Abstract
Purpose
Metastatic cervical cancer is a prototypical chemotherapy-refractory epithelial malignancy for which better treatments are needed. Adoptive T-cell therapy (ACT) is emerging as a promising cancer treatment, but its study in epithelial malignancies has been limited. This study was conducted to determine if ACT could mediate regression of metastatic cervical cancer.
Patients and Methods
Patients enrolled onto this protocol were diagnosed with metastatic cervical cancer and had previously received platinum-based chemotherapy or chemoradiotherapy. Patients were treated with a single infusion of tumor-infiltrating T cells selected when possible for human papillomavirus (HPV) E6 and E7 reactivity (HPV-TILs). Cell infusion was preceded by lymphocyte-depleting chemotherapy and was followed by administration of aldesleukin.
Results
Three of nine patients experienced objective tumor responses (two complete responses and one partial response). The two complete responses were ongoing 22 and 15 months after treatment, respectively. One partial response was 3 months in duration. The HPV reactivity of T cells in the infusion product (as measured by interferon gamma production, enzyme-linked immunospot, and CD137 upregulation assays) correlated positively with clinical response (P = .0238 for all three assays). In addition, the frequency of HPV-reactive T cells in peripheral blood 1 month after treatment was positively associated with clinical response (P = .0238).
Conclusion
Durable, complete regression of metastatic cervical cancer can occur after a single infusion of HPV-TILs. Exploratory studies suggest a correlation between HPV reactivity of the infusion product and clinical response. Continued investigation of this therapy is warranted.
INTRODUCTION
Although it is hoped that in the future cervical cancer will be prevented by human papillomavirus (HPV) vaccines and cancer screening, it currently causes the deaths of more than 4,000 women in the United States each year.1 In the advanced stage, cervical cancer is a chemotherapy-refractory disease for which durable palliation or cure is rarely achieved.2 Cervical cancers harbor the HPV oncoproteins, cancer-driving viral antigens that are highly attractive therapeutic targets.3,4 However, efforts to target the HPV oncoproteins with therapeutic vaccines have been unsuccessful in advanced cervical cancer, and evidence that immunotherapy can induce regression of this disease has been lacking.
Adoptive T-cell therapy (ACT), infusion of autologous tumor-reactive T cells, can mediate complete clinical responses in some patients with B-cell malignancies and metastatic melanoma.5–12 Study of ACT is expanding, but its evaluation in epithelial malignancies has been limited,3,4,13 and it is unknown if it can mediate regression of metastatic cervical cancer. We developed a method for generating T-cell cultures from HPV-positive cancers and for selecting when possible HPV oncoprotein–reactive cultures for administration to patients. We initiated a clinical protocol to study if infusion of these cells (HPV-TILs) can induce cancer regression in patients. Here we report the clinical and immunologic findings from treatment of a cohort of women with metastatic cervical cancer.
PATIENTS AND METHODS
Patients
Patients age 18 to 66 years with a pathologically confirmed diagnosis of metastatic or locally advanced refractory or recurrent cervical cancer were eligible for the clinical trial. All patients had received prior platinum-based chemotherapy or chemoradiotherapy. Patients with ≤ three brain metastases that were < 1 cm in diameter and asymptomatic were permitted to participate. An Eastern Cooperative Oncology Group performance status of 0 or 1 was required.
Study Design
The clinical trial was designed to determine if HPV-TILs could mediate regression of advanced HPV-positive cancers. Patients were treated in two cohorts (cervical cancer and noncervical cancer diagnoses). Patients from the cervical cancer cohort are reported here. The protocol was approved by the National Cancer Institute Institutional Review Board at the National Institutes of Health Clinical Center, and informed consent was obtained from all patients. The treatment schema is shown in the Data Supplement. Treatment consisted of a lymphocyte-depleting conditioning chemotherapy regimen (cyclophosphamide 60 mg/kg intravenously [IV] daily for 2 days and fludarabine 25 mg/m2 daily for 5 days), HPV-TIL infusion IV as a single dose, and aldesleukin 720,000 IU/kg/dose IV bolus every 8 hours to tolerance or a maximum of 15 doses. Tumor responses were determined using RECIST (version 1.0). Additional details are provided in the Data Supplement.
Generation of HPV-TIL Cell Products
HPV-TIL cell products were generated as described in the Data Supplement. Briefly, T-cell cultures were initiated from fragments of metastatic tumors and expanded using interleukin-2–containing culture media.14 Cultures with lymphocyte outgrowth were tested for reactivity against HPV-16 or HPV-18 E6 and E7. Cultures were selected for additional expansion14,15 for patient administration based on HPV oncoprotein reactivity, rapid growth, high T-cell purity, and high frequency of CD8+ T cells.
Immunologic Assays
Infusion product and peripheral blood (PB) T-cell reactivity against the HPV antigens was determined as described in the Data Supplement. Briefly, assays were performed by coculture of T cells with autologous dendritic cells loaded with peptide pools (15-mer peptides overlapping by 11 amino acids) spanning E6, E7, or gp100 (negative control). The HPV type of the peptide pools used in assays was matched to that of the patient's tumor. Interferon gamma (IFN-γ) enzyme-linked immunospot (ELISPOT; Mabtech, Cincinnati, OH) assay and enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN; Thermo Fisher Scientific, West Palm Beach, FL) were performed according to manufacturer instructions. CD137 upregulation assays were performed by flow cytometric analysis after 20 to 24 hours of coculture.16
Statistical Analysis
The Mann-Whitney U test was used to test for correlations between HPV reactivity and clinical response (GraphPad Prism software [version 6.0]; http://www.graphpad.com/scientific-software/prism). Reported P values are two tailed and not adjusted for multiple comparisons. P values < .05 were considered statistically significant.
RESULTS
Patient Characteristics
Between April 24, 2012, and May 1, 2014, nine women with metastatic cervical cancer were treated (Table 1). The median age was 37 years (range, 30 to 59 years). The patients' tumors were squamous cell carcinomas (n = 4), adenocarcinomas (n = 3), or adenosquamous carcinomas (n = 2). The predominant HPV serotype was HPV-18 (n = 7), with HPV-16 considerably less common (n = 2). All patients had multiple sites of distant metastatic disease and had been previously treated with platinum chemotherapy. Six patients had previously received combination chemotherapy regimens. A median of 80 × 109 (range, 33 to 152 × 109) T cells were administered, consisting of both CD4+ and CD8+ T-cell subsets. The median number of interleukin-2 doses was five (range, one to nine).
Table 1.
Characteristics of Patients and Administered T Cells
| Patient | Age (years) | Histology | HPV Type | Sites of Disease | Prior RT | Prior Systemic Treatment | Cells (× 109) | Within CD3+ (%) |
No. of IL-2 Doses | Response |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4+ | CD8+ | Type | Duration or TTP (months) | |||||||||
| 1 | 30 | ASC | 18 | Iliac lymph nodes, lung, lung hilum, retroperitoneum, vaginal cuff | Yes | Cisplatin | 101.4 | 29 | 72 | 7 | PD | 1 |
| 2 | 53 | SCC | 18 | Bone, liver, lung, lung hilum, mediastinum, pelvis | Yes | Cisplatin, carboplatin, paclitaxel, topotecan, ixabepilone dimethane sulfonate | 126.0 | 10 | 94 | 3 | PR | 3 |
| 3 | 36 | SCC | 16 | Iliac lymph nodes, lung hilum, mediastinum, retroperitoneum | Yes | Cisplatin, vincristine, bleomycin, gemcitabine, paclitaxel, topotecan | 152.0 | 21 | 83 | 2 | CR | 22+ |
| 4 | 55 | SCC | 16 | Axilla, breast, liver, omentum, pleura, soft tissue | Yes | Cisplatin, carboplatin, paclitaxel, fluorouracil, irinotecan, dovitinib, pemetrexed | 80.1 | 23 | 76 | 7 | PD | 2 |
| 5 | 44 | SCC | 18 | Brain, mediastinum, supraclavicular nodes | Yes | Cisplatin | 90.0 | 66 | 29 | 5 | PD | 2 |
| 6 | 36 | AC | 18 | Abdominal wall, liver, paracolic, pelvis, retroperitoneum | Yes | Cisplatin | 74.7 | 13 | 86 | 8 | CR | 15+ |
| 7 | 59 | AC | 18 | Abdominal wall, lung | Yes | Cisplatin, paclitaxel, carboplatin, bevacizumab | 33.4 | 36 | 58 | 8 | PD | 1 |
| 8 | 31 | ASC | 18 | Pelvis, perihepatic mass | No | Cisplatin, paclitaxel | 46.1 | 64 | 29 | 9 | PD | 2 |
| 9 | 37 | AC | 18 | Axilla, bone, lung, mediastinum, pelvis, retroperitoneum | Yes | Cisplatin, carboplatin, paclitaxel, ipilimumab | 70.2 | 33 | 59 | 1 | PD | 1 |
Abbrevations: AC, adenocarcinoma; ASC, adenosquamous cell carcinoma; CR, complete response; HPV, human papillomavirus; IL-2, interleukin-2; PD, progressive disease; PR, partial response; RT, radiotherapy; SCC, squamous cell carcinoma; TTP, time to progression.
Clinical Responses
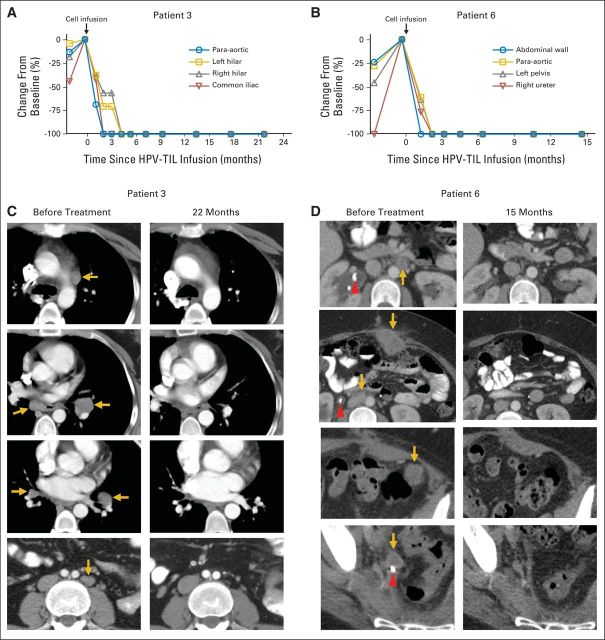
Three of nine women attained objective tumor responses (two complete responses and one partial response; Table 1; Figs 1A to 1D). The partial response was 3 months in duration. The two complete responses were ongoing 22 and 15 months after treatment, respectively. One woman with a complete response (patient 3) had metastatic squamous cell carcinoma and had received multiple combination chemotherapy regimens. Her initial treatment consisted of induction cisplatin, vincristine, and bleomycin followed by chemoradiotherapy with gemcitabine plus cisplatin. She then developed disease in paratracheal (biopsy confirmed), subcarinal, and bilateral hilar lymph nodes and received topotecan and paclitaxel. At the time of HPV-TIL treatment, she had progressing metastatic cervical cancer involving para-aortic, bilateral hilar, subcarinal, and iliac sites (Figs 1A and 1C). After treatment, she experienced complete regression at all sites of disease (Figs 1A and 1C). The other patient with a complete response (patient 6) had metastatic adenocarcinoma. Her primary tumor was refractory to chemoradiotherapy. Salvage surgery identified para-aortic and iliac lymph node involvement and additional pelvic disease. Her cancer progressed to involve additional retroperitoneal lymph nodes and the liver surface, and she developed right hydroureteronephrosis and bilateral pulmonary emboli, which required a ureteral stent and anticoagulation therapy. At the time of HPV-TIL treatment, she had progressing tumors at retroperitoneal, abdominal wall, paracolic, parahepatic, and pelvic sites (Figs 1B and 1D). After treatment with HPV-TILs, she experienced a complete clinical response (Figs 1B and 1D; Data Supplement).
Fig 1.
Complete tumor responses in two patients with metastatic cervical cancer treated with tumor-infiltrating T cells selected for human papillomavirus E6 and E7 reactivity (HPV-TILs). Change from pretreatment baseline in longest diameter of individual metastatic tumors after treatment with HPV-TILs for patients (A) 3 and (B) 6. Contrast-enhanced computed tomography scans obtained before treatment and at most recent follow-up for these patients. (C) Patient 3 had disease involving para-aortic, bilateral hilar, subcarinal, and left iliac lymph nodes (gold arrows). Left hilar tumor seen on second and third images from top is same tumor at different slice levels. Patient had no evidence of disease 22 months after treatment. (D) Patient 6 had metastatic disease in para-aortic lymph node, abdominal wall, aortocaval lymph node, left pericolic pelvic mass, and right ureteral nodule (gold arrows). Additional tumors were present on liver surface and were visualized best with magnetic resonance imaging (Data Supplement). Patient had no evidence of disease 15 months after treatment. Red arrowhead indicates ureteral stent that was removed after right ureteral tumor regressed.
Adverse Events
There were no acute toxicities related to cell infusion. No autoimmune adverse events occurred. Grade 3 and 4 adverse events are summarized in Table 2. The most common severe toxicities were hematologic and the expected result of the lymphocyte-depleting conditioning regimen. Aldesleukin was dosed to tolerance, and its toxicities were generally not severe and resolved with discontinuation of the drug. No patients were intubated or required hemodialysis, and no deaths occurred. Serum cytokine levels were examined in the two patients with complete responses (Data Supplement). Both patients displayed transient cytokine elevations that were associated with fevers, but neither they nor the other patients manifested severe cytokine release syndrome.
Table 2.
Adverse Events (grades 3 and 4)
| Adverse Event | No. of Patients |
|---|---|
| Anemia | 9 |
| Lymphopenia | 9 |
| Leukopenia | 9 |
| Neutropenia | 9 |
| Thrombocytopenia | 9 |
| Infection* | 6 |
| Febrile neutropenia | 5 |
| Metabolic disorders | 5 |
| Nausea/vomiting | 4 |
| Fatigue | 3 |
| Diarrhea | 2 |
| Hypoxia | 2 |
| Syncope | 1 |
| Hypotension | 1 |
| Hemorrhage† | 1 |
| Ureteral obstruction | 1 |
Includes positive surveillance blood cultures.
Associated with radiation cystitis and colitis.
Correlation of Infused T-Cell HPV Reactivity With Clinical Response
The frequency of HPV-reactive T cells in the infusion products was assessed by IFN-γ ELISPOT and CD137 upregulation assays (Figs 2A and 2B). The three patients with the highest frequency of HPV-reactive T cells in their infusion products (as determined by either assay) were also the three patients who demonstrated objective tumor responses. HPV reactivity as measured by IFN-γ production was also greatest in the three responding patients (Fig 2C). Two patients with no apparent HPV reactivity by any of the three assays did not have tumor responses. Overall, HPV reactivity, as assessed by each of the three immunologic assays, was positively associated with tumor response (P = .0238 for each assay). However, the number of patients in these exploratory studies was small, and the results must be interpreted cautiously.
Fig 2.
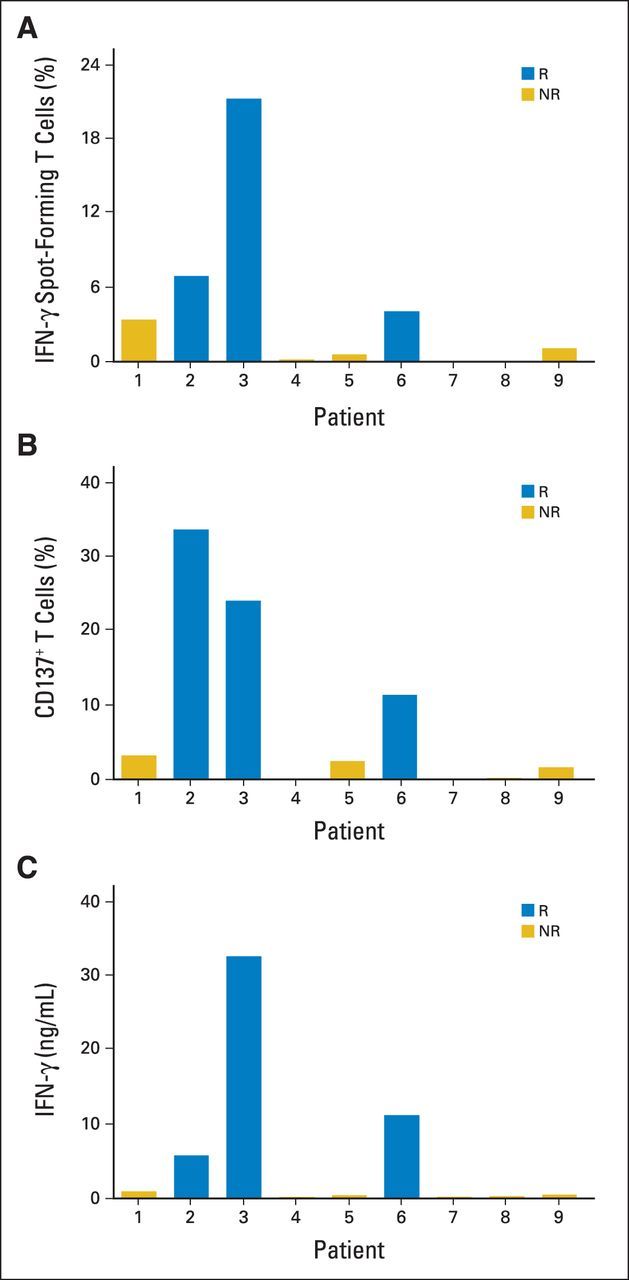
Human papillomavirus (HPV) reactivity of infused T cells. Infusion products for each patient were assessed for reactivity against HPV type–specific E6 and E7 oncoproteins using (A) interferon gamma (IFN-γ) enzyme-linked immunospot, (B) CD137 upregulation, and (C) IFN-γ production assays. For patients 3 and 4, HPV-16–positive oncoprotein peptide pools were used; for patients 1, 2, 5, 6, 7, 8 and 9, HPV-18–positive oncoprotein peptide pools were used (Table 1). Values shown represent sum of E6 and E7 reactivity after background subtraction (data for each antigen and negative control are provided in Data Supplement). Data are representative of ≥ two independent experiments, each performed in duplicate wells. NR, nonresponding patient; R, responding patient.
HPV reactivity was observed in CD4+ and CD8+ T cells, as determined by CD137 upregulation, and the target epitopes for these T cells varied among patients (Data Supplement). Although CD137 is better established as an activation marker for antigen-specific CD8+ T cells, it has also been used to identify viral antigen-specific CD4+ T cells.16–18 In our data set, CD137 upregulation correlated with IFN-γ ELISPOT reactivity (Figs 2A and 2B). HPV-reactive infused T cells were capable of producing multiple effector cytokines (Data Supplement). Cytokine production by TILs was not assessed before their expansion in culture. Infusion products were not assessed for the presence of T-regulatory cells; however, in our experience with melanoma, T-regulatory cells present in excised tumors19 were not detectable in infusion products generated with our methods.20 Analyses of tumor major histocompatibility complex expression and infiltration by CD3+, CD4+, and CD8+ cells did not reveal statistically significant correlations with response to treatment (data not shown).
Correlation of Repopulation With HPV-Reactive T Cells With Clinical Response
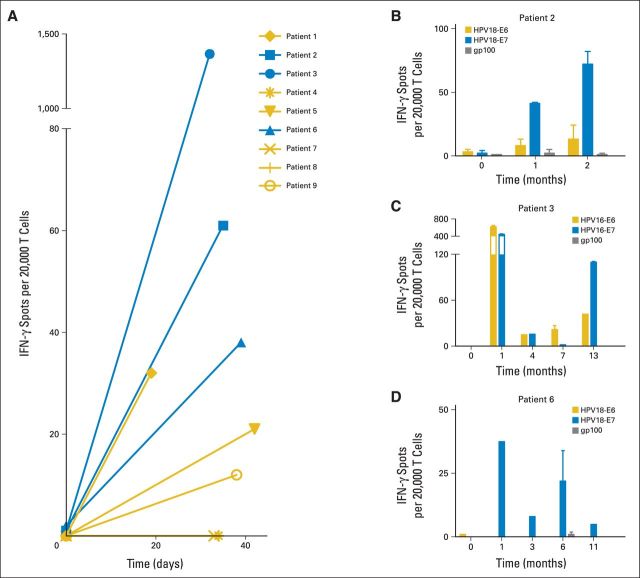
PB samples were studied to determine if infusion of HPV-TILs induced an increase in the frequency of HPV-reactive T cells approximately 1 month after treatment (Figs 3A to 3D). Patients displayed minimal, if any, T-cell HPV reactivity before treatment. One month after treatment, six of nine patients showed an increase in T-cell reactivity against E6 and E7 (two patients who received cells lacking HPV reactivity showed no acquisition of T-cell HPV reactivity after treatment; Fig 3A). The three patients with the highest frequency of HPV-reactive T cells in their PB after treatment experienced objective tumor responses. Overall, the frequency of HPV-reactive T cells in PB approximately 1 month after treatment correlated positively with clinical response (P = .0238). These results suggest a possible relationship between the repopulation of patients with HPV-reactive T cells and tumor response; however, study of additional patients will be required to confirm this finding. One month after treatment, oncoprotein-reactive T cells from patients 3 and 6 showed the capacity to produce multiple cytokines (Data Supplement).
Fig 3.
Repopulation of patients with human papillomavirus (HPV) –reactive T cells after treatment with tumor-infiltrating T cells selected for HPV E6 and E7 reactivity. Peripheral blood (PB) samples from before and after treatment were assessed by interferon gamma (IFN-γ) enzyme-linked immunospot for reactivity against HPV oncoproteins. For patients 3 and 4, HPV-16–positive oncoprotein peptide pools were used; for patients 1, 2, 5, 6, 7, 8 and 9, HPV-18–positive oncoprotein peptide pools were used (Table 1). (A) HPV reactivity of PB T cells from responding (blue) and nonresponding (gold) patients before and 19 to 42 days after treatment. (B, C, D) HPV reactivity of PB T cells from responding patients (B) 2, (C) 3, and (D) 6 at late time points after treatment. Error bars represent standard deviations of duplicate wells in same experiment except in patients 3 (month 4) and 6 (months 1, 3, and 11), which are single determinations. This experiment was repeated once with slightly different time points and similar results.
Prolonged Repopulation With HPV-Reactive T Cells in Responding Patients
The PB of patients who experienced a clinical response was analyzed to determine if the frequency of HPV-reactive T cells remained elevated at later time points after treatment (Figs 3B and 3C). No samples were available from patients who did not respond to treatment, because they were taken off protocol after disease progression. T cells were isolated directly from PB and analyzed without in vitro expansion. All three patients displayed elevated PB T-cell reactivity against E6 and/or E7 but not gp100 (negative control) at late time points after treatment (up to 2, 13, and 11 months for patients 2, 3, and 6, respectively). The particular oncoprotein (E6 v E7) targeted by HPV-reactive PB T cells after treatment was consistent with that targeted by the infused T cells (Data Supplement). These results suggest that responding patients experienced prolonged repopulation with the infused HPV-reactive T cells.
DISCUSSION
We report objective tumor regression in patients with metastatic cervical cancer after treatment with tumor-infiltrating T cells selected for reactivity against HPV E6 and E7. Two patients, one with squamous cell carcinoma and the other with adenocarcinoma, experienced complete cancer remissions that were ongoing 22 and 15 months, respectively, after a single infusion of T cells. These results may have important implications for immunotherapy of cervical cancer and for the expanded application of cellular therapy.
Metastatic cervical cancer is a difficult-to-treat condition that is generally incurable. First-line chemotherapy consists of platinum-based combinations that rarely provide durable disease control.2 Addition of bevacizumab to first-line chemotherapy improves median overall survival by a few months and has been a step forward,21 but better treatments are still needed. Second-line chemotherapy has low response rates and no demonstrated survival benefit,2 and clinical trials of molecularly targeted small molecules have not identified new agents with greater response rates.22–24 Innovative therapeutic approaches that circumvent the inherent limitations of traditional oncology drugs are needed.
Immunotherapy acts through mechanisms that are distinct from traditional systemic therapies. It is an attractive strategy for cervical cancers because these tumors nearly universally harbor the HPV E6 and E7 antigens.3,4 E6 and E7 are appealing therapeutic targets because they are constitutively expressed, tumor specific, and functionally important, and they can be recognized and attacked by the human adaptive immune system.3,4,25 Prior efforts to treat cervical cancer by directing immune responses against E6 and E7 have focused primarily on the induction of endogenous T-cell responses through therapeutic vaccination. In premalignant HPV-positive disease (eg, vulvar intraepithelial neoplasia), encouraging results have been attained with a long-peptide vaccine.26 However, in invasive cervical cancer, vaccines have failed to demonstrate clear clinical activity.27–31 Immune checkpoint blockade is an active area of investigation for wide-ranging cancers, but the use of this approach for cervical cancer has not been reported. The results of our study demonstrate that immunotherapy can mediate long-lasting regression of chemotherapy-refractory metastatic cervical cancer, and they provide additional support for the investigation of immune-based treatments for this disease.
The patients in this study had cancers that constitutively expressed the HPV oncoproteins, and the administered T cells were selected to target these antigens. The HPV reactivity of T cells in the infusion products and the frequency of HPV-reactive T cells in patients' PB after treatment correlated with tumor response. These results suggest the infused HPV-reactive T cells played a role in the clinical responses. However, they do not definitively demonstrate such a role, because so-called bystander T cells with other antigen specificities were also administered and may have been responsible for the observed clinical responses. Tumor responses to TIL therapy in melanoma can be mediated by T cells with reactivity against mutated-gene products.32 Cervical cancers also harbor somatic gene mutations,33,34 and the cells administered to our patients may have included T cells targeting these mutations. Studies of TIL reactivity against non-HPV tumor antigens may provide additional insight into the mechanisms of tumor regression in this therapy. Also, when a greater number of patient samples can be studied, comparisons between the frequencies of persisting tumor-reactive T cells in melanoma and cervical cancer TIL therapies may be interesting, although they may be confounded by differences in the antigens expressed by these cancers. Finally, post-treatment studies of samples from regressing tumors rather than PB may provide additional insight into how this cellular therapy mediates tumor regression.
The identification of mechanism-based predictive biomarkers that could guide treatment decisions is an important goal in oncology drug development. HPV-TILs are a highly personalized treatment permitting biomarker studies that are not possible with traditional off-the-shelf drugs. In HPV-TIL therapy, the drug itself (ie, cell infusion product) consists of the patient's cells and can be analyzed for characteristics that might correlate with clinical response. We found that the magnitude of HPV reactivity of the infusion products (measured by IFN-γ production, ELISPOT, or CD137 upregulation) was associated with clinical response (Figs 2A to 2C). The number of patients studied is small, so the data must be interpreted with caution. However, the results of this exploratory analysis merit further study in a larger number of patients. With TIL therapy for melanoma, expression of CD27 and telomere length of the infused T cells are associated with clinical response, but there is considerable overlap between responding and nonresponding patients8; such investigations may be informative for HPV-TILs when a greater number of patients can be studied.
The lymphocyte-depleting conditioning regimen used in this protocol increases the toxicity of therapy but enhances the antitumor activity of infused T cells through indirect mechanisms.3,35 Whether it has direct cytotoxic clinical activity in platinum-treated cervical cancer is unknown. Fludarabine has no direct antitumor activity in cervical cancer.36 Cyclophosphamide has clinical activity in diverse malignancies, but it has not been studied as a single agent for cervical cancers in the era of objective response criteria. Cyclophosphamide is not used in the treatment of cervical cancer, but its analog, ifosfamide, has a response rate of 16% in platinum-naive patients.37 In platinum-treated patients, like those in this trial, the response rate is 11%, and the response duration is short (three of 27 partial responses, ranging from 1.8 to 3.1 months in duration).38 Thus, the direct antitumor effect of the single cycle of conditioning chemotherapy administered to the patients in this trial is uncertain, but it probably does not account for the durable, complete responses that were observed. It did, however, contribute to the severe hematologic toxicities noted in all patients, and strategies to reduce or eliminate the preparative regimen are an area of active investigation.
In this protocol, tumor responses occurred in patients with two distinct cervical cancer histologies: squamous cell carcinoma and adenocarcinoma. Additional research will be required to determine if squamous and glandular carcinomas from noncervical anatomic sites also can respond to HPV-TILs and other cellular therapies. Extension of ACT to common epithelial cancers may depend more on the presence of antigens that can be targeted without severe autoimmune toxicity than on the sensitivity of certain malignancies to T cell–mediated recognition and killing.4 This notion is supported by the diversity of tumor types that seem to respond to ACT (melanoma,7,8 synovial cell sarcoma,7 and B-cell malignancies5,6,9,11,12) as well as by our report of tumor regression in two novel epithelial histologies. Whether HPV-TILs can also mediate regression of HPV-positive carcinomas of the oropharynx, anus, vulva, vagina, or penis is under investigation in the noncervical cancer cohort in this clinical trial. However, at this time, accrual and follow-up are insufficient to draw conclusions about potential clinical benefit.
Cervical cancer is a major worldwide health problem that is best addressed with preventative vaccines and screening programs. However, vaccine uptake in the United States has been lower than hoped, and screening is imperfect (both patients with complete tumor responses described here had endocervical cancers that were reportedly missed by screening). Patients continue to develop advanced disease for which better treatments are needed. ACT is a highly personalized, novel therapeutic approach that may circumvent the inherent limitations of chemotherapy in cervical cancer. Further study of HPV-TILs is warranted.
Supplementary Material
Acknowledgment
We thank the Milstein Family Foundation for its generous support. We also thank Robert P.T. Somerville and Sadik Kassim for clinical cell manufacturing; Eric Tran, Alena Gros, Anna Pasetto, and Drew C. Deniger for technical assistance and helpful discussions; Sid Kerkar and Mark Raffeld for immunohistologic analyses; and the National Cancer Institute Surgery Branch immunotherapy clinical fellows and nurses for their care of the patients.
Glossary Terms
- adoptive T-cell therapy:
the culture and expansion of T lymphocytes outside the body and then the infusion of those lymphocytes into patients for therapeutic purposes.
- HPV E6:
a protein that forms a complex with an E3 ubiquitin ligase, E6-associated protein (E6AP), and ubiquitinates the p53 tumor suppressor protein. The ubiquitination causes rapid degradation or destabilization of p53, thus resulting in deregulation of the cell cycle and proliferation, induction of cellular immortalization, and antiapoptosis.
- HPV E7:
a protein that binds to the cullin 2 ubiquitin ligase complex and ubiquitinates the retinoblastoma tumor suppressor protein. The ubiquitination causes rapid degradation or destabilization of the retinoblastoma tumor suppressor protein, thus resulting in deregulation of the cell cycle and proliferation, induction of cellular immortalization, and antiapoptosis.
- human papillomavirus (HPV):
a double-stranded DNA virus from the papillomaviridae family. Human papillomavirus is a cause of cervical cancer as well as of a subset of cancers of the anus, oropharynx, penis, vagina, and vulva.
- immunotherapy:
a therapeutic approach that uses cellular and/or humoral elements of the immune system to fight a disease.
- Response Evaluation Criteria in Solid Tumors (RECIST):
a model proposed by the Response Evaluation Criteria Group by which a combined assessment of all existing lesions, characterized by target lesions (to be measured) and nontarget lesions, is used to extrapolate an overall response to treatment.
Footnotes
See accompanying editorial on page 1521
Processed as a Rapid Communication manuscript.
Supported by the Intramural Research Program of the National Institutes of Health and the Milstein Family Foundation.
Presented in part at the 87th Semiannual Meeting of the Gynecologic Oncology Group, San Antonio, TX, July 18-21, 2013; 28th Annual Meeting of the Society for Immunotherapy of Cancer, National Harbor, MD, November 8-10, 2013; 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014; and 29th International Papillomavirus Conference and Clinical Workshop, Seattle, WA, August 20-25, 2014.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01585428.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Sanja Stevanović, Lindsey M. Draper, Michelle M. Langhan, John R. Wunderlich, Mark E. Dudley, Nicholas P. Restifo, Christian S. Hinrichs
Provision of study materials or patients: James C. Yang
Collection and assembly of data: Sanja Stevanović, Tracy E. Campbell, Mei Li Kwong, James C. Yang, Christian S. Hinrichs
Data analysis and interpretation: Sanja Stevanović, Mei Li Kwong, Richard M. Sherry, Udai S. Kammula, Nicholas P. Restifo, Steven A. Rosenberg, Christian S. Hinrichs
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Complete Regression of Metastatic Cervical Cancer After Treatment With Human Papillomavirus–Targeted Tumor-Infiltrating T Cells
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Sanja Stevanović
No relationship to disclose
Lindsey M. Draper
No relationship to disclose
Michelle M. Langhan
No relationship to disclose
Tracy E. Campbell
No relationship to disclose
Mei Li Kwong
No relationship to disclose
John R. Wunderlich
Patents, Royalties, Other Intellectual Property: Immunotherapy with in vitro-selected antigen-specific lymphocytes after nonmyeloablative lymphodepleting chemotherapy: US Patent Application No. US 8287857 B2 (Inst)
Mark E. Dudley
Employment: Novartis
Research Funding: Kite Pharma (Inst), Lion Biotechnology (Inst)
Patents, Royalties, Other Intellectual Property: Immunotherapy with in vitro-selected antigen-specific lymphocytes after nonmyeloablative lymphodepleting chemotherapy: US Patent Application No. US 8287857 B2 (Inst)
James C. Yang
Patents, Royalties, Other Intellectual Property: US Patents No. 7, 711, 702 and 7, 820, 174; not related to this work (Inst)
Richard M. Sherry
No relationship to disclose
Udai S. Kammula
Stock or Other Ownership: Merck, Pfizer
Nicholas P. Restifo
No relationship to disclose
Steven A. Rosenberg
Research Funding: Kite Pharma (Inst), Lion Biotechnologies (Inst)
Patents, Royalties, Other Intellectual Property: Methods of preparing anti-human papillomavirus antigen T cells: US Provisional Patent Application No. 61/846, 161 (Inst), Methods of preparing anti-human papillomavirus antigen T cells: International Patent Application No. PCT/US14/46478 (Inst), Immunotherapy with in vitro–selected antigen-specific lymphocytes after nonmyeloablative lymphodepleting chemotherapy: US Patent Application No. US 8287857 B2 (Inst)
Christian S. Hinrichs
Employment: MedImmune (I)
Patents, Royalties, Other Intellectual Property: Methods of preparing anti-human papillomavirus antigen T cells: US Provisional Patent Application No. 61/846,161 (Inst), Methods of preparing anti-human papillomavirus antigen T cells: International Patent Application No. PCT/US14/46478 (Inst)
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ramondetta L. What is the appropriate approach to treating women with incurable cervical cancer? J Natl Compr Canc Netw. 2013;11:348–355. doi: 10.6004/jnccn.2013.0044. [DOI] [PubMed] [Google Scholar]
- 3.Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T cell therapy for cancer. Immunol Rev. 2014;257:56–71. doi: 10.1111/imr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinrichs CS, Restifo NP. Reassessing target antigens for adoptive T cell therapy. Nat Biotechnol. 2013;31:999–1008. doi: 10.1038/nbt.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollard CM, Gottschalk S, Torrano V, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014;32:798–808. doi: 10.1200/JCO.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudley ME, Wunderlich JR, Shelton TE, et al. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin J, Sabatino M, Somerville R, et al. Simplified method of the growth of human tumor infiltrating lymphocytes in gas-permeable flasks to numbers needed for patient treatment. J Immunother. 2012;35:283–292. doi: 10.1097/CJI.0b013e31824e801f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfl M, Kuball J, Ho WY, et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110:201–210. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wehler TC, Karg M, Distler E, et al. Rapid identification and sorting of viable virus-reactive CD4+ and CD8+ T cells based on antigen-triggered CD137 expression. J Immunol Methods. 2008;339:23–37. doi: 10.1016/j.jim.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Zandvliet ML, van Liempt E, Jedema I, et al. Simultaneous isolation of CD8(+) and CD4(+) T cells specific for multiple viruses for broad antiviral immune reconstitution after allogeneic stem cell transplantation. J Immunother. 2011;34:307–319. doi: 10.1097/CJI.0b013e318213cb90. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadzadeh M, Felipe-Silva A, Heemskerk B, et al. FOXP3 expression accurately defines the population of intratumoral regulatory T-cells that selectively accumulate in metastatic melanoma lesions. Blood. 2008;112:4953–4960. doi: 10.1182/blood-2008-06-163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao X, Ahmadzadeh M, Lu YC, et al. Levels of peripheral CD4(+)FoxP3(+) regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood. 2012;119:5688–5696. doi: 10.1182/blood-2011-10-386482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tewari KS, Sill MW, Long HJ, 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monk BJ, Mas Lopez L, Zarba JJ, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol. 2010;28:3562–3569. doi: 10.1200/JCO.2009.26.9571. [DOI] [PubMed] [Google Scholar]
- 23.Santin AD, Sill MW, McMeekin DS, et al. Phase II trial of cetuximab in the treatment of persistent or recurrent squamous or non-squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. Gynecol Oncol. 2011;122:495–500. doi: 10.1016/j.ygyno.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackay HJ, Tinker A, Winquist E, et al. A phase II study of sunitinib in patients with locally advanced or metastatic cervical carcinoma: NCIC CTG Trial IND.184. Gynecol Oncol. 2010;116:163–167. doi: 10.1016/j.ygyno.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Moody CA, Laimins LA. Human papillomavirus oncoproteins: Pathways to transformation. Nat Rev Cancer. 2010;10:550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 26.Kenter GG, Welters MJP, Valentijn ARPM, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 27.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: A phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–3983. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 28.Borysiewicz LK, Fiander A, Nimako M, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–1527. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 29.Steller MA, Gurski KJ, Murakami M, et al. Cell-mediated immunological responses in cervical and vaginal cancer patients immunized with a lipidated epitope of human papillomavirus type 16 E7. Clin Cancer Res. 1998;4:2103–2109. [PubMed] [Google Scholar]
- 30.Ferrara A, Nonn M, Sehr P, et al. Dendritic cell-based tumor vaccine for cervical cancer II: Results of a clinical pilot study in 15 individual patients. J Cancer Res Clin Oncol. 2003;129:521–530. doi: 10.1007/s00432-003-0463-5. [DOI] [PubMed] [Google Scholar]
- 31.Van Poelgeest MI, Welters MJ, van Esch EM, et al. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J Transl Med. 2013;11:88. doi: 10.1186/1479-5876-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins PF, Lu YC, El-Gamil M, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19:747–752. doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ojesina AI, Lichtenstein L, Freeman SS, et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014;506:371–375. doi: 10.1038/nature12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright AA, Howitt BE, Myers AP, et al. Oncogenic mutations in cervical cancer: Genomic differences between adenocarcinomas and squamous cell carcinomas of the cervix. Cancer. 2013;119:3776–3783. doi: 10.1002/cncr.28288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Von Hoff DD, Green S, Surwit EA, et al. Phase II study of fludarabine phosphate (NSC 312887) in patients with advanced cervical cancer: A Southwest Oncology Group study. Am J Clin Oncol. 1990;13:433–435. doi: 10.1097/00000421-199010000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Sutton GP, Blessing JA, McGuire WP, et al. Phase II trial of ifosfamide and mesna in patients with advanced or recurrent squamous carcinoma of the cervix who had never received chemotherapy: A Gynecologic Oncology Group study. Am J Obstet Gynecol. 1993;168:805–807. doi: 10.1016/s0002-9378(12)90824-8. [DOI] [PubMed] [Google Scholar]
- 38.Sutton GP, Blessing JA, Photopulos G, et al. Gynecologic Oncology Group experience with ifosfamide. Semin Oncol. 1990;17:6–10. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.