Abstract
Purpose
Allogeneic stem-cell transplantation (SCT) induces long-term remission in a fraction of patients with high-risk chronic lymphocytic leukemia (CLL) or Richter's transformation (RT). Our purpose was to determine the outcomes of patients whose disease progressed after allogeneic SCT.
Patients and Methods
We retrospectively analyzed the outcomes of 72 patients (52 with CLL and 20 with RT) who underwent allogeneic SCT between 1998 and 2011 and had documented progression after transplantation. Twenty-two (31%) never had a response, and 50 (69%) had a response but experienced relapse after a median of 7 months (range, 2 to 85 months). Forty-eight patients who were receiving or were candidates to receive post-SCT cell-based therapies were not included in this analysis.
Results
The median age at time of transplantation was 58 years (range, 30 to 72 years). Sixty-two patients (86%) received more than two treatment regimens and 37 (51%) received more than three treatment regimens before SCT. Sixty-six patients (92%) had active disease at the time of transplantation. The 2- and 5-year survival rates were 67% and 38% (patients with CLL) and 36% and 0% (patients with RT). The patients who developed acute or chronic graft-versus-host disease had a longer overall survival (OS; P = .05). In a multivariable analysis, RT or low hemoglobin at the time of SCT predicted shorter OS. Chronic graft-versus-host disease and an initial response to SCT predicted longer OS.
Conclusion
Patients with CLL in whom allogeneic SCT fails may have a response to and benefit from salvage therapies, and their prognosis is relatively good.
INTRODUCTION
The majority of patients with chronic lymphocytic leukemia (CLL) who receive a combination of chemotherapy and immunotherapy will experience a response.1,2 In most cases, however, the disease will ultimately relapse and, with time, will become refractory. The prognosis of patients with refractory CLL is dismal, and the median survival is measured in months.3
Allogeneic hematopoietic stem-cell transplantation (SCT) is a treatment option offered to selected patients with CLL on the basis of risk-benefit assessment and the patient's preferences. Most patients who undergo allogeneic SCT for CLL have refractory disease or Richter's transformation (RT), and many of them are heavily pretreated.4
Early myeloablative SCT studies established that long-term remission or cure is attainable in CLL. However, a myeloablative preparative regimen has limited benefits because the rates of treatment-related mortality have been as high as 50%.5–10 High response rates and long-term remissions have also been attained with reduced-intensity conditioning (RIC) regimens. These regimens significantly reduce treatment-related mortality11 and have increased the 5-year survival rate to 50% to 70%.12–16 However, the nonrelapse mortality within the first 2 years amounts to 15% to 25%,6,12–14,17 and approximately half the surviving patients develop chronic graft-versus-host disease (GVHD), which contributes to debilitating morbidity and nonrelapse mortality.18
The efficacy of RIC-SCT has been attributed to a graft-versus-leukemia (GVL) effect.19,20 We previously reported an effective GVL response in patients who experienced relapse after RIC-SCT and were treated with a combination of donor lymphocyte infusions (DLIs) and rituximab.21 Whether GVL also contributed to a favorable outcome in patients who were not in remission after SCT is not known.
Our purpose was to characterize the outcomes of patients with CLL or RT whose disease progressed after SCT. Retrospective review and analysis of patient records revealed 2- and 5-year survival rates from time of progression of 73% and 29%: for patients with CLL, the rates were 67% and 38%, respectively; for those with RT, 38% and 0%. Patients who developed chronic GVHD had a more favorable outcome.
PATIENTS AND METHODS
We searched the clinical databases of the Department of Leukemia and of the Bone Marrow Transplantation Program at The University of Texas MD Anderson Cancer Center to identify patients who underwent allogeneic SCT at the center and who, at the time of post-transplantation response assessment, had had no response (refractory disease) or had experienced relapse after an initial documented response and were referred to the Leukemia Clinic for further treatment. Patients with progressive or residual disease were usually treated with step-wise DLI. DLI was not administered to patients with acute GVHD or rapidly progressing disease. In this analysis, we excluded patients with documented post-SCT relapsed/refractory disease who were undergoing, or were candidates to undergo, treatment with DLI as their sole therapy and considered them to be on an ongoing cell therapy regimen.
All patients included in this study had provided written informed consent for treatment in various clinical trials that had been approved by the institutional review board. Separate institutional review board approval was obtained, which allowed us to retrospectively collect and analyze data from the patients' electronic medical records. Some of the patients' data were previously reported.15,21 However, the post-transplantation follow-up and long-term outcomes of these patients are provided here for the first time.
Patient characteristics were analyzed as frequencies (percentages) for categorical variables and as median and range for continuous variables. The response criteria were those defined by the National Cancer Institute's Sponsored Working Group on CLL.22 Before transplantation, all patients underwent computed tomography scans of the chest, abdomen, and pelvis and a gallium or positron emission tomography scan. Biopsies were performed when RT was suspected. The patients with RT reported here are those who had histologically confirmed RT.
Overall response rates included partial response (PR) or complete response (CR). Refractory disease was defined as failure to achieve CR or PR. Relapsed disease was defined as disease progression 6 months or more after attaining CR or PR, and progressive disease was defined as disease progression within 6 months after completion of a given therapy.23 Overall survival (OS) was demarcated as the time between the date of progression and the date of death or last follow-up contact, whichever occurred first. Patients who were alive at last follow-up contact were censored at that time.
To compare groups of patients on the basis of categorical variables, we used the χ2 test. For categorical time-dependent (paired) variables, we used the McNemar's test. The probability of OS was estimated by the Kaplan-Meier method. The log-rank test was used to compare survival distributions. To determine whether resistant disease before transplantation predicts poor response to SCT, we estimated the odds ratio of the pretransplantation immunotherapy and chemotherapy sensitivity by the exp(β) in a logistic regression model with the post-transplantation response as a dichotomous dependent variable. Univariable and multivariable Cox proportional hazards regression models were fit to assess association between OS and the following variables: age, Eastern Cooperative Oncology Group performance status, hemoglobin level, platelet count, WBC count, cytogenetic abnormalities, p53 deletion or mutation, the diagnosis at time of transplantation (CLL or RT), the type of SCT (myeloablative v nonmyeloablative), presence of acute GVHD or chronic GVHD, best chimerism response, post-transplantation response assessment, and response to first post-transplantation treatment. For the multivariable model, we used a backwards stepwise elimination of nonsignificant covariates on the basis of the likelihood ratio. Significance levels were set at 0.05. Statistical analyses were performed by using SPSS version 21 software (SPSS, Chicago, IL) and GraphPad Prism version 6.0 software (GraphPad Software, San Diego, CA).
RESULTS
Patient Characteristics
Our retrospective review of the Bone Marrow Transplantation Program database identified 358 patients who underwent allogeneic SCT between 1998 and 2011 (159 months). From those patients, we identified 72 who had been referred to the Leukemia Clinic for further treatment for active disease after a median of 74 months (range, 13 to 196 months) from diagnosis and a median of 7 months (range, 7 to 87 months) after transplantation. Forty-eight patients with documented post-SCT relapsed/refractory disease who were undergoing, or were candidates to undergo, cell-based therapies such as DLI were excluded from this analysis. The median time from progression to last follow-up was 30 months (range, 13 to 137 months) and 23 patients (32%) were still alive at time of last follow-up. The patient and disease characteristics at time of diagnosis are summarized in Table 1.
Table 1.
Patient and Disease Characteristics at Time of Diagnosis
| Characteristic | No./Total Available | % |
|---|---|---|
| Total No. of patients | 72 | |
| Age, years | ||
| Median | 51 | |
| Range | 28-70 | |
| Sex | ||
| Male | 54 | 75 |
| Female | 18 | 25 |
| Rai staging | ||
| 0 | 6 | 8 |
| 1 | 37 | 51 |
| 2 | 5 | 7 |
| 3 | 6 | 8 |
| 4 | 18 | 25 |
| Cytogenetics by FISH | ||
| Normal karyotype | 15/51 | 29 |
| del(13q) | 6/51 | 12 |
| T12 | 3/51 | 6 |
| del(11q) | 12/51 | 24 |
| del(17p) | 15/51 | 29 |
| VH mutation status | ||
| Mutated | 6/38 | 16 |
| Unmutated | 32/38 | 84 |
| Mean β2 microglobulin, mg/L | 4.0 | |
Abbreviations: FISH, fluorescent in situ hybridization; VH, immunoglobulin heavy chain.
Patient Clinical Characteristics at the Time of SCT and Transplantation Procedure
Patient clinical characteristics and treatment history at the time of transplantation are summarized in Table 2. Details of the SCT procedures are summarized in Table 3. Specifics of the SCT preparative regimens, infection and GVHD prophylaxis, and supportive care were previously published.21,24,25 The most common preparative regimen comprised fludarabine 30 mg/m2 and cyclophosphamide 750 mg/m2 on days −5 to −3 and rituximab 375 mg/m2 on day −13 and 1,000 mg/m2 on days −6, +1, and +8.
Table 2.
Patient Clinical Characteristics at Time of SCT
| Characteristic | Primary Diagnosis at Time of SCT |
P | |||
|---|---|---|---|---|---|
| CLL |
RT |
||||
| No. | % | No. | % | ||
| Total No. of patients | 52 | 72 | 20 | 28 | |
| Age at time of transplantation, years | .94 | ||||
| Median | 58 | 58 | |||
| Range | 30-72 | 32-72 | |||
| < 50 | 6 | 11 | 4 | 20 | |
| ≥ 50 to < 65 | 39 | 75 | 11 | 55 | |
| ≥ 65 | 7 | 14 | 5 | 25 | |
| ECOG performance status | |||||
| 0 | 4 | 20 | 16 | 31 | .59 |
| 1 | 13 | 65 | 31 | 60 | |
| 2 or more | 3 | 15 | 5 | 9 | |
| No. of prior treatments | |||||
| 1 | 9 | 17 | 1 | 5 | .30 |
| 2 | 16 | 31 | 9 | 45 | |
| 3 or more | 27 | 52 | 10 | 50 | |
| Response to last treatment | |||||
| CR | 3 | 6 | 0 | .48 | |
| PR | 21 | 43 | 8 | 40 | |
| Refractory | 25 | 51 | 12 | 60 | |
| Time from diagnosis of CLL to SCT, months | .43 | ||||
| Median | 70 | 45 | |||
| Range | 11-167 | 12-161 | |||
| Bone marrow cellularity | .9 | ||||
| Percent | 50 | 45 | |||
| Range | 15-100 | 20-95 | |||
| Fludarabine refractory | 13 | 26 | 6 | 32 | .7 |
Abbreviations: CLL, chronic lymphocytic leukemia; CR, complete response; ECOG, Eastern Cooperative Oncology Group; PR, partial response; RT, Richter's transformation; SCT, stem-cell transplantation.
Table 3.
SCT Conditioning and Outcome Data
| SCT Parameters | Primary Diagnosis at Time of SCT |
|||
|---|---|---|---|---|
| RT (n = 20) |
CLL (n = 52) |
|||
| No. | % | No. | % | |
| Donor | ||||
| Matched related | 7 | 35 | 28 | 54 |
| Matched unrelated | 11 | 55 | 23 | 44 |
| Haplo-identical | 2 | 10 | 1 | 2 |
| Stem-cell source | ||||
| Peripheral blood | 14 | 70 | 36 | 69 |
| Bone marrow | 6 | 30 | 11 | 21 |
| Cord blood | 0 | 5 | 10 | |
| Conditioning regimen | ||||
| Myeloablative | 6 | 30 | 14 | 27 |
| Nonmyeloablative | 14 | 70 | 38 | 73 |
| Engraftment | ||||
| Yes | 20 | 100 | 48 | 92 |
| No | 0 | 4 | 8 | |
| Acute GVHD | ||||
| Yes | 12 | 60 | 25 | 48 |
| No | 8 | 40 | 27 | 52 |
| Chronic GVHD | 11 | 51 | 24 | 46 |
| Limited | 3 | 27 | 4 | 17 |
| Extensive | 8 | 73 | 20 | 83 |
| Best donor/recipient chimerism | ||||
| Only recipient | 0 | 2 | 4 | |
| Mixed donor/recipient | 12 | 63 | 24 | 51 |
| Only donor | 7 | 37 | 21 | 45 |
| Post-transplantation response | ||||
| Response | 13 | 65 | 37 | 71 |
| CR | 7 | 54 | 18 | 49 |
| PR | 6 | 46 | 19 | 51 |
| No response | 7 | 35 | 15 | 29 |
Abbreviations: CLL, chronic lymphocytic leukemia; CR, complete response; GVHD, graft-versus-host disease; PR, partial response; RT, Richter's transformation; SCT, stem-cell transplantation.
Post-Transplantation Response Assessment
RT is a major risk in patients who are undergoing SCT, and RT was diagnosed in 16 patients with CLL (31%) after transplantation. Conversely, CLL was diagnosed in four (20%) of the 20 patients who had RT before SCT but were without evidence of RT at post-transplantation response assessment. In all these patients, the same immunoglobulin light chain, fluorescent in situ hybridization, and p53 gene abnormalities were detected before and after transplantation. These tests did not detect an unrelated clone or clonal evolution during post-transplantation follow-up. One patient with pretransplantation CLL developed treatment-related acute myeloid leukemia (AML) 6 months after allogeneic SCT. His bone marrow (BM) cytogenetic analysis before transplantation revealed del(7). When AML was diagnosed, the patient's BM analysis showed mixed chimerism and del(7), suggesting that the AML clone was of recipient's origin.
Twenty-two patients (30%) had refractory disease. Fifty (70%) of the patients who had an initial response to SCT (25 with CR and 25 with PR) subsequently experienced relapse, and the median time from transplantation to relapse was 11 months (range, 2 to 85 months). The response rates in patients with CLL and RT were similar (Table 3). To determine whether any of the factors tested were associated with post-transplantation failure, we performed a match-paired analysis. We identified in our database patients with CLL or RT who underwent SCT about the same time, attained CR, and did not relapse. For 47 of our analyzed patients, we identified a match within the same calendar year and for 61 patients within two calendar years. The matched patients who remained in CR following SCT had higher rates of acute GVHD (60% [n = 41] v 40% [n = 27]; P = .004) and/or chronic GVHD (59% [n = 41] v 41% [n = 28]; P = .011). The estimated median BM cellularity of patients for whom SCT failed was 50% (range, 15% to 100%) whereas the BM cellularity of patients who remained in remission was 35% (range, 5% to 90%; P < .001).
Post-Transplantation Treatments
Most patients who experienced relapse after transplantation received additional treatments. Patients with pre-SCT RT and those who transformed to RT after SCT (n = 16) underwent post-transplantation treatments. Patients with post-transplantation progressive disease were treated for constitutional symptoms and/or severe fatigue (20 patients), granulocytopenia with recurrent infections (two patients), and anemia and/or thrombocytopenia (nine patients). Twenty-one patients were treated for worsening lymphadenopathy (eight patients), massive BM involvement (five patients), or both (eight patients). Five patients did not receive treatment. Four patients had stable disease and did not require treatment, and one patient died before treatment was initiated. The patient who developed secondary AML was treated accordingly but died soon thereafter.
Because there is no consensus on treatment for relapsed/refractory disease after transplantation, the post-transplantation treatment these patients received varied. These treatments are summarized in Table 4. Thirty-two patients (44%) received DLIs and most patients with CLL received an anti-CD20 antibody–based regimen with either rituximab (40%; n = 16) or ofatumumab (28%; n = 11). Five patients received the Bruton tyrosine kinase inhibitor ibrutinib, which did not become available until 2010. Most patients with post-SCT RT received chemoimmunotherapy with either hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine (modified hyperCVAD)26 or oxaliplatin, fludarabine, rituximab, and pegfilgrastim (OFAR).27 Four patients who had a response to the first post-transplantation treatment regimen underwent a second allogeneic SCT.
Table 4.
Post-Transplantation Treatment Regimens
| Treatment Regimen | Post-Transplantation Diagnosis |
|||||||
|---|---|---|---|---|---|---|---|---|
| CLL (n = 40) |
RT (n = 32) |
|||||||
| Given First |
Given |
Given First |
Given |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Rituximab* | 13 | 33 | 17 | 43 | 5 | 20 | 8 | 25 |
| Thalidomide or lenalidomide with or without rituximab | 3 | 8 | 10 | 25 | 3 | 9 | 8 | 25 |
| Alemtuzumab with or without rituximab | 5 | 13 | 10 | 28 | 1 | 3 | 3 | 9 |
| Ofatumumab | 3 | 8 | 11 | 28 | 2 | 6 | 6 | 19 |
| Purine analog-based regimen† | 3 | 8 | 8 | 20 | 0 | 3 | 9 | |
| BR/FBR | 1 | 3 | 6 | 15 | 0 | 2 | 6 | |
| R-hyperCVAD/OFAR | 2 | 5 | 11 | 28 | 9 | 28 | 30 | 94 |
| R-ICE/R-EPOCH/R-DHAP/R-ESHAP | 0 | 0 | 4 | 13 | 6 | 19 | ||
| R-CHOP/R-COP | 1 | 3 | 2 | 5 | 1 | 3 | 1 | 3 |
| Ibrutinib | 1 | 3 | 5 | 13 | 0 | 0 | ||
| Radiation | 0 | 3 | 8 | 0 | 6 | 19 | ||
| Donor lymphocyte infusion‡ | NA | 16 | 40 | NA | 17 | 53 | ||
| Second allogeneic SCT | 0 | 2 | 5 | 0 | 2 | 6 | ||
| Other treatment | 2 | 5 | 4 | 10 | 2 | 6 | 14 | 44 |
| No treatment§ | 0 | 4 | 10 | 0 | 1 | 3 | ||
Abbreviations: BR, bendamustine, rituximab; CLL, chronic lymphocytic leukemia; FBR, cyclophosphamide, bendamustine, rituximab; NA, not available; R-hyperCVAD, rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone; OFAR, oxaliplatin, fludarabine, cytarabine, rituximab; R-ICE, rituximab, ifosfamide, cisplatin, etoposide; R-EPOCH, rituximab, etoposide, prednisone, vincristine, doxorubicin; R-DHAP, dexamethasone, doxorubicin, cytarabine, cisplatin; R-ESHAP, rituximab, etoposide, methylprednisolone, cytarabine, cisplatin; R-CHOP, rituximab, cyclophosphamide, vincristine, prednisone, doxorubicin; R-COP, rituximab, cyclophosphamide, vincristine, prednisone; RT, Richter's transformation.
Rituximab given as single treatment or in combination with a steroid and/or granulocyte-macrophage colony-stimulating factor.
Purine analog–based regimens included fludarabine, cyclophosphamide, and rituximab (FCR); cyclophosphamide, fludarabine, cytarabine, and rituximab (CFAR); or pentostatin, cyclophosphamide, and rituximab (PCR).
Patients who received only donor lymphocyte infusion are not included.
Other treatments included IPI-145; chimeric antigen receptor T cells; cytarabine with cladribine; flavopiridol; bafetinib; anti-CD23 antibodies; 8-chloroadenosine; 6-mercaptopurine, vincristine, methotrexate, and prednisone (POMP); azacitidine; and clofarabine.
The overall rate of response to the first post-transplantation treatment protocol was 45% (n = 34). The response was CR in four patients (6%; three with CLL, one with RT) and PR in 26 (36%; 18 with CLL, eight with RT). Patients who did not respond to post-SCT failure first-line salvage therapy could still be given salvage therapy. Twenty-nine patients for whom first-line therapy failed received additional treatments, and 12 (41%) of those patients responded. Overall, the patients received a median of two different post-transplantation treatments (range, 0 to 8 treatments; Table 4).
Survival
The median OS from time of progression of the 72 patients in the study was 35 months (95% CI, 30 to 40 months; Fig 1A). The strongest predictor of OS was the diagnosis at time of SCT. OS duration was 36 months (95% CI, 24 to 48 months) in patients with CLL (n = 52) and 15 months (95% CI, 2 to 28 months) in patients with RT (n = 20; P < .001; Fig 1B). The 2- and 5-year OS rates were 67% and 38% in patients with CLL and 36% and 0% in patients with RT. Patients with CLL for whom the first post-transplantation regimen failed and who received additional treatment(s) had a relatively good survival expectancy; the post second-line treatment OS in these patients was 21 months (95% CI, 12 to 30 months).
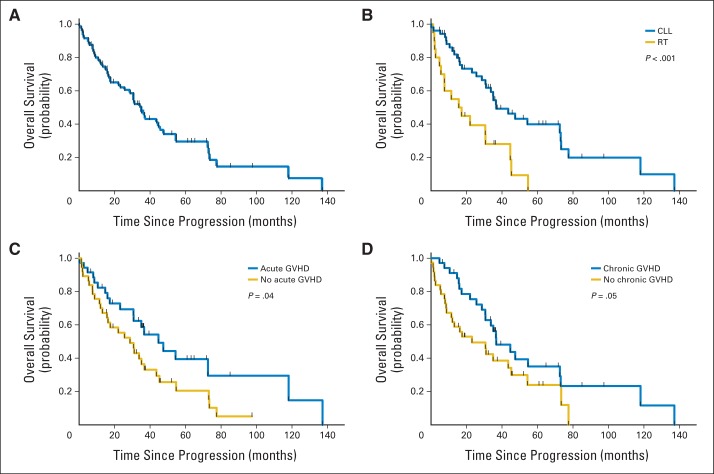
Fig 1.
Survival from time of progression in patients for whom allogeneic stem-cell transplantation failed. (A) The median overall survival (OS) of the entire cohort was 35 months (95% CI, 30 to 40 months). (B) Diagnosis at the time of transplantation predicted survival: OS is shown for patients who had chronic lymphocytic leukemia (CLL; median OS, 36 months; 95% CI, 24 to 48 months) or Richter's transformation (RT) at the time of transplantation (median OS, 15 months; 95% CI, 2 to 28 months). (C) Presence of acute graft-versus-host disease (GVHD) predicted survival: OS is shown for patients who developed acute GVHD after stem-cell transplantation (median OS, 45 months; 95% CI, 28 to 61 months) and those who had no acute GVHD (median OS, 29 months; 95% CI, 17 to 41 months). (D) Presence of chronic GVHD predicted survival: OS is shown for patients who developed chronic GVHD after stem-cell transplantation (median OS, 37 months; 95% CI, 23 to 51 months) and patients who did not develop chronic GVHD (median OS, 23 months; 95% CI, 5 to 41 months).
Patients who had a response to SCT had longer OS than those who did not have a response (P = .003; Fig 1C), and GVL likely contributed to favorable survival outcome as evinced by the significantly longer OS in patients who experienced acute (Fig 1C) or chronic (Fig 1D) GVHD following SCT than in those who did not have GVHD (P = .04 and P = .05, respectively).
Conversely, sensitivity to chemotherapy was not predictive of OS: patients with chemotherapy-sensitive disease before SCT had a similar OS rate to those with refractory disease before SCT. Likewise, a similar survival outcome was observed in patients who received or did not receive DLIs and in patients who did or did not have a response to the first post hematopoietic stem-cell transplantation treatment.
In a multivariable analysis, only low hemoglobin level and a diagnosis of RT at time of SCT were associated with a shorter OS, whereas chronic GVHD and response to the first post-transplantation treatment assessment predicted a longer OS (Table 5).
Table 5.
Prognostic Factor Analysis for Patients With Primary Diagnosis of CLL for Whom Allogeneic SCT Failed
| Prognostic Factor | HR | 95% CI | P |
|---|---|---|---|
| Univariable analysis | |||
| Age ≥ 5 v < 55 years | 1.6 | 0.88 to 3.0 | .12 |
| At time of transplantation | |||
| ECOG performance status | |||
| 0 | 1 | ||
| 1 | 1.6 | 0.78 to 3.43 | .19 |
| 2 to 3 | 4.37 | 1.5 to 12.5 | .006 |
| Hemoglobin | 0.74 | 0.63 to 0.87 | < .001 |
| Albumin | 0.54 | 0.39 to 0.75 | < .001 |
| Bone marrow cellularity | 0.99 | 0.98 to 1.01 | .93 |
| Diagnosis (RT v CLL) | 2.75 | 1.48 to 5.11 | .001 |
| Complex cytogenetics | 0.59 | 0.25 to 1.39 | .23 |
| del(17)/p53 mutated | 0.75 | 0.36 to 1.52 | .42 |
| Conditioning regimen* | 0.71 | 0.37 to 1.38 | .31 |
| After transplantation | |||
| Acute GVHD | 0.55 | 0.30 to 0.99 | .049 |
| Chronic GVHD | 0.57 | 0.32 to 1.01 | .055 |
| Best chimerism response† | 0.54 | 0.28 to 1.01 | .055 |
| Post-transplantation response‡ | 0.40 | 0.22 to 0.75 | .006 |
| Multivariable analysis | |||
| Hemoglobin | 0.76 | 0.64 to 0.90 | .002 |
| RT at time of transplantation | 3.54 | 1.74 to 7.22 | < .001 |
| Response§ | 0.35 | 0.17 to 0.71 | .004 |
| Chronic GVHD | 0.53 | 0.28 to 1.00 | .05 |
Abbreviations: CLL, chronic lymphocytic leukemia; ECOG, Eastern Cooperative Oncology Group; GVHD, graft-versus-host disease; HR, hazard ratio; RT, Richter's transformation; SCT, stem-cell transplantation.
Myeloablative versus nonablative.
Complete donor versus mixed/autologous.
Response versus no response.
At post-transplantation assessment.
DISCUSSION
In CLL, allogeneic SCT is commonly perceived as a last resort, offered to patients after all other options have been exhausted. We report here that the prognosis after SCT has failed for patients with CLL is relatively good, unlike for those with acute leukemia. Similar to all other patients with relapsed/refractory CLL, post-SCT relapsed patients were treated according to our standard treatment criteria. Four of those patients (8%) did not require treatment for a median follow-up of 45 months.
The patients in our study tolerated one to eight lines of post-SCT therapy with a median OS of nearly 3 years from time of documented progression. Although selection of younger patients and patients who were eligible for transplantation may partly account for the unexpectedly prolonged survival in our cohort, in a multivariable analysis, age and performance status were not significantly associated with OS.
Remissions obtained in post-transplantation CLL following DLI are considered evidence for the GVL effect. The alloimmune cells are thought to play a key role in immune surveillance and suppression of the leukemic clone.5,19–21,28–30 Nearly half our patients with active CLL after transplantation had chronic GVHD, and the association of chronic GVHD with achieving cure and its power to predict OS among patients for whom transplantation failed suggests that the GVL effect contributes to prolonged survival even in patients with a high burden of disease.
The relatively long post-transplantation survival rates were largely restricted to patients with CLL rather than RT. Case reports and small case series have suggested that allogeneic SCT might improve treatment outcome in RT.31–33 In a recent study, the cumulative survival rate at 3 years was 75%, and remission after allogeneic SCT correlated independently with prolonged survival.33 In our cohort, the 5-year survival rate in CLL was 36%, but none of the patients with well-documented RT survived 5 years. Our findings indicate that, in RT, like other aggressive lymphomas but not like CLL, the benefit from allogeneic SCT is restricted to patients who achieve a durable response. A recent study of whole-exome sequencing and copy number variation analysis revealed that, in most cases, RT was derived from the CLL clone.34 By using our standard laboratory tests, we did not detect unrelated clones in patients who developed RT after transplantation or in the four patients who had RT before and CLL after BM transplantation.
After ibrutinib became available, questions were raised about the role of SCT and other immunotherapies in patients with relapsed/refractory CLL.35 As a single agent, ibrutinib is well tolerated, induces durable responses, and prolongs progression-free survival and OS in high-risk patients, regardless of adverse cytogenetic abnormalities.36 SCT often results in a durable eradication of minimal residual disease and offers the potential for cure.37 Furthermore, our data suggest that SCT is beneficial, even in patients in whom a durable response is not maintained. In contrast, the rates of CR have been low with ibrutinib, and the resistance acquired by a proportion of patients38 seems to be difficult to overcome.
The variety of salvage treatments administered to our patients limited our ability to compare the efficacies and toxicities of these regimens. Because of the favorable outcomes with ibrutinib in relapsed/refractory CLL, we believe that ibrutinib might have a role in the treatment of disease progression following transplantation failure. In our cohort, ibrutinib was administered after three to five post-transplantation treatments. Of the five patients who received ibrutinib, four responded and are alive after a median follow-up duration of 16 months.
The patients reported here received several lines of therapy that failed, including SCT. In such heavily treated patients the association between response and OS is not entirely clear. In heavily treated patients, therapeutic intervention might not be required unless clearly indicated. Among our patients for whom BM transplantation failed, four patients did not receive post-transplantation treatment. In 37 patients, treatment was administered because of progressive disease or transformation, and in 31 patients treatment was administered because of symptomatic disease. Whether a subset of these patients would do well with a watch-and-wait approach is not clear.
Allogeneic SCT was associated with a significant risk for transformation; 16 patients (30%) with CLL developed RT after transplantation. The opposite effect also occurred: four patients who had RT before transplantation had CLL but no signs of aggressive lymphoma at the post-transplantation work-up. The outcomes of these four patients were similar to those of patients with CLL rather than RT, with a median OS of 31 months. A turn in the course of the disease after transplantation was also observed in seven patients whose disease was refractory to fludarabine before transplantation but responded to regimens that included fludarabine after the transplantation. Taken together, these findings suggest that allogeneic SCT may reset the clock and dramatically change the course of the disease, either through the GVL effect or another mechanism yet to be determined.
In conclusion, whereas in acute leukemia SCT failure is associated with poor outcome, the estimated 5-year survival after SCT failure in CLL was 38%. Patients who developed chronic GVHD had significantly higher OS, suggesting that a donor GVL effect contributes to controlling the disease, even in the absence of overt response.
Acknowledgment
We thank Kathryn Hale from the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for editing the manuscript and Susan Smith for obtaining the clinical data.
Footnotes
See accompanying editorial on page 1527
Supported by a grant from the Chronic Lymphocytic Leukemia Global Research Foundation and by Grant No. CA01667 from the National Institutes of Health through MD Anderson's Cancer Center Support 2.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Michael Keating, Zeev Estrov
Provision of study materials or patients: William G. Wierda, Susan O'Brien, Jan A. Burger, Alessandra Ferrajoli, Stefan Faderl, Michael Keating
Collection and assembly of data: Uri Rozovski, Ohad Benjamini, Preetesh Jain, Philip A. Thompson, William G. Wierda, Susan O'Brien, Jan A. Burger, Alessandra Ferrajoli, Stefan Faderl, Elizabeth Shpall, Chitra Hosing, Issa F. Khouri, Richard Champlin
Data analysis and interpretation: Uri Rozovski
Manuscript writing: Uri Rozovski, Zeev Estrov
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Outcomes of Patients With Chronic Lymphocytic Leukemia and Richter's Transformation After Transplantation Failure
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Uri Rozovski
No relationship to disclose
Ohad Benjamini
No relationship to disclose
Preetesh Jain
No relationship to disclose
Philip A. Thompson
No relationship to disclose
William G. Wierda
No relationship to disclose
Susan O'Brien
No relationship to disclose
Jan A. Burger
Consulting or Advisory Role: Pharmacyclics, Gilead Sciences, Janssen Pharmaceuticals
Research Funding: Pharmacyclics, Gilead Sciences, Noxxon Pharma
Alessandra Ferrajoli
No relationship to disclose
Stefan Faderl
No relationship to disclose
Elizabeth Shpall
No relationship to disclose
Chitra Hosing
Honoraria: Sanofi
Research Funding: Celgene (Inst)
Travel, Accommodations, Expenses: Kyowa Hakko Kirrin
Issa F. Khouri
No relationship to disclose
Richard Champlin
No relationship to disclose
Michael J. Keating
No relationship to disclose
Zeev Estrov
No relationship to disclose
REFERENCES
- 1.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 2.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 3.Montserrat E, Moreno C, Esteve J, et al. How I treat refractory CLL. Blood. 2006;107:1276–1283. doi: 10.1182/blood-2005-02-0819. [DOI] [PubMed] [Google Scholar]
- 4.Dreger P European Group for Blood and Marrow Transplantation (EBMT) The evolving role of stem cell transplantation in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2013;27:355–369. doi: 10.1016/j.hoc.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Gribben JG, Zahrieh D, Stephans K, et al. Autologous and allogeneic stem cell transplantations for poor-risk chronic lymphocytic leukemia. Blood. 2005;106:4389–4396. doi: 10.1182/blood-2005-05-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khouri IF, Keating MJ, Saliba RM, et al. Long-term follow-up of patients with CLL treated with allogeneic hematopoietic transplantation. Cytotherapy. 2002;4:217–221. doi: 10.1080/146532402320219736. [DOI] [PubMed] [Google Scholar]
- 7.Khouri IF, Keating MJ, Vriesendorp HM, et al. Autologous and allogeneic bone marrow transplantation for chronic lymphocytic leukemia: Preliminary results. J Clin Oncol. 1994;12:748–758. doi: 10.1200/JCO.1994.12.4.748. [DOI] [PubMed] [Google Scholar]
- 8.Khouri IF, Przepiorka D, van Besien K, et al. Allogeneic blood or marrow transplantation for chronic lymphocytic leukaemia: Timing of transplantation and potential effect of fludarabine on acute graft-versus-host disease. Br J Haematol. 1997;97:466–473. doi: 10.1046/j.1365-2141.1997.272673.x. [DOI] [PubMed] [Google Scholar]
- 9.Malhotra P, Hogan WJ, Litzow MR, et al. Long-term outcome of allogeneic stem cell transplantation in chronic lymphocytic leukemia: Analysis after a minimum follow-up of 5 years. Leuk Lymphoma. 2008;49:1724–1730. doi: 10.1080/10428190802263535. [DOI] [PubMed] [Google Scholar]
- 10.Rabinowe SN, Soiffer RJ, Gribben JG, et al. Autologous and allogeneic bone marrow transplantation for poor prognosis patients with B-cell chronic lymphocytic leukemia. Blood. 1993;82:1366–1376. [PubMed] [Google Scholar]
- 11.Dreger P, Brand R, Milligan D, et al. Reduced-intensity conditioning lowers treatment-related mortality of allogeneic stem cell transplantation for chronic lymphocytic leukemia: A population-matched analysis. Leukemia. 2005;19:1029–1033. doi: 10.1038/sj.leu.2403745. [DOI] [PubMed] [Google Scholar]
- 12.Sorror ML, Storer BE, Sandmaier BM, et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol. 2008;26:4912–4920. doi: 10.1200/JCO.2007.15.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown JR, Kim HT, Armand P, et al. Long-term follow-up of reduced-intensity allogeneic stem cell transplantation for chronic lymphocytic leukemia: Prognostic model to predict outcome. Leukemia. 2013;27:362–369. doi: 10.1038/leu.2012.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreger P, Döhner H, Ritgen M, et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: Long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood. 2010;116:2438–2447. doi: 10.1182/blood-2010-03-275420. [DOI] [PubMed] [Google Scholar]
- 15.Khouri IF, Bassett R, Poindexter N, et al. Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia: Long-term follow-up, prognostic factors, and effect of human leukocyte histocompatibility antigen subtype on outcome. Cancer. 2011;117:4679–4688. doi: 10.1002/cncr.26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorror ML, Storer BE, Maloney DG, et al. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111:446–452. doi: 10.1182/blood-2007-07-098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schetelig J, van Biezen A, Brand R, et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: A retrospective European Group for Blood and Marrow Transplantation analysis. J Clin Oncol. 2008;26:5094–5100. doi: 10.1200/JCO.2008.16.2982. [DOI] [PubMed] [Google Scholar]
- 18.Garnier A, Robin M, Larosa F, et al. Allogeneic hematopoietic stem cell transplantation allows long-term complete remission and curability in high-risk Waldenström's macroglobulinemia: Results of a retrospective analysis of the Société Française de Greffe de Moelle et de Thérapie Cellulaire. Haematologica. 2010;95:950–955. doi: 10.3324/haematol.2009.017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritgen M, Stilgenbauer S, von Neuhoff N, et al. Graft-versus-leukemia activity may overcome therapeutic resistance of chronic lymphocytic leukemia with unmutated immunoglobulin variable heavy-chain gene status: Implications of minimal residual disease measurement with quantitative PCR. Blood. 2004;104:2600–2602. doi: 10.1182/blood-2003-12-4321. [DOI] [PubMed] [Google Scholar]
- 20.Rondón G, Giralt S, Huh Y, et al. Graft-versus-leukemia effect after allogeneic bone marrow transplantation for chronic lymphocytic leukemia. Bone Marrow Transplant. 1996;18:669–672. [PubMed] [Google Scholar]
- 21.Khouri IF, Lee MS, Saliba RM, et al. Nonablative allogeneic stem cell transplantation for chronic lymphocytic leukemia: Impact of rituximab on immunomodulation and survival. Exp Hematol. 2004;32:28–35. doi: 10.1016/j.exphem.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 23.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khouri IF, Keating M, Körbling M, et al. Transplant-lite: Induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998;16:2817–2824. doi: 10.1200/JCO.1998.16.8.2817. [DOI] [PubMed] [Google Scholar]
- 25.Khouri IF, Saliba RM, Admirand J, et al. Graft-versus-leukaemia effect after non-myeloablative haematopoietic transplantation can overcome the unfavourable expression of ZAP-70 in refractory chronic lymphocytic leukaemia. Br J Haematol. 2007;137:355–363. doi: 10.1111/j.1365-2141.2007.06591.x. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Manero G, Kantarjian HM. The hyper-CVAD regimen in adult acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2000;14:1381–1396. doi: 10.1016/s0889-8588(05)70192-1. [DOI] [PubMed] [Google Scholar]
- 27.Tsimberidou AM, Wierda WG, Plunkett W, et al. Phase I-II study of oxaliplatin, fludarabine, cytarabine, and rituximab combination therapy in patients with Richter's syndrome or fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2008;26:196–203. doi: 10.1200/JCO.2007.11.8513. [DOI] [PubMed] [Google Scholar]
- 28.Marks DI, Lush R, Cavenagh J, et al. The toxicity and efficacy of donor lymphocyte infusions given after reduced-intensity conditioning allogeneic stem cell transplantation. Blood. 2002;100:3108–3114. doi: 10.1182/blood-2002-02-0506. [DOI] [PubMed] [Google Scholar]
- 29.Mattsson J, Uzunel M, Remberger M, et al. Minimal residual disease is common after allogeneic stem cell transplantation in patients with B cell chronic lymphocytic leukemia and may be controlled by graft-versus-host disease. Leukemia. 2000;14:247–254. doi: 10.1038/sj.leu.2401669. [DOI] [PubMed] [Google Scholar]
- 30.Moreno C, Villamor N, Colomer D, et al. Clinical significance of minimal residual disease, as assessed by different techniques, after stem cell transplantation for chronic lymphocytic leukemia. Blood. 2006;107:4563–4569. doi: 10.1182/blood-2005-09-3634. [DOI] [PubMed] [Google Scholar]
- 31.Cwynarski K, van Biezen A, de Wreede L, et al. Autologous and allogeneic stem-cell transplantation for transformed chronic lymphocytic leukemia (Richter's syndrome): A retrospective analysis from the chronic lymphocytic leukemia subcommittee of the chronic leukemia working party and lymphoma working party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2012;30:2211–2217. doi: 10.1200/JCO.2011.37.4108. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez J, Keating MJ, O'Brien S, et al. Allogeneic haematopoietic transplantation for Richter's syndrome. Br J Haematol. 2000;110:897–899. doi: 10.1046/j.1365-2141.2000.02295.x. [DOI] [PubMed] [Google Scholar]
- 33.Tsimberidou AM, O'Brien S, Khouri I, et al. Clinical outcomes and prognostic factors in patients with Richter's syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J Clin Oncol. 2006;24:2343–2351. doi: 10.1200/JCO.2005.05.0187. [DOI] [PubMed] [Google Scholar]
- 34.Fabbri G, Khiabanian H, Holmes AB, et al. Genetic lesions associated with chronic lymphocytic leukemia transformation to Richter syndrome. J Exp Med. 2013;210:2273–2288. doi: 10.1084/jem.20131448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kharfan-Dabaja MA, Wierda WG, Cooper LJ. Immunotherapy for chronic lymphocytic leukemia in the era of BTK inhibitors. Leukemia. 2014;28:507–517. doi: 10.1038/leu.2013.311. [DOI] [PubMed] [Google Scholar]
- 36.Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dreger P, Schnaiter A, Zenz T, et al. TP53, SF3B1, and NOTCH1 mutations and outcome of allotransplantation for chronic lymphocytic leukemia: Six-year follow-up of the GCLLSG CLL3X trial. Blood. 2013;121:3284–3288. doi: 10.1182/blood-2012-11-469627. [DOI] [PubMed] [Google Scholar]
- 38.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370:2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]