Abstract
Purpose
To evaluate preferences for and experiences with genetic testing in a diverse cohort of patients with breast cancer identified through population-based registries, with attention to differences by race/ethnicity.
Methods
We surveyed women diagnosed with nonmetastatic breast cancer from 2005 to 2007, as reported to the SEER registries of metropolitan Los Angeles and Detroit, about experiences with hereditary risk evaluation. Multivariable models evaluated correlates of a strong desire for genetic testing, unmet need for discussion with a health care professional, and receipt of testing.
Results
Among 1,536 patients who completed the survey, 35% expressed strong desire for genetic testing, 28% reported discussing testing with a health care professional, and 19% reported test receipt. Strong desire for testing was more common in younger women, Latinas, and those with family history. Minority patients were significantly more likely to have unmet need for discussion (failure to discuss genetic testing with a health professional when they had a strong desire for testing): odds ratios of 1.68, 2.44, and 7.39 for blacks, English-speaking Latinas, and Spanish-speaking Latinas compared with whites, respectively. Worry in the long-term survivorship period was higher among those with unmet need for discussion (48.7% v 24.9%; P <.001). Patients who received genetic testing were younger, less likely to be black, and more likely to have a family cancer history.
Conclusion
Many patients, especially minorities, express a strong desire for genetic testing and may benefit from discussion to clarify risks. Clinicians should discuss genetic risk even with patients they perceive to be at low risk, as this may reduce worry.
INTRODUCTION
A diagnosis of breast cancer triggers a cascade of increasingly complicated decisions about treatment options. An important consideration for some patients making decisions about the initial course of locoregional treatment is the potential risk of a second primary cancer. Approximately 5% to 10% of patients with breast cancer have germline mutations that predispose them to developing additional cancers,1 and this risk may extend to other relatives who carry the same mutation. BRCA1/2 mutations have been identified across all racial/ethnic subgroups.2
Discovery of a genetic mutation has important implications for a patient's treatment decision making in the context of a new breast cancer diagnosis.3 Patients with high inherited risk of new primary cancers may be more inclined toward mastectomy and contralateral risk-reducing mastectomy; those with BRCA1/2 mutations may also consider risk-reducing salpingo-oophorectomy; those with Li-Fraumeni syndrome should avoid approaches that incorporate radiotherapy.4 Implications of genetic mutations also extend to the survivorship period, when high-risk women who did not choose bilateral mastectomy may benefit from additional measures, such as MRI surveillance. Identification of a familial mutation also affects relatives, who may or may not wish to know this information, test for the mutation themselves, and consider risk-reducing interventions.5
The rapidly expanding scope and availability of genetic testing for cancer risk6 motivates research to examine patients' perspectives and experiences with testing. Most studies to date have focused on general or high-risk populations and have raised concerns about racial/ethnic disparities in knowledge and access.7 The few studies that included cancer patients have been limited by smaller convenience samples, low participation rates, and few racial/ethnic minority patients. These studies have yielded conflicting results regarding whether patients with a personal history of cancer are more likely than others to desire or receive testing. In a 2002 survey of women with early-onset breast cancer who were identified through two breast cancer support groups, among the 21% completing questionnaires, 83% were aware of BRCA testing, and 12.5% had received testing.8 More generalizable research is needed to understand which women diagnosed with breast cancer in the community desire and receive genetic testing.
Given gaps in knowledge about desire for and receipt of testing in patients with breast cancer, particularly racial/ethnic minorities and those not at high risk, we considered a diverse cohort of breast cancer survivors identified through population-based registries to examine patients' self-reported desire to receive genetic testing, whether they had unmet need for discussion (failed to discuss genetic testing with a health professional when they had a strong desire for testing), and whether they had received testing. We described reasons for test receipt and nonreceipt and explored associations between unmet need for discussion and worry about breast cancer during survivorship. Finally, we evaluated the correlates of desire for genetic testing, unmet need for discussion, and test receipt.
METHODS
Study Sample
Our study sample originated from a longitudinal cohort study of women diagnosed with breast cancer in metropolitan Los Angeles and Detroit. We included patients age 20 to 79 years who were diagnosed with stage 0-III breast cancer between June 2005 and February 2007, as reported to the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) population-based program registries in those regions.
We excluded patients with stage IV breast cancer and those who could not complete a questionnaire in English or Spanish. Asian women in Los Angeles were excluded because of enrollment in other studies. Latina (in Los Angeles) and black (in both Los Angeles and Detroit) patients were oversampled to ensure sufficient minority representation.
Questionnaire Design and Content
Questionnaires (Data Supplement) were based on existing literature, measures previously developed to assess relevant constructs, and theoretical models. We utilized standard techniques of content validation,9 including systematic review by design experts10–12 and pretesting with 40 patients in three waves, including 12 detailed cognitive interviews.13,14
Data Collection
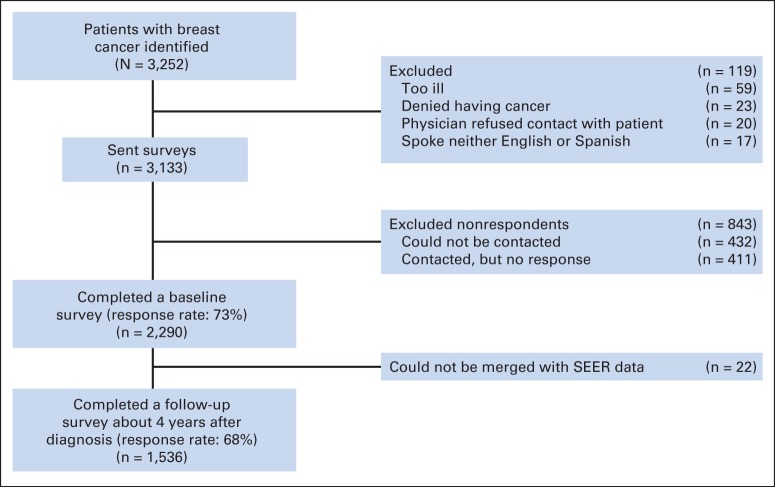
After IRB approval, patients were identified via rapid case ascertainment and surveyed a mean of 9 months after diagnosis (mean, 288 days; SD, 100), and again approximately four years later (mean, 1,524 days; SD, 143). To encourage response, we provided a $10 cash incentive and used a modified Dillman method.15 All materials were sent in English and Spanish to those with Spanish surnames.16 The response rate to the baseline survey was 73%, and the response rate to the follow-up survey was 68%. Survey responses were combined into a single data set, into which clinical data from SEER were merged. More details regarding the flow of patients into the sample are provided in Appendix Figure A1 (online only).
Measures
SEER records provided clinical stage. In the baseline survey, we measured age, race/ethnicity (white, black, English-speaking Latina, and Spanish-speaking Latina), education (≤ high school v at least some college), insurance status (none, private, Medicare, or Medicaid), and family history of breast and/or ovarian cancer (in none v ≥ 1 first-degree relatives). We measured worry about implications for family in the baseline survey, by asking how true it was that the respondent had “worry that other members of my family might someday get the same illness I have” in the past 7 days.
We introduced the concept of genetic testing for cancer risk in the follow-up survey by describing tests that “look for gene mutations or changes, to see if women and their families have a greater risk of developing breast cancer in the future.” We evaluated desire for testing by asking “How much did you want to have a genetic test for breast cancer risk?” We dichotomized responses for analysis (defining a “strong desire” as responses of “quite a bit” or “very much” rather than “somewhat,” “a little bit,” or “not at all”). We evaluated whether the patient had discussed testing with a health care professional through an item that inquired: “Did a genetic counselor, doctor, or other health professional talk with you about having a genetic test for breast cancer risk?” We further defined “unmet need for discussion” by using responses from these two items to define a subset of patients who expressed strong desire for testing but denied discussion with a health care professional. Finally, we evaluated test receipt with an item that inquired: “Have you ever had a genetic test for breast cancer risk?”
Patients who indicated they did receive the test were asked to check all that applied from among a list of reasons for desiring testing (“my doctor thought I should get tested,” “I wanted more information about my own health,” “I wanted more information for my family members,” “my family wanted me to be tested,” and “other”). Those who indicated they did not were asked reasons for not receiving testing (from among “my doctor didn't recommend it,” “I didn't want it,” “my family didn't want me to get it,” “it was too expensive,” and “other”).
We measured worry about breast cancer in the follow-up survey by asking how much the respondent worried about breast cancer coming back in the same breast, occurring in the other breast, or spreading to other parts of her body. Responses to the worry items were dichotomized for analysis as “very much” or “quite a bit” versus “somewhat,” “a little bit,” or “not at all.”
Analytic Approach
To allow statistical inferences to represent the original targeted population, we applied complex survey weights to the calculation of percentages and regression analyses. Design weights compensated for the oversampling of minorities and disproportionate selection across SEER sites; nonresponse weights compensated for the fact that women with certain characteristics were not as likely to respond to the surveys at each time point (Data Supplement). Analyses were conducted using SAS 9·2 (Cary, NC).
We first generated descriptive statistics for the sample, including rates of strong desire for testing, discussion of testing with a health care professional, and receipt of testing. We then evaluated correlates of expressing a strong desire for genetic testing, unmet need for discussion (a strong desire for testing but without a discussion with a health care professional), and genetic testing receipt. In each set of analyses, we first considered the following independent variables on bivariable analyses: age, race/ethnicity (white, black, English-speaking Latina, and Spanish-speaking Latina), disease type (in situ v invasive), education (high school or less v at least some college), insurance status (none, private, or governmental—Medicaid/Medicare), and family history of breast and/or ovarian cancer (in none v one or more first-degree relatives). Best multiple variable models were constructed using a backward elimination strategy, with all covariates first offered to the model, and covariates iteratively removed and the model recalculated, after consideration of the covariate's significance, its possible effect modification on the remaining covariates, and its impact on the overall model fit as computed by Akaike's information criterion for nested models. We also described concerns about impact on family, reasons for receipt and nonreceipt of testing, and evaluated associations between unmet need for discussion and worry about breast cancer during survivorship. Frequencies between groups were compared statistically using the Rao-Scott χ2 test. For all statistic tests, P values ≤ .05 were considered significant.
RESULTS
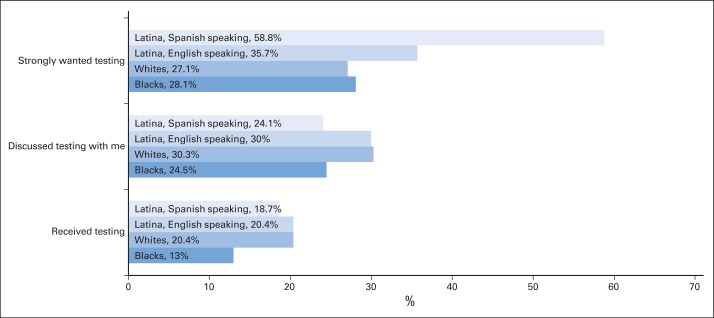
Of 3,133 women surveyed, 2,290 (73%) completed the baseline survey. Of these, 1,536 (68%) completed the follow-up survey and constituted the analytic sample. Table 1 reports the characteristics of these 1,536 patients. The sample was diverse, with 17% black and 39% Latina. A total of 42% had a high school education or less, 52% had stage 0-I disease, and 32% reported a family history of breast or ovarian cancer in a first degree relative. About a third of patients (35%) expressed a strong desire for genetic testing; 28% reported discussing testing with a health care professional; and 19% reported receipt of genetic testing. Figure 1 depicts variations by race/ethnicity in these responses.
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | No. | % | Weighted Mean or Weighted % |
|---|---|---|---|
| SEER site | |||
| Detroit | 694 | 45.2 | |
| Los Angeles | 842 | 54.8 | |
| Age at time of baseline survey, years | |||
| Mean | 57.5 | 57.2 | |
| SD | 11.2 | ||
| Race/ethnicity | |||
| White | 728 | 47.4 | 41.8 |
| Black | 380 | 24.7 | 17.1 |
| Latina, English speaking | 191 | 12.4 | 19.2 |
| Latina, Spanish speaking | 203 | 13.2 | 20.0 |
| Other | 34 | 2.2 | 1.8 |
| Education | |||
| High school or less | 564 | 36.7 | 41.7 |
| At least some college | 945 | 61.5 | 56.1 |
| Missing/unknown | 27 | 1.8 | 2.2 |
| Insurance status | |||
| None | 104 | 6.8 | 9.2 |
| Private | 1054 | 68.6 | 63.6 |
| Medicaid | 120 | 7.8 | 9.6 |
| Medicare | 202 | 13.2 | 13.5 |
| Missing/unknown | 56 | 3.6 | 4.1 |
| Disease stage | |||
| DCIS | 380 | 24.7 | 18.1 |
| 1 | 553 | 36.0 | 34.3 |
| 2 | 425 | 27.7 | 32.7 |
| 3 | 141 | 9.2 | 11.7 |
| Missing/unknown | 37 | 2.4 | 3.2 |
| Family history of breast and/or ovarian cancer | |||
| None | 964 | 62.8 | 62.6 |
| ≥ One first-degree relative | 504 | 32.8 | 32.4 |
| Missing/unknown | 68 | 4.4 | 5.0 |
| Strongly wanted testing | |||
| Yes | 493 | 32.1 | 35.3 |
| No | 966 | 62.9 | 60.1 |
| Missing/unknown | 77 | 5.0 | 4.6 |
| Discussed genetic testing with a genetic counselor, physician, or other health professional? | |||
| Yes | 432 | 28.1 | 27.9 |
| No | 957 | 62.3 | 62.5 |
| I don't know | 136 | 8.9 | 8.8 |
| Missing/unknown | 11 | 0.7 | 0.9 |
| Received genetic testing | |||
| Yes | 269 | 17.5 | 18.6 |
| No | 1118 | 72.8 | 71.9 |
| I don't know | 105 | 6.8 | 7.6 |
| Missing/unknown | 44 | 2.9 | 2.0 |
Abbreviations: DCIS, ductal carcinoma in situ; SD, standard deviation.
Fig 1.
Weighted percentages for wanting, discussing, and receiving genetic testing for breast cancer risk by race/ethnicity in a survey sample of 1,536 women diagnosed with early-stage breast cancer between 2005 and 2007 and reported to the population-based SEER registries of metropolitan Los Angeles and Detroit. Weighting includes both design weights compensating for oversampling of minorities and disproportionate selection across SEER sites, as well as nonresponse weights, as detailed in Methods and the Data Supplement.
Table 2 presents a multivariable model of the correlates of a strong desire for genetic testing. Strong desire for genetic testing was more common in younger women, Spanish-speaking Latinas, and those with a family cancer history. Of note, the strong desire for testing among Latinas was highly consistent with racial/ethnic differences in the expression of worry about implications for family members: 31.1% of blacks, 38.4% of whites, 56.7% of English-speaking Latinas, and 83.1% of Spanish-speaking Latinas (P < .001) reported that they were quite a bit or very worried that other members of the family might get breast cancer in the future.
Table 2.
Characteristics Associated With a Strong Desire for Genetic Testing
| Characteristic | Bivariate Associations |
Best Multivariable Model |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age at diagnosis (+ 1 year)* | 0.95 | 0.93 to 0.96 | < .001 | 0.95 | 0.93 to 0.96 | < .001 |
| Race/ethnicity | < .001 | < .001 | ||||
| White | 1.00 | 1.00 | ||||
| Black | 1.14 | 0.82 to 1.58 | 0.96 | 0.67 to 1.67 | ||
| Latina, English speaking | 1.57 | 1.07 to 2.30 | 1.32 | 0.86 to 2.03 | ||
| Latina, Spanish speaking | 4.32 | 2.95 to 6.35 | 3.64 | 2.34 to 5.66 | ||
| Other | 1.00 | 0.38 to 2.64 | 1.09 | 0.30 to 3.95 | ||
| Disease type | .040 | |||||
| In situ | 1.00 | |||||
| Invasive | 1.38 | 1.02 to 1.87 | ||||
| Family history of breast or ovarian cancer | .003 | < .001 | ||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.57 | 1.17 to 2.11 | 1.73 | 1.26 to 2.39 | ||
| Education | .011 | |||||
| High school or less | 1.00 | |||||
| At least some college | 0.71 | 0.54 to 0.93 | ||||
| Insurance | < .001 | |||||
| None | 2.43 | 1.52 to 3.89 | ||||
| Private | 1.00 | |||||
| Medicaid | 1.40 | 0.88 to 2.23 | ||||
| Medicare | 0.66 | 0.43 to 1.01 | ||||
Abbreviation: OR, odds ratio.
Another way of summarizing the information regarding the association between age and strong desire would be that for a woman 20 years older than another—for example, a 65-year-old versus a 45-year-old woman—the OR for having strong desire would be 0.33 (95% CI, 0.26 to 0.43) in the bivariable analysis and 0.34 (95% CI, 0.25 to 0.46) in the multivariable analysis.
Of the 493 patients who expressed a strong desire for testing, 196 (43.4%) did not have a relevant discussion with a health care professional (“unmet need for discussion”). Table 3 shows that minority patients were more likely to express unmet need for discussion after controlling for other factors (see also Fig 2). Spanish-speaking Latinas were nearly five times more likely to have unmet need for discussion about testing than white non-Latina patients. Worry about local recurrence, contralateral new primary, and/or distant metastases during survivorship was considerably higher among those who had unmet need for discussion: 48.7% of those with unmet need for discussion worried about breast cancer compared with 24.9% of those without unmet need (P < .001).
Table 3.
Characteristics Associated With Unmet Need for Discussion (a strong desire for testing but without a discussion of genetic testing with a medical professional)
| Characteristic | Bivariate Associations |
Best Multivariable Model |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age at diagnosis (+ 1 year) | 0.99 | 0.97 to 1.00 | .075 | |||
| Race/ethnicity | < .001 | < .001 | ||||
| White | 1.00 | 1.00 | ||||
| Black | 1.68 | 1.00 to 2.80 | 1.64 | 0.94 to 2.88 | ||
| Latina, English speaking | 2.44 | 1.41 to 4.23 | 2.59 | 1.44 to 4.63 | ||
| Latina, Spanish speaking | 7.39 | 4.58 to 11.93 | 7.08 | 4.22 to 11.89 | ||
| Other | 3.36 | 1.00 to 11.29 | 4.41 | 1.10 to 17.62 | ||
| Disease type | .686 | |||||
| In situ | 1.08 | 0.73 to 1.61 | ||||
| Invasive | 1.00 | |||||
| Family history of breast or ovarian cancer | .028 | .060 | ||||
| No | 1.60 | 1.05 to 2.42 | 1.57 | 0.98 to 2.50 | ||
| Yes | 1.00 | 1.00 | ||||
| Education | < .001 | |||||
| High school or less | 2.46 | 1.73 to 3.51 | ||||
| At least some college | 1.00 | |||||
| Insurance | < .001 | |||||
| None | 3.76 | 2.25 to 6.29 | ||||
| Private | 1.00 | |||||
| Medicaid | 1.79 | 1.01 to 3.15 | ||||
| Medicare | 1.06 | 0.59 to 1.90 | ||||
Abbreviation: OR, odds ratio.
Fig 2.
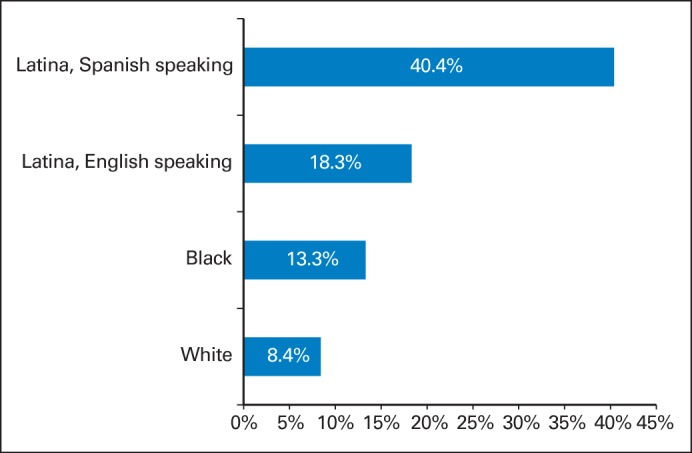
Weighted percentages, by race/ethnicity, of unmet need for discussion (reporting a strong desire for genetic testing but failure to discuss this with a genetics counselor, physician, or other health professional) from a survey sample of 1,536 women diagnosed with early-stage breast cancer between 2005 and 2007 and reported to the population-based SEER registries of metropolitan Los Angeles and Detroit. Weighting includes both design weights compensating for oversampling of minorities and disproportionate selection across SEER sites, as well as nonresponse weights, as detailed in Methods and the Data Supplement.
Table 4 shows the correlates of receipt of genetic testing in this sample. Patients who ultimately received genetic testing were younger, more likely to be white than black, and more likely to have a family history of breast and/or ovarian cancer. Among patients who expressed strong desire for testing, 41.3% had testing. Of the 269 patients who had testing, 20.5% did not express a strong desire for testing. Those who received testing endorsed various reasons (Appendix Fig A2A, online only). These included perceived physician recommendation (65.2%), patients' desire for information relevant to family members (53.6%). Those who did not receiving testing also indicated a variety of reasons (Appendix Fig A2B, online only). These included physician recommendation (64.9%) and personal choice (8.9%). Financial expense was cited as a reason for nonreceipt by 7.0%.
Table 4.
Characteristics Associated With Receipt of Genetic Testing
| Characteristic | Bivariate Associations |
Best Multivariable Model |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age at diagnosis (+ 1 year) | 0.92 | 0.91 to 0.94 | < .001 | 0.91 | 0.89 to 0.93 | < .001 |
| Race/ethnicity | .133 | .012 | ||||
| White | 1.00 | 1.00 | ||||
| Black | 0.67 | 0.43 to 1.03 | 0.45 | 0.28 to 0.73 | ||
| Latina, English speaking | 1.00 | 0.65 to 1.55 | 0.75 | 0.45 to 1.22 | ||
| Latina, Spanish speaking | 1.02 | 0.65 to 1.62 | 0.65 | 0.39 to 1.10 | ||
| Other | 0.33 | 0.10 to 1.06 | 0.30 | 0.07 to 1.19 | ||
| Disease type | .036 | |||||
| In situ | 1.00 | |||||
| Invasive | 1.51 | 1.03 to 2.23 | ||||
| Family history of breast or ovarian cancer | < .001 | < .001 | ||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.75 | 1.94 to 3.90 | 3.11 | 2.14 to 4.53 | ||
| Education | .012 | |||||
| High school or less | 1.00 | |||||
| At least some college | 1.58 | 1.11 to 2.26 | ||||
| Insurance | .023 | |||||
| None | 1.00 | 0.57 to 1.75 | ||||
| Private | 1.00 | |||||
| Medicaid | 1.06 | 0.58 to 1.94 | ||||
| Medicare | 0.40 | 0.23 to 0.73 | ||||
Abbreviation: OR, odds ratio.
DISCUSSION
In this study of diverse patients with breast cancer identified through population-based registries, about one-third strongly desired genetic testing. One in five reported test receipt, which was more common in whites than blacks, as well as those who were younger and had a family cancer history. Minority patients were significantly more likely to have unmet need for discussion in this context, and those with this unmet need were much more likely to express worry about breast cancer as long-term survivors.
Previous studies of desire for and uptake of genetic counseling in cancer-free patients have largely focused on populations of affluent white women, but noteworthy exceptions have identified racial/ethnic differences in attitudes, preferences, and decisions regarding genetic testing.17–24 One study found that African American women with a family history of breast or ovarian cancer were less likely to receive genetic counseling than white women with a similar family history, even after adjustment for socioeconomic status, estimated probability of BRCA1/2 mutation carriage, risk perception and worry, attitudes about testing, and primary care physician discussion.7 Studies of ovarian cancer patients have documented lack of awareness about BRCA1/2 mutation testing, particularly in minorities.25 Our findings suggest marked unmet need for discussion, particularly among Latinas with breast cancer, a group who may have elevated risk of BRCA1 mutation carriage.26 Attention to this disparity is necessary to ensure that all women diagnosed with breast cancer can make informed and preference-concordant decisions.
Previous studies have explored barriers to genetic testing, including affordability and insurance concerns,27 including coverage. In one study, cost was described as very important by 23% of patients in a cancer risk assessment clinic20; numerous others reported that cost appears to influence testing uptake.25,28,29 A survey of patients who experienced cancer genetic counseling identified misperceptions about insurance coverage.30 A relatively small proportion of our respondents endorsed financial concerns as a reason for nontesting, although it is possible that some women who had not discussed testing were unaware of the potential costs.
Of note, not all patients who express a strong desire for testing have significant probability of carrying a genetic mutation,5 and some may misunderstand the distinction between a second primary cancer and a recurrence of the first cancer; simply discussing these issues might alleviate anxiety and not necessarily require testing to follow. Furthermore, studies have documented that patients tend to overestimate risk before genetic counseling.31 Therefore, some patients with unmet need for discussion may harbor anxiety resulting from inaccurate risk perception, which might be resolved by provider discussion when testing is not clinically indicated. Our observation that patients with unmet need for discussion are more likely to express elevated levels of worry during survivorship supports this idea. Given the prevalence of misconceptions about genetic testing that may distort its use,32 the infrequency of relevant discussion in breast cancer decision making that we observed in our 2006 cohort is concerning. These findings are even more relevant today given the exponential growth in news about genetic risk and rapidly increasing access to an expanded array of available genetic tests. Especially in today's climate, our results suggest that clinicians should proactively discuss genetic risk even with patients whom they perceive to be at low risk. Addressing this potentially missed clinical opportunity may alleviate worry and reduce confusion about the risks of subsequent primary cancers versus recurrence of the incident cancer.
Our finding that genetic testing receipt correlated with younger age and family cancer history is reassuring, since these are among the strongest predictors of deleterious mutation carriage. Women diagnosed with breast cancer younger than age 40 years have a 10% frequency of BRCA1/2 mutation carriage even in the absence of family history,33 which substantially exceeds the general population frequency (1 in 400).34–37 Prior studies reported higher uptake of genetic testing among those with first-degree relatives affected by cancer and those diagnosed at younger ages.8,20,28,38,39
Although this study has substantial strengths, including a high response rate and a racially/ethnically diverse sample of patients with breast cancer from population-based registries, it also has limitations. First, it was limited to two geographic areas; women from other areas, particularly rural areas, may have different experiences and concerns. Second, like most studies of this topic, we relied on patient self-report. Although we tried to explain the concept of genetic testing clearly and carefully evaluated our questions with intensive pretesting, it is possible that some respondents misconstrued the questions or remembered experiences inaccurately. Future research should consider complementary methods, such as analysis of taped interactions between providers and patients, to confirm these observations. Third, it is possible that associations observed were not causal. For example, the association between unmet need for discussion and worry might be confounded by an unmeasured variable (such as personality predisposition). Finally, we included only women; men with breast cancer may have different experiences with genetic testing that merit additional exploration in future work.40
In conclusion, we observed significant racial/ethnic variation in experiences with genetic evaluation and discussions of hereditary risk in this diverse sample of patients with breast cancer drawn from two population-based registries. Notably, we found a concerning unmet need for discussion that was more common among minorities. Although clinicians may fear that discussion of genetic risk will amplify the stress of a breast cancer diagnosis,41 many patients, especially minorities, appear likely to benefit from discussion to clarify their true risks. Public awareness of genetic testing has increased rapidly, in relation to recent judicial opinions, celebrity testing disclosures, and direct-to-consumer marketing.42–44 Therefore, it is critical to recognize that patients with breast cancer, even those lacking recognized risk factors for deleterious mutation carriage, may nevertheless desire and benefit from an explicit discussion of genetic risk and its implications for their care.
Testing itself cannot and should not take the place of considered discussions of risk between physician and patient. This study suggests that discussions regarding the actual risk of a hereditary syndrome are critical, particularly in vulnerable populations. Physicians must take care to explain the difference between the risk of new primary cancer and recurrence of the incident cancer. Such discussions are essential to help patients at higher risk to access testing while also helping patients at lower risk to appropriately avoid testing without leaving lingering worry.
Supplementary Material
Acknowledgment
We acknowledge the outstanding work of our project staff: Barbara Salem, MS, MSW, Rebecca Morrison, MPH, and Ashley Gay, BA (University of Michigan); Ain Boone, BA, Cathey Boyer, MSA, and Deborah Wilson, BA (Wayne State University); and Alma Acosta, Mary Lo, MS, Norma Caldera, Marlene Caldera, and Maria Isabel Gaeta (University of Southern California). All of these individuals received compensation for their assistance. We thank the American College of Surgeons Commission on Cancer (David Winchester, MD, and Connie Bura) and the National Cancer Institute Outcomes Branch (Neeraj Arora, PhD, and Steven Clauser, PhD) for their support. We acknowledge with gratitude the patients with breast cancer who responded to our survey.
Glossary Terms
- BRCA1:
a tumor suppressor gene known to play a role in repairing DNA breaks. Mutations in this gene are associated with increased risks of developing breast or ovarian cancer.
- BRCA2:
a tumor suppressor gene whose protein product is involved in repairing chromosomal damage. Although structurally different from BRCA1, BRCA2 has cellular functions similar to BRCA1. BRCA2 binds to RAD51 to fix DNA breaks caused by irradiation and other environmental agents. Also known as the breast cancer 2 early onset gene.
- germline mutation:
an inherited variation in the lineage of germ cells. Germline mutations can be passed on to offspring.
- Surveillance, Epidemiology, and End Results (SEER):
a national cancer registry that collects information from all incident malignancies in multiple geographic areas of the United States.
Appendix
Fig A1.
Patient flow into the study. This figure depicts the flow of patients into the study from those initially identified to the final analytic sample.
Fig A2.
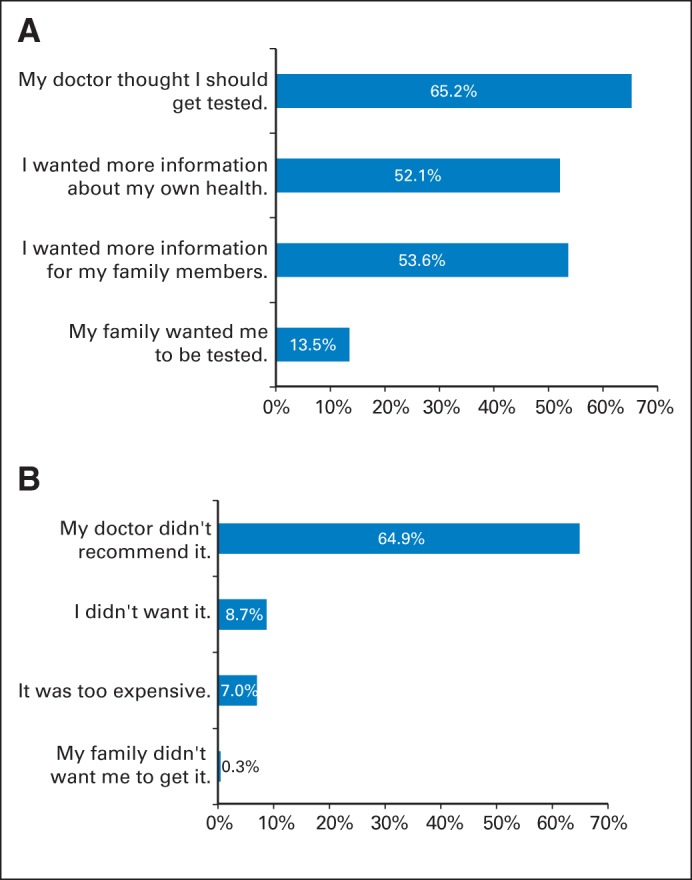
The data in this figure derive from responses of a survey sample of 1,536 women diagnosed with early-stage breast cancer between 2005 and 2007 and reported to the population-based SEER registries of metropolitan Los Angeles and Detroit. (A) Weighted percentages for reasons for getting tested among the population who received testing (n = 269). (B) Weighted percentages for reasons for not getting tested among population who did not receive testing (n = 1,118). Weighting includes both design weights compensating for oversampling of minorities and disproportionate selection across SEER sites, as well as nonresponse weights, as detailed in Methods and the Data Supplement.
Listen to the podcast by Dr Axilbund at www.jco.org.podcasts
Support information appears at the end of this article.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Support
Supported by Grants No. R01 CA109696 and R01 CA088370 from the National Cancer Institute (NCI) to the University of Michigan. R.J. was supported by Mentored Research Scholar Grant No. MRSG-09-145-01 from the American Cancer Society. S.J.K. was supported by an Established Investigator Award in Cancer Prevention, Control, Behavioral, and Population Sciences Research from the NCI (K05CA111340). The collection of Los Angeles County cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885, by the NCI SEER program under Contract No. N01-PC-35139 awarded to the University of Southern California and Contract No. N01-PC-54404 awarded to the Public Health Institute, and by the Centers for Disease Control and Prevention National Program of Cancer Registries under Agreement No. 1U58DP00807-01 awarded to the Public Health Institute. The collection of metropolitan Detroit cancer incidence data was supported by NCI SEER program Contract No. N01-PC-35145.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Reshma Jagsi, Monica Morrow, Ann S. Hamilton, John J. Graff, Steven J. Katz, Sarah T. Hawley
Financial support: Sarah T. Hawley
Administrative support: Steven J. Katz
Collection and assembly of data: Kent A. Griffith, Ann S. Hamilton, John J. Graff
Data analysis and interpretation: Reshma Jagsi, Kent A. Griffith, Allison W. Kurian, Monica Morrow, Steven J. Katz, Sarah T. Hawley
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Concerns About Cancer Risk and Experiences With Genetic Testing in a Diverse Population of Patients With Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Reshma Jagsi
Employment: University of Michigan
Honoraria: International Journal of Radiation Oncology Biology Physics
Consulting or Advisory Role: Eviti
Research Funding: AbbVie (Inst)
Kent A. Griffith
No relationship to disclose
Allison W. Kurian
Research Funding: Myriad Genetics, Invitae
Monica Morrow
No relationship to disclose
Ann S. Hamilton
No relationship to disclose
John J. Graff
No relationship to disclose
Steven J. Katz
No relationship to disclose
Sarah T. Hawley
No relationship to disclose
REFERENCES
- 1.Ford D, Easton D. The genetics of breast and ovarian cancer. Br J Cancer. 1995 Oct;72:805–812. doi: 10.1038/bjc.1995.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72–78. doi: 10.1097/GCO.0b013e328332dca3. [DOI] [PubMed] [Google Scholar]
- 3.Wevers MR, Hahn DE, Verhoef S, et al. Breast cancer genetic counseling after diagnosis but before treatment: a pilot study on treatment consequences and psychological impact. Patient Educ Couns. 2012;89:89–95. doi: 10.1016/j.pec.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Schneider K, Zelley K, Nichols K, et al. Li-Fraumeni Syndrome. In: Pagon R, editor. GeneReviews. Seattle, WA: University of Washington, Seattle; 1999. [PubMed] [Google Scholar]
- 5.Daly MB, Pilarski R, Axilbund JE, et al. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw. 2014;12:1326–1338. doi: 10.6004/jnccn.2014.0127. [DOI] [PubMed] [Google Scholar]
- 6.Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32:2001–2009. doi: 10.1200/JCO.2013.53.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong K, Micco E, Carney A, et al. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293:1729–1736. doi: 10.1001/jama.293.14.1729. [DOI] [PubMed] [Google Scholar]
- 8.Peters N, Domchek SM, Rose A, et al. Knowledge, attitudes, and utilization of BRCA 1/2 testing among women with early-onset breast cancer. Genet Test. 2005;9:48–53. doi: 10.1089/gte.2005.9.48. [DOI] [PubMed] [Google Scholar]
- 9.Fowler FJ. Improving Survey Questions: Design and Evaluation (Applied Social Research Methods) Thousand Oaks, CA: SAGE Publications; 1995. [Google Scholar]
- 10.Katz SJ, Lantz PM, Paredes Y, et al. Breast cancer treatment experiences of Latinas in Los Angeles county. Am J Public Health. 2005;95:2225–2230. doi: 10.2105/AJPH.2004.057950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawley ST, Griggs JJ, Hamilton AS, et al. Decision involvement and receipt of mastectomy among racially and ethnically diverse patients with breast cancer. J Natl Cancer Inst. 2009;101:1337–1347. doi: 10.1093/jnci/djp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zikmund-Fisher BJ, Smith DM, Ubel PA, et al. Validation of the subjective numeracy scale: Effects of low numeracy on comprehension of risk communications and utility elicitations. Med Decis Making. 2007;27:663–671. doi: 10.1177/0272989X07303824. [DOI] [PubMed] [Google Scholar]
- 13.Willis GB. Cognitive Interviewing: A Tool for Improving Questionnaire Design. Thousand Oaks, CA: Sage Publications, Inc; 2005. [Google Scholar]
- 14.Mauceri S. Cognitive interviewing: A tool for improving questionnaire design. Int J Soc Res Methodol. 2008;11:79–83. [Google Scholar]
- 15.Dillman DA, Smyth JD, Christian LM. Internet, Mail, and Mixed-Mode Surveys: The Tailored Design Method. ed 3. Hoboken, NJ: John Wiley & Sons; 2009. [Google Scholar]
- 16.Hamilton AS, Hofer TP, Hawley ST, et al. Latinas and breast cancer outcomes: Population based sampling, ethnic identity, and acculturation assessment. Cancer Epidemiol Biomarkers Prev. 2009;18:2022–2029. doi: 10.1158/1055-9965.EPI-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes C, Gomez-Caminero A, Benkendorf J, et al. Ethnic differences in knowledge and attitudes about BRCA1 testing in women at increased risk. Patient Educ Couns. 1997;32:51–62. doi: 10.1016/s0738-3991(97)00064-5. [DOI] [PubMed] [Google Scholar]
- 18.Mogilner A, Otten M, Cunningham J, et al. Awareness and attitudes concerning BRCA gene testing. Ann Surg Oncol. 1998;5:608–612. doi: 10.1007/BF02303830. [DOI] [PubMed] [Google Scholar]
- 19.Lerman C, Hughes C, Benkendorf JL, et al. Racial differences in testing motivation and psychological distress following pretest education for BRCA1 gene testing. Cancer Epidemiol Biomarkers Prev. 1999;8:361–367. [PubMed] [Google Scholar]
- 20.Armstrong K, Calzone K, Stopfer J, et al. Factors associated with decisions about clinical BRCA1/2 testing. Cancer Epidemiol Biomarkers Prev. 2000;9:1251–1254. [PubMed] [Google Scholar]
- 21.Armstrong K, Weber B, Stopfer J, et al. Early use of clinical BRCA1/2 testing: Associations with race and breast cancer risk. Am J Surg Genet A. 2003;117A:154–160. doi: 10.1002/ajmg.a.10928. [DOI] [PubMed] [Google Scholar]
- 22.Meiser B, Butow P, Barratt A, et al. Attitudes to genetic testing for breast cancer susceptibility in women at increased risk of developing hereditary breast cancer. J Med Genet. 2000;37:472–476. doi: 10.1136/jmg.37.6.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinney A, Croyle RT, Dudley WN, et al. Knowledge, attitudes, and interest in breast-ovarian cancer gene testing: A survey of a large African-American kindred with a BRCA1 mutation. Prev Med. 2001;33:543–551. doi: 10.1006/pmed.2001.0920. [DOI] [PubMed] [Google Scholar]
- 24.Peters N, Rose A, Armstrong K. The association between race and attitudes about predictive genetic testing. Cancer Epidemiol Biomarkers Prev. 2004;13:361–365. [PubMed] [Google Scholar]
- 25.Lacour RA, Daniels MS, Westin SN, et al. What women with ovarian cancer think and know about genetic testing. Gynecol Oncol. 2008;111:132–136. doi: 10.1016/j.ygyno.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John EM, Miron A, Gong G, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 U.S. racial/ethnic groups. JAMA. 2007;298:2869–2876. doi: 10.1001/jama.298.24.2869. [DOI] [PubMed] [Google Scholar]
- 27.Peterson EA, Milliron KJ, Lewis KE, et al. Health insurance and discrimination concerns and BRCA1/2 testing in a clinic population. Cancer Epidemiol Biomarkers Prev. 2002;11:79–87. [PubMed] [Google Scholar]
- 28.Chaliki H, Loader S, Levenkron JC, et al. Women's receptivity to testing for a genetic susceptibility to breast cancer. Am J Public Health. 1995;85:1133–1135. doi: 10.2105/ajph.85.8_pt_1.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SC, Bernhardt BA, Helzlsouer KJ. Utilization of BRCA1/2 genetic testing in the clinical setting. Cancer. 2002;94:1876–1885. doi: 10.1002/cncr.10420. [DOI] [PubMed] [Google Scholar]
- 30.Kausmeyer DT, Lengerich EJ, Kluhsman BC, et al. A survey of patients' experiences with the cancer genetic counseling process: Recommendations for cancer genetics programs. J Genet Couns. 2006;15:409–431. doi: 10.1007/s10897-006-9039-2. [DOI] [PubMed] [Google Scholar]
- 31.Rantala J, Platten U, Lindgren G, et al. Risk perception after genetic counseling in patients with increased risk of cancer. Hered Cancer Clin Pract. 2009;7:15. doi: 10.1186/1897-4287-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose AL, Peters N, Shea JA, et al. Attitudes and misconceptions about predictive genetic testing for cancer risk. Community Genet. 2005;8:145–151. doi: 10.1159/000086757. [DOI] [PubMed] [Google Scholar]
- 33.Domchek SM, Eisen A, Calzone K, et al. Application of breast cancer risk prediction models in clinical practice. J Clin Oncol. 2003;21:593–601. doi: 10.1200/JCO.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Risch HA, McLaughlin JR, Cole DE, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: A kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694–1706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 35.Couch FJ, DeShano ML, Blackwood MA, et al. BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. N Engl J Med. 1997;336:1409–1415. doi: 10.1056/NEJM199705153362002. [DOI] [PubMed] [Google Scholar]
- 36.Malone KE, Daling JR, Thompson JD, et al. BRCA1 mutations and breast cancer in the general population: Analyses in women before age 35 years and in women before age 45 years with first-degree family history. JAMA. 1998;279:922–929. doi: 10.1001/jama.279.12.922. [DOI] [PubMed] [Google Scholar]
- 37.Whittemore AS, Gong G, John EM, et al. Prevalence of BRCA1 mutation carriers among U.S. non-Hispanic Whites. Cancer Epidemiol Biomarkers Prev. 2004;13:2078–2083. [PubMed] [Google Scholar]
- 38.Lerman C, Seay J, Balshem A, et al. Interest in genetic testing among first-degree relatives of patients with breast cancer. Am J Med Genet. 1995;57:385–392. doi: 10.1002/ajmg.1320570304. [DOI] [PubMed] [Google Scholar]
- 39.Lodder L, Frets PG, Trijsburg RW, et al. Attitudes and distress levels in women at risk to carry a BRCA1/BRCA2 gene mutation who decline genetic testing. Am J Med Genet. 2003;119A:266–272. doi: 10.1002/ajmg.a.10168. [DOI] [PubMed] [Google Scholar]
- 40.Hallowell N, Ardern-Jones A, Eeles R, et al. Men's decision-making about predictive BRCA1/2 testing: The role of family. J Genet Couns. 2005;14:207–217. doi: 10.1007/s10897-005-0384-3. [DOI] [PubMed] [Google Scholar]
- 41.Ardern-Jones A, Kenen R, Eeles R. Too much, too soon? Patients and health professionals' views concerning the impact of genetic testing at the time of breast cancer diagnosis in women under the age of 40. Eur J Cancer Care (Engl) 2005;14:272–281. doi: 10.1111/j.1365-2354.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- 42.Offit K, Bradbury A, Storm C, et al. Gene patents and personalized cancer care: Impact of the myriad case on clinical oncology. J Clin Oncol. 2013;31:2743–2748. doi: 10.1200/JCO.2013.49.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borzekowski DL, Guan Y, Smith KC, et al. The Angelina effect: Immediate reach, grasp, and impact of going public. Genet Med. 2014;16:516–521. doi: 10.1038/gim.2013.181. [DOI] [PubMed] [Google Scholar]
- 44.Mouchawar J, Hensley-Alford S, Laurion S, et al. Impact of direct-to-consumer advertising for hereditary breast cancer testing on genetic services at a managed care organization: A naturally-occurring experiment. Genet Med. 2005;7:191–197. doi: 10.1097/01.gim.0000156526.16967.7a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.