dynamics hypothesis we predicted for this stimulus enhancement task that observers would handle an object (target object) longer than any of four other available objects if a sibling model had been handling this object before, but not if the model had been a non-sibling. However, juvenile observer birds did not handle the target object significantly longer than any other object (Fig. 2). This was true both for the sibling (N = 14, Z = −0.754, p = 0.451) and the non-sibling condition (N = 13, Z = −0.315, p = 0.753). We also tested the probability that a bird copies the behaviour of a conspecific depending on the behaviour of the model. But comparing the time the model was manipulating the target object in the sibling and in the non-sibling condition did not reveal a significant difference between conditions (Wilcoxon signed-ranks test: N = 16, Z = −1.138, p = 0.255).
Fig. 2.
Comparison of handling time (s) of the target object and average objects in the sibling and non-sibling condition of experiment 1. Boxes represent mean handling times of 18 juvenile jackdaw observers. Boxes indicate median, 25th and 75th percentiles, whiskers indicate 10th and 90th percentiles and dots indicate outliers. Open boxes indicate observers’ handling time of the target object while black boxes indicate the average handling time of the other four available objects. Wilcoxon signed-ranks test.
3.3. Experiment 2: juveniles, food context
For this colour discrimination task, we again predicted that observers would handle and/or visit the former rewarded colour of the demonstration longer and/or more often if the model bird had been a sibling, but not if it had been a non-sibling if the existence of food-sharing between affiliated individuals faciliates social learning. On the contrary, if food-sharing inhibits social learning, observers should handle and/or visit the former rewarded colour of the demonstration longer and/or more often if the model bird had been a non-sibling, but not if it had been a sibling.
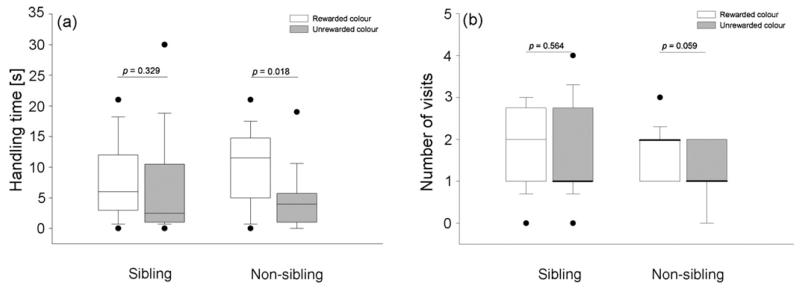
Juvenile observer birds that had previously seen a model bird feeding from a certain coloured (rewarded) box showed significant differences with regard to handling time only in the non-sibling but not in the sibling condition. Non-sibling observers manipulated the previously rewarded coloured box significantly longer than the unrewarded one (N = 15, Z = −2.359, p = 0.018, Fig. 3a) and there was a trend towards visiting the rewarded colour more often than the unrewarded one (N = 12, Z = −1.89, p = 0.059, Fig. 3b). On the contrary, in the sibling condition there was no significant difference neither with regard to handling time (N = 14, Z = −0.976, p = 0.329, Fig. 3a) nor to visits (N = 12, Z = −0.577, p = 0.564, Fig. 3b) to the rewarded coloured box in comparison to the unrewarded one.
Fig. 3.
Comparison of (a) handling time (s) and (b) number of visits of rewarded and unrewarded coloured filmboxes in the sibling and non-sibling condition of experiment 2. Boxes represent mean handling time and number of visits of 16 juvenile jackdaw observers. Boxes indicate median, 25th and 75th percentiles, whiskers indicate 10th and 90th percentiles and dots indicate outliers. Open boxes indicate observers’ handling time and number of visits of the rewarded colour while black boxes indicate observers’ handling time and number of visits of the unrewarded colour. Wilcoxon signed-ranks test.
Juvenile birds did not show any significant preference for a certain colour of a pair of boxes or a certain side (left–right). Comparisons of paired colours did not result in significant differences neither with regard to handling time (colour pair 1: N = 8, T+ = 30, p = 0.109; colour pair 2: N = 6, T+ = 14.5, p = 0.563; colour pair 3: N = 8, T+ = 24.5, p = 0.461; colour pair 4: N = 7, T+ = 19, p = 0.469) nor with regard to number of visits (colour pair 1: N = 6, T+ = 14, p = 0.563; colour pair 2: N = 6, T+ = 17.5, p = 0.219; colour pair 3: N = 5, T+ = 7.5, p > 0.999; colour pair 4: N = 7, T+ = 17.5, p = 0.688). Also, juvenile birds did not show a significant preference for left or right boxes, either with regard to handling time (N = 15, Z = −0.313, p = 0.754) or with regard to number of visits (N = 11, Z = −0.549, p = 0.552).
3.4. Experiment 3: adults, food context
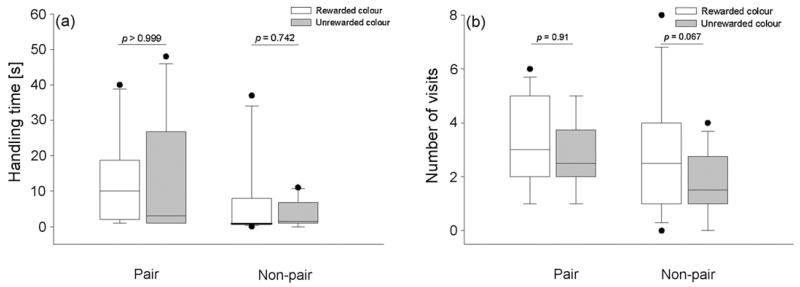
For this colour discrimination task, we predicted the same outcome as for experiment 2 but now for pair and non-pair partners instead of siblings and non-siblings. Adult birds showed a less pronounced, but similar pattern than the juveniles in experiment 2. In the non-pair condition they did not differ significantly with regard to handling time of the previously rewarded coloured box (N = 8, T+ = 21.5, p = 0.742, Fig. 4a). However, non-pair partners tended to visit the rewarded colour more often than the unrewarded one (N = 11, T+ = 54, p = 0.067, Fig. 4b) as did juvenile observer birds. On the contrary, in the pair condition there was no significant difference between the rewarded and the unrewarded coloured box neither with regard to handling time (N = 9, T+ = 23, p > 0.999, Fig. 4a) nor with regard to number of visits (N = 9, T+ = 24, p = 0.91, Fig. 4b).
Fig. 4.
Comparison of (a) handling time (s) and (b) number of visits of rewarded and unrewarded coloured filmboxes in the pair and non-pair condition of experiment 3. Boxes represent mean handling time and number of visits of 12 adult jackdaw observers. Boxes indicate median, 25th and 75th percentiles, whiskers indicate 10th and 90th percentiles and dots indicate outliers. Open boxes indicate observers’ handling time and number of visits of the rewarded colour while black boxes indicate observers’ handling time and number of visits of the unrewarded colour. Wilcoxon signed-ranks test.
Adult observer birds did not show any significant preference for a certain colour of a pair of boxes neither with regard to handling time (colour pair 1: N = 5, T+ = 9, p = 0.812; colour pair 2: N = 3, T+ = 0, p > 0.999; colour pair 3: N = 4, T+ = 7, p = 0.625; colour pair 4: N = 5, T+ = 12, p = 0.313) nor with regard to number of visits (colour pair 1: N = 6, T+ = 13, p = 0.688; colour pair 2: N = 5, T+ = 5, p > 0.999; colour pair 3: N = 5, T+ = 10.5, p = 0.625; colour pair 4: N = 4, T+ = 5, p > 0.999). Adult birds handled boxes to the left for longer (N = 10, T+ = 53.5, p = 0.006) and visited them more often (N = 12, T+ = 76, p = 0.001) than boxes to the right.
4. Discussion
In our social learning experiments jackdaws either did not show any significant behavioural modification in response to the performance of a model bird (experiment 1: juveniles, non-food context), or they learned from non-affiliated rather than affiliated individuals (experiment 2: juveniles, food context, and experiment 3: adults, food context). To our knowledge, this is the first study showing enhancement effects of non-affiliated individuals on social learning because other studies dealing with affiliation patterns and social learning found positive effects between affiliated individuals (Bonnie and de Waal, 2006; Russon and Galdikas, 1995; Schwab et al., 2008).
Contrary to our prediction for experiment 1, observers did not handle an object for a longer period of time than any of four other available objects if a sibling model has been handling this object before in experiment 1 (juveniles, non-food context). These non-significant results were not due to a general avoidance of handling objects. Juvenile jackdaws did manipulate the presented objects in experiment 1, but did not prefer to handle the demonstrator’s object over the other objects presented. Hence, a lack of interest in artificial objects per se, or neophobic reactions to the test objects, can be excluded as explanations for our results.
In the same experimental set-up, hand-raised juvenile ravens handled objects significantly longer when they had been manipulated by sibling models before, whereas non-sibling models produced no such effect. Furthermore, ravens also matched their decisions to cache or not to cache with their siblings but not with non-siblings (Schwab et al., 2008). We suggest two not mutually exclusive explanations for these different results in the two studies. First, raven models showed a tendency of handling the target object longer in the sibling than in the non-sibling condition (Schwab et al., 2008), which was not true for jackdaw models. Hence, the quality of demonstration may have been different in ravens than in jackdaws, enhancing potential effects on social learning in the former but not in the latter. Second, because social relations of juvenile jackdaws and juvenile ravens showed the same pattern with siblings maintaining closer affiliated relations than non-siblings, differences between jackdaws and ravens in this non-food experiment could be caused by differences in caching behaviour. Jackdaws hardly, if ever, cache and if, they do it superficially (Henty, 1975). Ravens on the other hand intensely cache food (Bugnyar and Kotrschal, 2002; Heinrich, 1999; Heinrich and Pepper, 1998) and even show play-caching of objects (Bugnyar et al., 2007; Heinrich and Smolker, 1998). Caching of objects may be important for ravens to develop cache protection strategies (Bugnyar et al., 2007). Therefore, caching ravens generally may be more interested in objects than non-caching jackdaws. This could result in ravens being more attentive to an object handling conspecific and learning socially in this non-food context experiment as opposed to jackdaws.
Experiments 2 (juveniles) and 3 (adults) were conducted in a food context. When paired with a non-affiliated model, juvenile observers handled the rewarded coloured box for significantly longer than the unrewarded one and observers tended to visit the rewarded box more often in both non-sibling and non-pair conditions. There was no negative carry-over effect from experiment 1 to experiments 2 and 3, indicating that jackdaws could be motivated to learn by a change in the set-up. Wechsler (1988b) also found no enhanced learning from pair partners in adult jackdaws. However, the latter study was conducted in a group context with several potential models being present at the same time. In the present study, birds were tested in dyads with only one model present. Furthermore, Wechsler used a feeding apparatus which had to be manipulated in certain ways, while in the present study birds had to manipulate boxes without engaging in any sophisticated manipulation techniques. These methodological differences could explain the difference in results to our present study.
Naïve birds which did not observe a model, did not manipulate the boxes sufficiently to allow statistical analysis. It is unlikely that this was due to neophobia because naïve birds received an identical habituation phase to the boxes as observer birds and they did not show any behavioural signs of neophobia. More likely, the absence of conspecifics attenuated their interest in the objects as has been found in other species (Fragaszy and Mason, 1978). Therefore, we used test trials to calculate colour and side preferences, which were all non-significant with the exception of adult jackdaws who had developed a left-side preference. Although this may have influenced the birds’ performance and may have made our results more conservative, adults nevertheless tended to visit the rewarded colour more often. This result supports the clear results of juvenile observers in experiment 2.
All models readily ate the mealworms in all demonstrations of the experiments. The time they spent at the food source did not differ significantly between conditions, indicating that demonstration quality did not have an effect on the results of observer birds. Furthermore, one could argue that jackdaws, typically pairing in their first year (Lorenz, 1931), might have paid more attention to non-siblings as potential future pair partners. Although this might be generally true, none of the tested non-sibling dyads paired later on. Even more importantly, this could not explain the results of the birds in experiment 3, when they were already adult and paired.
Measuring the frequency and duration of looks of observer individuals in an independent experiment and testing dyads of jackdaws in a food and in a non-food context showed that the percentage of watching was higher for non-affiliated than for affiliated models in jackdaws, whereas the pattern was reversed in ravens (Scheid et al., 2007). This corroborates our current results and suggests that they may have been caused by paying different attention to affiliated and non-affiliated conspecifics.
Taken together, our results indicate that social relations do affect social learning in jackdaws, but differently than predicted by the social dynamics hypothesis (Coussi-Korbel and Fragaszy, 1995). Birds learned more readily from non-affiliated than from affiliated individuals. One outstanding characteristic of affiliation in jackdaws is that birds show high tolerance at food and even the active sharing of food (de Kort et al., 2006; von Bayern et al., 2007). Although the birds did not have the opportunity to share food, in the sense of tolerated scounging, during the current experiments they could have relied on their knowledgeable affiliated partners to secure food as has been proposed for female zebra finches (Beauchamp and Kacelnik, 1991). To interpret the lack of directed social learning expected between pair partners in jackdaws, Wechsler (1988b) suggested that pair partners might preferentially profit from each other’s food findings. Hence, non-affiliated individuals might be more important with regard to information about food than affiliated individuals, because food could be more likely shared with the latter than with the former. Furthermore, affiliated individuals spend most time in close spatial proximity to each other, which increases the probability that they simultaneously experience occurrences in their environment. On the contrary, spatially more distant individuals, which are more likely to be non-affiliated, may face different foraging situations and therefore provide different and/or more relevant information. Hence, physical distance, as an outcome of social relations, may increase the value of non-affiliated individuals because they might provide most useful discoveries based on the greater distance to the subject (Kummer, 1978). This may result in high attention towards non-affiliated individuals (Scheid et al., 2007) and preferential learning from them.
In sum, our results support the view that the choice of models in social learning experiments might be crucial and that it may depend on the social system and the quality of social relations between individuals. Interestingly, non-affiliated models were probably more valuable sources of information than affiliated conspecifics to the jackdaws of our study. In our experiments jackdaws were tested in dyads. Testing them in a more naturalistic setting, a group context, could show if the spread of a behaviour follows the same patterns as have been found here, or if other effects determine its transmission within the group.
Acknowledgements
The study was funded by FWF projects R31-B03 and P16939-B03. The Herzog v. Cumberland game park and the “Verein der Förderer KLF” provided permanent support. We thank Hans-Ulrich Stuiber for his help while obtaining jackdaws from the wild and the zoo in Stralsund for providing jackdaw chicks. We thank Christian Schloegl for helpful comments and discussion, Dorottya Újfalussy and Bruna Bonechi for their help with hand-raising the birds, and special thanks goes to Julian Hoskovec for constructing and keeping the aviary in good shape. The experiments comply with the legal requirements of Austria.
References
- Beauchamp G, Kacelnik A. Effects of the knowledge of partners on learning rates in zebra finches Taeniopygia guttata. Anim. Behav. 1991;41:247–253. [Google Scholar]
- Benskin CMH, Mann NI, Lachlan RF, Slater PJB. Social learning directs feeding preferences in the zebra finch, Taeniopygia guttata. Anim. Behav. 2002;64:823–828. [Google Scholar]
- Bonnie KE, de Waal FBM. Affiliation promotes the transmission of a social custom: handclasp grooming among captive chimpanzees. Primates. 2006;47:27–34. doi: 10.1007/s10329-005-0141-0. [DOI] [PubMed] [Google Scholar]
- Bugnyar T, Kotrschal K. Observational learning and the raiding of food caches in ravens, Corvus corax: is it ‘tactical’ deception? Anim. Behav. 2002;64:185–195. [Google Scholar]
- Bugnyar T, Schwab C, Schloegl C, Kotrschal K, Heinrich B. Ravens judge competitors through experience with play caching. Curr. Biol. 2007;17:1804–1808. doi: 10.1016/j.cub.2007.09.048. [DOI] [PubMed] [Google Scholar]
- Caldwell CA, Whiten A. Scrounging facilitates social learning in common mormosets, Callithrix jacchus. Anim. Behav. 2003;65:1085–1092. [Google Scholar]
- Choleris E, Guo C, Liu H, Mainardi M, Valsecchi P. The effect of demonstrator age and number on duration of socially-induced food preferences in house mouse (Mus domesticus) Behav. Process. 1997;41:69–77. doi: 10.1016/s0376-6357(97)00029-6. [DOI] [PubMed] [Google Scholar]
- Coussi-Korbel S, Fragaszy DM. On the relation between social dynamics and social learning. Anim. Behav. 1995;50:1441–1453. [Google Scholar]
- Danchin É, Giraldeau L-A, Valone TJ, Wagner RH. Public information: from nosy neighbors to cultural evolution. Science. 2004;305:487–491. doi: 10.1126/science.1098254. [DOI] [PubMed] [Google Scholar]
- de Kort SR, Emery NJ, Clayton NS. Food offering in jackdaws (Corvus monedula) Naturwiss. 2003;90:238–240. doi: 10.1007/s00114-003-0419-2. [DOI] [PubMed] [Google Scholar]
- de Kort SR, Emery NJ, Clayton NS. Food sharing in jackdaws, Corvus monedula: what, why and with whom? Anim. Behav. 2006;72:297–304. [Google Scholar]
- Fragaszy DM, Mason WA. Response to novelty in Saimiri and Callicebus: influence of social context. Primates. 1978;19:311–331. [Google Scholar]
- Fritz J, Kotrschal K. Social learning in common ravens, Corvus corax. Anim. Behav. 1999;57:785–793. doi: 10.1006/anbe.1998.1035. [DOI] [PubMed] [Google Scholar]
- Galef BGJ, Giraldeau L-A. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim. Behav. 2001;61:3–15. doi: 10.1006/anbe.2000.1557. [DOI] [PubMed] [Google Scholar]
- Galef BGJ, Whiskin EE. Effects of environmental stability and demonstrator age on social learning of food preferences by young Norway rats. Anim. Behav. 2004;68:897–902. [Google Scholar]
- Giraldeau L-A, Caraco T. Social Foraging Theory. Princeton University Press; Princeton, NJ: 2000. [Google Scholar]
- Giraldeau L-A, Lefebvre L. Scrounging prevents cultural transmission of food-finding behaviour in pigeons. Anim. Behav. 1987;35:387–394. [Google Scholar]
- Giraldeau L-A, Templeton JJ. Food scrounging and diffusion of foraging skills in pigeons, Columba livia: the importance of tutor and observer rewards. Ethology. 1991;89:63–72. [Google Scholar]
- Haffer J, Bauer KM. Corvidae—Rabenvögel. In: Glutz von Blotzheim UN, editor. Handbuch der Vögel Mitteleuropas. AULA-Verlag; Wiesbaden: 1993. pp. 1375–2023. [Google Scholar]
- Hatch KK, Lefebvre L. Does father know best? Social learning from kin and non-kin in juvenile ringdoves. Behav. Process. 1997;41:1–10. doi: 10.1016/s0376-6357(97)00022-3. [DOI] [PubMed] [Google Scholar]
- Heinrich B. Mind of the Raven. Harper Collins; New York: 1999. [Google Scholar]
- Heinrich B, Pepper JW. Influence of competitors on caching behaviour in the common raven, Corvus corax. Anim. Behav. 1998;56:1083–1090. doi: 10.1006/anbe.1998.0906. [DOI] [PubMed] [Google Scholar]
- Heinrich B, Smolker R. Play in common ravens (Corvus corax) In: Bekoff M, Byers JA, editors. Animal Play: Evolutionary, Comparative, and Ecological Perspectives. Cambridge University Press; Cambridge: 1998. pp. 27–44. [Google Scholar]
- Henderson IG, Hart PJB, Burke T. Strict monogamy in a semi-colonial passerine: the Jackdaw Corvus monedula. J. Avian Biol. 2000;31:177–182. [Google Scholar]
- Henty CJ. Feeding and food-hiding responses of Jackdaws and Magpies. Br. Birds. 1975;68:463–466. [Google Scholar]
- Katz M, Lachlan RF. Social learning of food types in zebra finches (Taenopygia guttata) is directed by demonstrator sex and feeding activity. Anim. Cogn. 2003;6:11–16. doi: 10.1007/s10071-003-0158-y. [DOI] [PubMed] [Google Scholar]
- Kummer H. On the value of social relationships to nonhuman primates: a heuristic scheme. Soc. Sci. Inform. 1978;17:687–705. [Google Scholar]
- Lachlan RF, Crooks L, Laland KN. Who follows whom? Shoaling preferences and social learning of foraging information in guppies. Anim. Behav. 1998;56:181–190. doi: 10.1006/anbe.1998.0760. [DOI] [PubMed] [Google Scholar]
- Laland KN. Social learning strategies. Learn. Behav. 2004;32:4–14. doi: 10.3758/bf03196002. [DOI] [PubMed] [Google Scholar]
- Lorenz K. Beiträge zur Ethologie sozialer Corviden. J. Ornithol. 1931;79:67–127. [Google Scholar]
- Mason JR, Reidinger RF. Effects of social facilitation and observational learning on feeding behavior of the red-winged blackbird (Agelaius phoeniceus) The Auk. 1981;98:778–784. [Google Scholar]
- Nicol CJ, Pope SJ. Social learning in small flocks of laying hens. Anim. Behav. 1994;47:1289–1296. doi: 10.1006/anbe.1998.0920. [DOI] [PubMed] [Google Scholar]
- Nicol CJ, Pope SJ. The effects of demonstrator social status and prior foraging success on social learning in laying hens. Anim. Behav. 1999;57:163–171. doi: 10.1006/anbe.1998.0920. [DOI] [PubMed] [Google Scholar]
- Röell A. Social behaviour of the jackdaw, Corvus monedula, in relation to its niche. Behaviour. 1978;64:1–124. [Google Scholar]
- Russon AE, Galdikas BMF. Constrains on great apes imitation: Model and action selectivity in rehabilitant orangutan (Pongo pygmaeus) imitation. J. Comp. Psychol. 1995;109:5–17. doi: 10.1037/0735-7036.109.1.5. [DOI] [PubMed] [Google Scholar]
- Scheid C, Range F, Bugnyar T. When, what, and whom to watch? Quantifying attention in ravens (Corvus corax) and jackdaws (Corvus monedula) J. Comp. Psychol. 2007;21:380–386. doi: 10.1037/0735-7036.121.4.380. [DOI] [PubMed] [Google Scholar]
- Schwab C, Bugnyar T, Schloegl C, Kotrschal K. Enhanced social learning between siblings in common ravens, Corvus corax. Anim. Behav. 2008;75:501–508. doi: 10.1016/j.anbehav.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab C, Bugnyar T, Swoboda R, Gaede D, Kotrschal K. Valuable relationships between adult pair partners and between juvenile nestmates in jackdaws (Corvus monedula) submitted. [Google Scholar]
- Siegel S, Castellan NJJ. Nonparametric Statistics for the Behavioral Sciences. McGraw-Hill; New York: 1988. [Google Scholar]
- Swaney W, Kendal J, Capon H, Brown C, Laland KN. Familiarity facilitates social learning of foraging behaviour in the guppy. Anim. Behav. 2001;62:591–598. [Google Scholar]
- Tamm S. Social dominance in captive jackdaws (Corvus monedula) Behav. Process. 1977;2:293–299. doi: 10.1016/0376-6357(77)90032-8. [DOI] [PubMed] [Google Scholar]
- Templeton JJ, Giraldeau L-A. Patch assessment in foraging flocks of European starlings: evidence for the use of public information. Behav. Ecol. 1995;6:65–72. [Google Scholar]
- Valsecchi P, Choleris E, Moles A, Guo C, Mainardi M. Kinship and familiarity as factors affecting social transfer of food preferences in adult Mongolian gerbils (Meriones unguiculatus) J. Comp. Psychol. 1996;110:243–251. doi: 10.1037/0735-7036.110.3.243. [DOI] [PubMed] [Google Scholar]
- van Schaik CP, Aureli F. The natural history of valuable relationships in primates. In: Aureli F, de Waal FBM, editors. Natural Conflict Resolution. University of California Press; Berkeley: 2000. pp. 307–333. [Google Scholar]
- von Bayern AMP, de Kort SR, Clayton NS, Emery NJ. The role of food- and object-sharing in the development of social bonds in juvenile jackdaws (Corvus monedula) Behaviour. 2007;144:711–733. [Google Scholar]
- Ward AJW, Hart PJB. Foraging benefits of shoaling with familiars may be exploited by outsiders. Anim. Behav. 2005;69:329–335. [Google Scholar]
- Wechsler B. Dominance relationships in jackdaws (Corvus monedula) Behaviour. 1988a;106:252–264. [Google Scholar]
- Wechsler B. The spread of food producing techniques in a captive flock of jackdaws. Behaviour. 1988b;107:267–277. [Google Scholar]
- Wechsler B. Measuring pair relationships in jackdaws. Ethology. 1989;80:307–317. [Google Scholar]