Abstract
Purpose
This study was undertaken to determine the magnitude of pulmonary dysfunction in childhood cancer survivors when compared with healthy controls and the extent (and predictors) of decline over time.
Patients and Methods
Survivors underwent baseline (t1) pulmonary function tests, followed by a second comprehensive evaluation (t2) after a median of 5 years (range, 1.0 to 10.3 years). Survivors were also compared with age- and sex-matched healthy controls at t2.
Results
Median age at cancer diagnosis was 16.5 years (range, 0.2 to 21.9 years), and time from diagnosis to t2 was 17.1 years (range, 6.3 to 40.1 years). Compared with odds for healthy controls, the odds of restrictive defects were increased 6.5-fold (odds ratio [OR], 6.5; 95% CI, 1.5 to 28.4; P < .01), and the odds of diffusion abnormalities were increased 5.2-fold (OR, 5.2; 95% CI, 1.8 to 15.5; P < .01). Among survivors, age younger than 16 years at diagnosis (OR, 3.0; 95% CI, 1.2 to 7.8; P = .02) and exposure to more than 20 Gy chest radiation (OR, 5.6; 95% CI, 1.5 to 21.0; P = .02, referent, no chest radiation) were associated with restrictive defects. Female sex (OR, 3.9; 95% CI, 1.7 to 9.5; P < .01) and chest radiation dose (referent: no chest radiation; ≤ 20 Gy: OR, 6.4; 95% CI, 1.7 to 24.4; P < .01; > 20 Gy: OR, 11.3; 95% CI, 2.6 to 49.5; P < .01) were associated with diffusion abnormalities. Among survivors with normal pulmonary function tests at t1, females and survivors treated with more than 20 Gy chest radiation demonstrated decline in diffusion function over time.
Conclusion
Childhood cancer survivors exposed to pulmonary-toxic therapy are significantly more likely to have restrictive and diffusion defects when compared with healthy controls. Diffusion capacity declines with time after exposure to pulmonary-toxic therapy, particularly among females and survivors treated with high-dose chest radiation. These individuals could benefit from subsequent monitoring.
INTRODUCTION
Childhood cancer survivors are at risk for late-occurring pulmonary complications resulting from therapeutic exposures such as lung irradiation, pulmonary-toxic chemotherapy, lung surgery, or as a result of hematopoietic cell transplantation (HCT) –associated chronic graft-versus-host disease (cGVHD).1,2 Five-year survivors have a nearly nine-fold excess risk of dying as a result of pulmonary compromise when compared with age- and sex-matched individuals without a history of cancer3; the cumulative incidence of pulmonary disease increases with time from diagnosis,4,5 suggesting that childhood cancer survivors increasingly face pulmonary morbidity as they age.
The prevalence of pulmonary dysfunction reported in previous cross-sectional studies ranges from 20% to 100%.2 The wide range can be attributed to small, convenience samples and the use of various definitions of pulmonary dysfunction.2,6–8 Prospective studies have focused on the early (< 5 years from diagnosis) period,2 providing us with little information regarding long-term changes in pulmonary function in survivors. Few studies have included age- and sex-matched noncancer controls,9,10 and none have examined the impact of abnormal lung function on health-related quality of life (HRQOL) or the role of blood biomarkers of lung injury11,12 (transforming growth factor beta1 [TGF-β1], platelet-derived growth factor A/B [PDGF-A/B], surfactant proteins A and D[SP-A/D]) in screening for pulmonary dysfunction long after completion of cancer therapy.
The Children's Oncology Group (COG) Long-Term Follow-Up (LTFU) Guidelines1,13 recommend that survivors exposed to pulmonary-toxic therapy undergo a one-time pulmonary function testing (PFT) and symptom assessment at the time of transition into LTFU care, with subsequent testing as clinically indicated. However, a paucity of information regarding the trajectory of change in pulmonary function with time has precluded the development of guidelines regarding the frequency and duration of subsequent screenings. This study addresses these gaps in knowledge by using both cross-sectional and longitudinal study designs to examine long-term pulmonary outcomes in childhood cancer survivors.
PATIENTS AND METHODS
Study participants were recruited from the Childhood Cancer Survivorship Clinic at City of Hope (COH); clinic eligibility criteria included cancer diagnosed at younger than age 22 years and ≥ 2 years since completion of cancer treatment. As previously described,6 all participants in this clinic undergo risk-based screening by using a research protocol that adheres to the recommendations of the COG LTFU Guidelines. Criteria for PFT screening are as follows: (1) previous exposure to pulmonary-toxic chemotherapy (bleomycin, busulfan, nitrosoureas), and/or (2) chest radiation (Data Supplement), and/or (3) history of allogeneic HCT with cGVHD, and/or (4) pulmonary surgery (lobectomy, metastectomy, or wedge resection).
One hundred fifty-five individuals had undergone a baseline (t1) PFT at entry into the COH Survivorship Clinic (median time from diagnosis, 12.2 years; range, 4.3 to 36.5 years). Among the 155 survivors who had undergone baseline (t1) PFTs, two (1.3%) died (one as a result of a second malignant neoplasm and one as a result of relapse), and four (2.6%) had relapsed or developed an second malignant neoplasm but were alive, leaving 149 individuals who were eligible for a second pulmonary function assessment (t2; Appendix Fig A1, online only). Of these 149 survivors, 25 (16.8%) were lost to follow-up, and two (1.3%) refused participation. This article includes results from the 121 survivors (81.2%) who completed both a baseline (t1) and follow-up evaluation (t2) at a median of 5.0 years (range, 1.0 to 10.3 years) from t1.
There were no statistically significant demographic or treatment-related differences between cancer survivors who underwent a PFT at t2 (n = 121) and those who did not (n = 34; Appendix Table A1, online only). Importantly, there were no differences in the prevalence of baseline lung function abnormalities between the two groups.
Healthy controls with no history of cancer or pulmonary disease were recruited at t2 from the general population, frequency-matched on sex and age at participation (Data Supplement). The study was approved by the COH institutional review board. All study participants and/or their parents or legal guardians provided written informed consent.
Pulmonary Function Evaluation
Study participants underwent a detailed physical examination, with special attention to signs and symptoms of pulmonary dysfunction, and completed a modified five-item Medical Research Council Dyspnea Questionnaire14 (Data Supplement). Individuals were considered to have symptomatic pulmonary disease if they answered “yes” to two or more of the Medical Research Council Dyspnea Questionnaire items. PFTs were performed on the day of clinical evaluation according to the American Thoracic Society recommendations15,16 and included total lung capacity (TLC), forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), FEV1/FVC ratio, diffusing capacity of the lungs for carbon monoxide (DLCO), and DLCO/volume of air (DLCO/VA). Percent of predicted normal values were calculated by using established reference values. If DLCO was less than 80% of the predicted value, then DLCO corrected for hemoglobin content (DLCOcorr), age, and sex was calculated.17 All PFTs were interpreted by the study pulmonologist (D.H.) who was blinded to the status of the study participants.
Blood Biomarkers
Blood samples collected on the day of clinical assessment were used to measure TGF-β1 and PDGF-A/B (Luminex xMAP Technology Kit, Millipore, St. Charles, MO; lower limit of detection: TGF-β1, 6.0 pg/mL; PDGF-A, 0.4 pg/mL; PDGF-B, 2.2 pg/mL) and SP-A and SP-D (Surfactant Protein ELISA, MyBioSource, San Diego, CA; range, 0.5 to 60 ng/mL and 7.8 to 500 ng/mL, respectively).
Clinical Data Collection
Demographics and health behaviors.
Self-reported questionnaires completed by survivors and controls were used to obtain data on demographics, insurance, physical activity, smoking history, and history of cardiomyopathy/heart failure.18
HRQOL.
Study participants (survivors and controls) who were age 18 years or older at study participation (94.5% of all participants) completed the Medical Outcomes Study 36-item Short-Form Health Survey (SF-36).
Clinical information.
Medical records (survivors only) provided the date of diagnosis; type of cancer; history of cardiomyopathy and/or heart failure; cGVHD; cumulative exposure to bleomycin, busulfan, and nitrosoureas; and receipt of and total prescribed dose of chest-directed (Data Supplement) radiation and surgery.
Statistical Analysis
Cancer survivors and controls were compared at t2. The primary outcome was pulmonary dysfunction, as defined by moderate-to-severe obstructive lung disease (FEV1/FVC < 0.7 and FEV1 < 80% of predicted [Global Initiative for Chronic Obstructive Lung Disease; GOLD19 criteria]), restrictive lung disease (TLC < 75% and FEV1 ≥ 80% predicted [grade ≥ 2 according to Common Terminology Criteria for Adverse Events v3.0; CTCAE v3.0]20), or diffusion capacity abnormality (DLCOcorr < 75% predicted [grade ≥ 2 per CTCAE v3.0]). The prevalence of obstructive disease, restrictive disease, and diffusion abnormalities among the survivors exposed to pulmonary-toxic therapy was projected to be 3%, 20%, and 35%, respectively8; the expected prevalence in controls was estimated to be 1%, 2%, and 4%, respectively.21–23 Assuming a type I error of 0.017 (accounting for multiple testing), enrolling approximately 120 childhood cancer survivors and 40 healthy controls provided more than 80% power to detect a significant difference in the prevalence of defects between cancer survivors and controls.
Descriptive statistics for pulmonary function indices and blood biomarkers were generated for cancer survivors and controls. Among cancer survivors, pulmonary function indices were compared by therapeutic exposures and clinical characteristics. Categorical variables were compared by using χ2 tests. Mean domain-specific scores were compared by using independent two-sample t tests or analysis of variance. Pearson's correlation was calculated between continuous variables (pulmonary function indices and blood biomarkers).
Predictors of Pulmonary Disease at t2
Dependent variables included restrictive lung disease and diffusion capacity abnormality. The low prevalence of obstructive lung disease precluded further analysis.
Survivors versus controls.
Independent variables assessed were age at examination (< 30/≥ 30 years old), sex, race/ethnicity (non-Hispanic white, Hispanic, other), health insurance at evaluation (yes/no), exercise (< 3/≥ 3 days per week), body mass index at examination (< 25/≥ 25 kg/m2), smoking history (ever, never), and cardiomyopathy and/or heart failure at the time of evaluation (yes/no).
Within childhood cancer survivors analyses.
Additional independent variables assessed included diagnosis (lymphoma, leukemia, solid malignancy), age at cancer diagnosis (< 16/≥ 16 years old), time since diagnosis (< 17/≥ 17 years), bleomycin (none, any), busulfan (none, any), nitrosoureas (none, any), chest radiation (none, ≤ 20 Gy, > 20 Gy), and HCT (none, autologous, allogeneic).
Variables included in the multivariable logistic regression analysis were those associated (P < .2) with the dependent variable in the univariable analysis. Data were analyzed by using SPSS Version 18.0 (IBM, Armonk, NY). All statistical tests were two-sided, and P < .05 was considered statistically significant.
Predictors of Change in Pulmonary Function Over Time (t1 to t2)
Multivariable regression analysis was used to identify variables associated with progressive diffusion abnormality (decline in function between t1 and t2) in survivors with normal function at baseline. The low prevalence of progressive obstructive or restrictive lung disease in individuals with normal function at baseline precluded similar analyses for these outcomes.
RESULTS
Patient Characteristics
Cancer survivors versus controls.
Cancer survivors and controls were comparable with respect to sex, age, body mass index, employment status, self-reported exercise, smoking history, and heart failure (Table 1). Controls were more likely to be non-Hispanic white (51.2% v 37.2%; P < .01) and to have health insurance (93.0% v 71.9%; P < .01).
Table 1.
Patient and Treatment Characteristics
| Characteristic | Survivors (n = 121) |
Controls* (n = 43) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Male sex | 61 | 50.4 | 23 | 53.4 | .73 |
| Age at examination, years | .80 | ||||
| Median | 32.3 | 33.5 | |||
| Range | 14.6-58.9 | 14.8-56.9 | |||
| Race/ethnicity | |||||
| Non-Hispanic white | 45 | 37.2 | 22 | 51.2 | |
| Hispanic | 59 | 48.8 | 9 | 20.9 | |
| Other | 17 | 14.0 | 12 | 27.9 | < .01 |
| Body mass index ≥ 25 at examination | 55 | 45.5 | 19 | 44.2 | .89 |
| Currently employed part-time or full-time | 81 | 68.1 | 35 | 81.4 | .10 |
| Currently insured | 87 | 71.9 | 40 | 93.0 | < .01 |
| Exercise ≥ 3 days per week | 71 | 58.7 | 22 | 51.2 | .39 |
| Minutes per week of exercise | .30 | ||||
| Median | 150 | 120 | |||
| Range | 0-840 | 0-1,260 | |||
| Ever-smoker | 6 | 5.0 | 5 | 11.6 | .13 |
| Cardiomyopathy | 8 | 6.6 | 0 | .09 | |
| Diagnosis | |||||
| Lymphoma | 48 | 39.7 | — | — | |
| Hodgkin | 41 | 33.9 | — | — | |
| Non-Hodgkin | 7 | 5.8 | — | — | |
| Leukemia | 43 | 35.5 | — | — | |
| Acute lymphoblastic | 20 | 16.5 | — | — | |
| Acute myeloid | 17 | 14.0 | — | — | |
| Other | 6 | 5.0 | |||
| Solid malignancy | 30 | 24.8 | — | — | |
| Sarcoma | 13 | 10.7 | — | — | |
| Other | 17 | 14.1 | — | — | |
| Age at diagnosis, years | |||||
| Median | 16.5 | — | — | ||
| Range | 0.2-21.9 | ||||
| Time since diagnosis, years | |||||
| Median | 17.1 | — | — | ||
| Range | 6.3-40.1 | ||||
| Treatment details | |||||
| Cumulative dose of bleomycin, IU/m2 | |||||
| Median | 60 | — | — | ||
| Range | 30-360 | ||||
| Any | 42 | 34.7 | — | — | |
| Cumulative dose of busulfan, mg/m2 | |||||
| Median | 436 | — | — | ||
| Range | 115-1,102 | ||||
| Any | 15 | 12.4 | — | — | |
| Cumulative dose of BCNU or CCNU, mg/m2 | |||||
| Median | 450 | — | — | ||
| Range | 225-987 | ||||
| Any | 12 | 9.9 | — | — | |
| Chest radiation therapy, Gy | |||||
| Median | 13.2 | — | — | ||
| Range | 2-76 | ||||
| None | 32 | 26.4 | — | — | |
| ≤ 20 Gy | 60 | 49.6 | — | — | |
| > 20 Gy | 29 | 24.0 | — | — | |
| Surgery (any) | — | — | |||
| Lobectomy, wedge resection, metastectomy | 7 | 5.8 | — | — | |
| Hematopoietic cell transplantation | |||||
| None | 57 | 47.1 | — | — | |
| Autologous | 20 | 16.5 | — | — | |
| Allogeneic | 44 | 36.4 | — | — | |
| Overall treatment | — | — | |||
| Chemotherapy only | 31 | 25.6 | — | — | |
| Radiation therapy only | 32 | 26.4 | — | — | |
| Chemotherapy + radiation therapy | 58 | 48.0 | — | — | |
Abbreviations: BCNU, carmustine; CCNU, chloroethylcyclohexylnitrosourea.
Frequency-matched to cancer survivors by sex and age at participation.
Cancer survivors.
Primary diagnoses (Table 1) included Hodgkin lymphoma (33.9%), acute lymphoblastic leukemia (16.5%), acute myeloid leukemia (14.1%), and other diagnoses (35.5%); year of cancer diagnosis ranged from 1972 to 2007. Nearly half (48.0%) had been treated with a combination of chest radiation and pulmonary-toxic chemotherapy; 52.9% had undergone HCT, and 29.5% of allogeneic HCT recipients had a history of cGVHD. However, none of the study participants had pulmonary involvement, and only 15.3% of the allogeneic HCT recipients were receiving systemic therapy for cGVHD at t2.
Pulmonary Dysfunction
Cancer survivors versus controls.
Cancer survivors were significantly more likely to have restrictive defects (24.0% v 4.8%; P < .01) and diffusion capacity abnormalities (34.7% v 9.5%; P < .01) when compared with controls (Fig 1); there was no statistically significant difference in the prevalence of obstructive lung disease between the two groups (4.1% v 0%; P = .18). Compared with controls, cancer survivors were significantly more likely to be symptomatic (21.5% v 4.7%; P < .01; Table 2); symptomatic disease was most prevalent in survivors with diffusion defects (Fig 1). Survivors with pulmonary dysfunction had significantly poorer HRQOL across all domains when compared with survivors without dysfunction, as well as healthy controls (Table 2).
Fig 1.
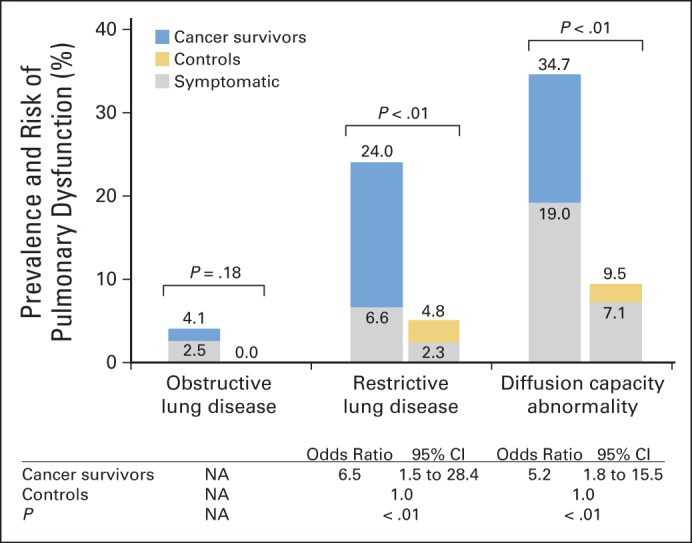
Prevalence and risk of pulmonary dysfunction in childhood cancer survivors versus healthy controls. Multivariable regression was adjusted for race/ethnicity, insurance status, smoking history, and cardiomyopathy and/or heart failure. NA, not applicable.
Table 2.
HRQOL in Survivors With Pulmonary Dysfunction Compared With Survivors Without Pulmonary Dysfunction and Controls
| SF-36 Domains | Survivors With Pulmonary Dysfunction* (n = 54) |
Survivors Without Pulmonary Dysfunction (n = 62) |
Controls (n = 39) |
P | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| General health | 52.3 | 21.4 | 59.1 | 22.3 | 77.9 | 16.4 | < .01 |
| Physical functioning | 71.3 | 27.2 | 87.4 | 17.9 | 96.2 | 9.7 | < .01 |
| Role limitations due to physical health | 69.9 | 35.1 | 87.7 | 22.3 | 95.4 | 17.3 | < .01 |
| Role limitations due to emotional problems | 73.8 | 43.0 | 80.0 | 35.8 | 99.1 | 5.3 | < .01 |
| Low energy/increased fatigue | 48.2 | 24.8 | 57.1 | 23.0 | 64.9 | 17.4 | < .01 |
| Emotional well-being | 67.5 | 22.4 | 71.1 | 19.9 | 80.3 | 12.1 | < .01 |
| Social functioning | 75.9 | 25.4 | 81.9 | 23.6 | 96.0 | 7.7 | < .01 |
| Pain | 73.2 | 27.0 | 82.3 | 18.4 | 91.6 | 11.4 | < .01 |
NOTE. Analysis was limited to symptomatic childhood cancer survivors (n = 116) and healthy controls (n = 39) who were age18 years or older at study enrollment.
Abbreviations: HRQOL, health-related quality of life; SF-36, Medical Outcomes Study 36-Item Short-Form Health Survey; SD, standard deviation.
Defined as obstructive lung disease, restrictive lung disease, or diffusion capacity abnormality.
In multivariable logistic regression analysis adjusted for race/ethnicity, health insurance status, smoking history, and history of cardiomyopathy and/or heart failure, the odds of restrictive defects were increased 6.5-fold among cancer survivors when compared with controls (Fig 1). The odds of diffusion defects were increased 5.2-fold among cancer survivors when compared with controls (Fig 1).
Cancer survivors.
The prevalence of any pulmonary dysfunction (obstructive, restrictive, and/or diffusion defects) among survivors was 45.5%.
Restrictive defects.
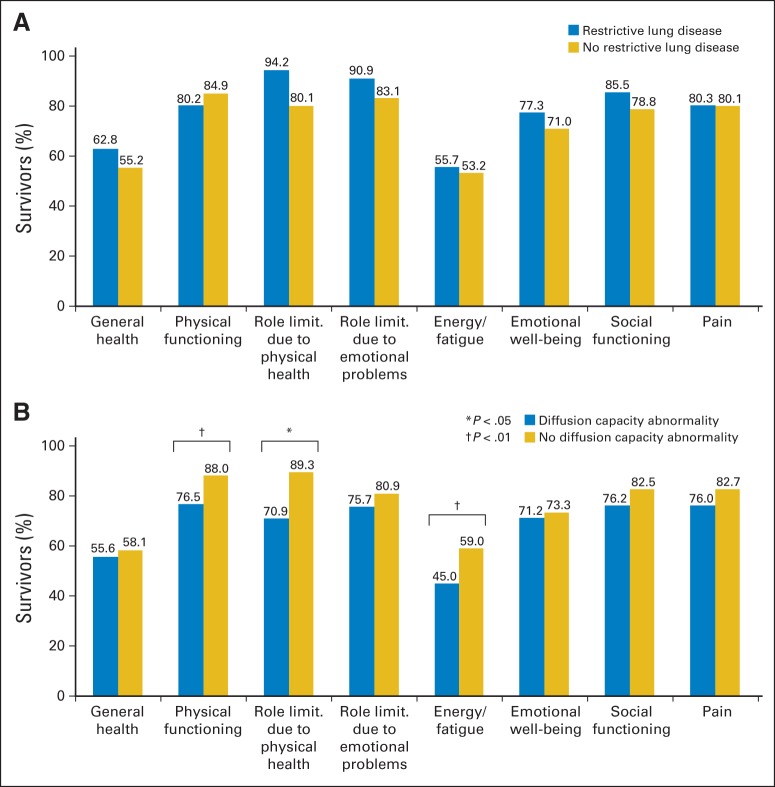
Multivariable logistic regression analysis revealed younger age (< 16 years) at diagnosis (odds ratio [OR], 3.0; 95% CI, 1.2 to 7.8; P = .02) and exposure to higher (> 20 Gy) radiation dose (OR, 5.6; 95% CI, 1.8 to 15.5; P = .02; referent, no radiation) to be associated with restrictive defects (Table 3). There were no differences in self-reported pulmonary symptoms (Appendix Table A2, online only) or HRQOL (Fig 2A) between survivors with and without restrictive defects.
Table 3.
Risk of Restrictive or Diffusion Abnormalities Among Childhood Cancer Survivors According to Demographic Characteristics, Lifestyle, and Treatment-Related Variables
| Characteristic | Restrictive Lung Disease (n = 29) |
No Restrictive Lung Disease (n = 92) |
Univariable Regression |
Multivariable Regression |
Diffusion Abnormality (n = 42) |
No Diffusion Abnormality (n = 79) |
Univariable Regression |
Multivariable Regression |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | OR | 95% CI | OR | 95% CI | No. | % | No. | % | OR | 95% CI | OR | 95% CI | |
| Sex | ||||||||||||||||
| Male | 15 | 51.7 | 46 | 50.0 | 1.0 | — | 12 | 28.6 | 49 | 62.0 | 1.0 | 1.0 | ||||
| Female | 14 | 48.3 | 46 | 50.0 | 0.9 | 0.4 to 2.2 | — | 30 | 71.4 | 30 | 38.0 | 4.1* | 1.8 to 9.2 | 3.9* | 1.7 to 9.5 | |
| Race/ethnicity | ||||||||||||||||
| Non-Hispanic white | 11 | 37.9 | 34 | 37.0 | 1.0 | — | 19 | 45.2 | 26 | 32.9 | 1.0 | — | ||||
| Hispanic | 12 | 41.4 | 47 | 51.1 | 0.8 | 0.3 to 2.0 | — | 16 | 38.1 | 43 | 54.4 | 0.5 | 0.2 to 1.2 | — | ||
| Other | 6 | 20.7 | 11 | 12.0 | 1.7 | 0.5 to 5.6 | — | 7 | 16.7 | 10 | 12.7 | 1.0 | 0.3 to 3.0 | — | ||
| BMI at examination, kg/m2 | ||||||||||||||||
| < 25 | 16 | 55.2 | 50 | 54.3 | 1.0 | — | 27 | 64.3 | 29 | 36.7 | 1.0 | 1.0 | ||||
| ≥ 25 | 13 | 44.8 | 42 | 45.7 | 1.0 | 0.4 to 2.2 | — | 15 | 35.7 | 50 | 50.6 | 0.5 | 0.3 to 1.2 | 0.5 | 0.2 to 1.1 | |
| Employment | ||||||||||||||||
| Employed | 19 | 65.5 | 62 | 68.9 | 1.0 | — | 29 | 69.0 | 52 | 67.5 | 1.0 | — | ||||
| Unemployed | 10 | 34.5 | 30 | 32.6 | 1.2 | 0.5 to 2.8 | — | 13 | 31.0 | 27 | 34.2 | 0.9 | 0.4 to 2.1 | — | ||
| Insurance status | ||||||||||||||||
| Insured | 19 | 65.5 | 68 | 73.9 | 1.0 | — | 29 | 69.0 | 58 | 73.4 | 1.0 | — | ||||
| Uninsured | 10 | 34.5 | 24 | 26.1 | 1.5 | 0.6 to 3.7 | — | 13 | 31.0 | 21 | 26.6 | 1.2 | 0.5 to 2.8 | — | ||
| Exercise, days per week | ||||||||||||||||
| ≥ 3 | 18 | 62.1 | 53 | 57.6 | 1.0 | — | 31 | 73.8 | 57 | 72.2 | 1.0 | — | ||||
| < 3 | 11 | 37.9 | 39 | 42.4 | 0.8 | 0.4 to 2.0 | — | 11 | 26.2 | 22 | 27.8 | 1.1 | 0.5 to 2.3 | — | ||
| Smoking history | ||||||||||||||||
| Never | 28 | 96.6 | 87 | 94.6 | 1.0 | — | 40 | 95.2 | 75 | 94.9 | 1.0 | — | ||||
| Ever | 1 | 3.4 | 5 | 5.4 | 0.9 | 0.7 to 1.9 | — | 2 | 4.8 | 4 | 5.1 | 0.9 | 0.2 to 5.3 | — | ||
| Diagnosis | ||||||||||||||||
| Lymphoma | 11 | 37.9 | 37 | 40.2 | 1.0 | — | 16 | 38.1 | 32 | 40.5 | 1.0 | — | ||||
| Leukemia | 12 | 41.4 | 36 | 39.1 | 1.1 | 0.4 to 2.9 | — | 18 | 42.9 | 30 | 38.0 | 1.2 | 0.5 to 2.8 | — | ||
| Solid malignancy | 6 | 20.7 | 19 | 20.7 | 1.1 | 0.3 to 3.3 | — | 8 | 19.0 | 17 | 21.5 | 0.9 | 0.3 to 2.7 | — | ||
| Age at diagnosis, years | ||||||||||||||||
| ≥ 16 | 10 | 34.5 | 52 | 56.5 | 1.0 | 1.0 | 21 | 50.0 | 42 | 53.2 | 1.0 | — | ||||
| <16 | 19 | 65.5 | 40 | 43.5 | 2.5† | 1.0 to 5.9 | 3.0† | 1.2 to 7.8 | 21 | 50.0 | 37 | 46.8 | 1.1 | 0.5 to 2.4 | — | |
| Time since diagnosis, years | ||||||||||||||||
| < 17 | 14 | 48.3 | 46 | 50.0 | 1.0 | — | 18 | 42.9 | 42 | 53.2 | 1.0 | — | ||||
| ≥ 17 | 15 | 51.7 | 46 | 50.0 | 1.1 | 0.5 to 2.5 | — | 24 | 57.1 | 37 | 46.8 | 1.5 | 0.7 to 3.2 | — | ||
| Cardiomyopathy | ||||||||||||||||
| No | 27 | 93.1 | 86 | 93.5 | 1.0 | — | 39 | 90.7 | 75 | 94.9 | 1.0 | — | ||||
| Yes | 2 | 6.9 | 6 | 6.5 | 1.1 | 0.2 to 5.6 | — | 4 | 9.3 | 4 | 5.1 | 2.0 | 0.5 to 8.3 | — | ||
| Treatment details | ||||||||||||||||
| Bleomycin, IU/m2 | ||||||||||||||||
| Median | 70.5 | 60 | — | 70.5 | 60 | |||||||||||
| Range | 34-120 | 30-360 | 34-120 | 30-360 | ||||||||||||
| None | 21 | 72.4 | 58 | 63.0 | 1.0 | — | 29 | 69.0 | 50 | 63.3 | 1.0 | — | ||||
| Any | 8 | 27.6 | 34 | 37.0 | 0.7 | 0.3 to 1.6 | — | 13 | 31.0 | 29 | 36.7 | 0.8 | 0.4 to 1.7 | — | ||
| Busulfan, mg/m2 | ||||||||||||||||
| Median | 480 | 436 | — | 480 | 436 | — | ||||||||||
| Range | 480-770 | 310-1,102 | 480-770 | 310-1,102 | ||||||||||||
| None | 26 | 89.7 | 80 | 87.0 | 1.0 | — | 39 | 92.8 | 67 | 84.8 | 1.0 | — | ||||
| Any | 3 | 10.3 | 12 | 13.0 | 0.8 | 0.2 to 2.9 | 3 | 7.1 | 12 | 15.2 | 0.4 | 0.1 to 1.6 | — | |||
| BCNU or CCNU, mg/m2 | ||||||||||||||||
| Median | 450 | 450 | — | 450 | 450 | — | ||||||||||
| Range | 225-450 | 300-987 | 225-450 | 300-987 | ||||||||||||
| None | 26 | 89.7 | 83 | 90.2 | 1.0 | — | 37 | 88.1 | 72 | 91.1 | 1.0 | — | ||||
| Any | 3 | 10.3 | 9 | 9.8 | 1.1 | 0.3 to 4.2 | — | 5 | 11.9 | 7 | 8.9 | 1.4 | 0.6 to 4.7 | — | ||
| Chest radiation therapy, Gy | ||||||||||||||||
| None | 4 | 13.8 | 28 | 30.4 | 1.0 | 1.0 | 3 | 7.1 | 29 | 36.7 | 1.0 | 1.0 | ||||
| ≤ 20 | 13 | 44.8 | 47 | 51.1 | 1.5 | 0.4 to 5.8 | 1.6 | 0.5 to 5.7 | 24 | 57.1 | 36 | 45.6 | 6.4* | 1.8 to 23.6 | 6.4* | 1.7 to 24.4 |
| > 20 | 12 | 41.4 | 17 | 18.5 | 4.9† | 1.4 to 17.8 | 5.6† | 1.5 to 21.0 | 15 | 35.7 | 14 | 17.7 | 10.4* | 2.6 to 41.8 | 11.3* | 2.6 to 49.5 |
| HCT | ||||||||||||||||
| None | 12 | 41.4 | 45 | 48.9 | 18 | 42.9 | 39 | 49.4 | 1.0 | — | ||||||
| Autologous | 4 | 13.8 | 16 | 17.4 | 7 | 16.7 | 13 | 16.5 | 1.2 | 0.4 to 3.4 | — | |||||
| Allogeneic | 13 | 44.8 | 31 | 33.7 | 17 | 40.5 | 27 | 34.2 | 1.4 | 0.6 to 3.1 | — | |||||
Abbreviations: BCNU, carmustine; BMI, body mass index; CCNU, chloroethylcyclohexylnitrosourea; HCT, hematopoietic cell transplantation; OR, odds ratio.
P < .01.
P < .05.
Fig 2.
Health-related quality of life in childhood cancer survivors with (A) restrictive lung disease or (B) diffusion capacity abnormality compared with those without. Analysis was limited to childhood cancer survivors (n = 116) who were age 18 years or older at study enrollment. limit., limitation.
Diffusion defects.
The odds of diffusion defects were increased 3.9-fold among females compared with males (OR, 3.9; 95% CI, 1.7 to 9.5; P < .01). There was a dose-dependent association with radiation exposure (referent, no radiation; ≤ 20 Gy: OR, 6.4; 95% CI, 1.7 to 24.4; P < .01; > 20 Gy: OR, 11.3; 95% CI, 2.6 to 49.5; P < .01; Table 3). Importantly, survivors with diffusion defects were significantly more likely to be symptomatic (35.7% v 13.9%; P < .01; Appendix Table A3, online only) and to have poorer HRQOL scores in the following domains: physical functioning (76.5 v 88; P < .01), role limitation as a result of physical health (70.9 p v 89.3; P = .02), and low energy/increased fatigue (45.0 v 59.0; P < .01) when compared with survivors without diffusion defects (Fig 2B).
Predictors of Decline in Lung Function
Among the 95 childhood cancer survivors with no evidence of restrictive lung disease at baseline (t1), only seven (7.4%) developed subsequent restrictive disease at t2. Conversely, among the 89 survivors with no evidence of diffusion defects at baseline (t1), 23 (25.8%) had diffusion defects at t2 (Fig 3). Female sex (OR, 4.5; 95% CI, 1.8 to 7.6; P = .02) and higher radiation dose (> 20 Gy: OR, 24.4; 95% CI, 5.7 to 38.3; P < .01; referent, no radiation) were associated with a decline in diffusion defects over time.
Fig 3.
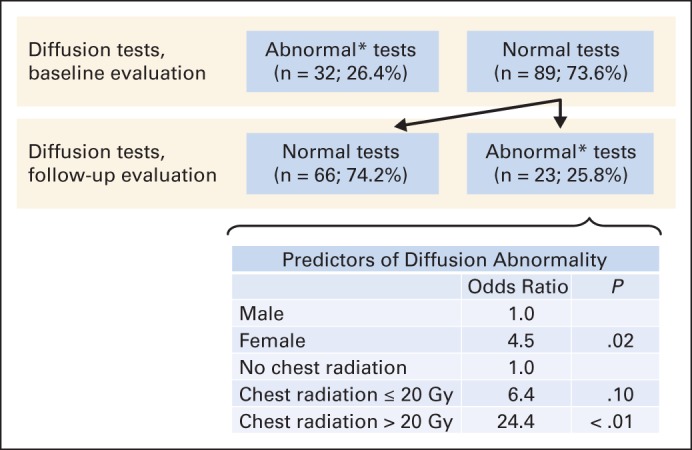
Prevalence of diffusion abnormalities at entry into Long-Term Follow-Up clinic and predictors of decline at follow-up. (*) Diffusing capacity of the lungs for carbon monoxide (corrected for hemoglobin content, age, and sex) less than 75% predicted.
Blood Biomarkers
This study failed to demonstrate a significant correlation between PFT indices (obstructive [FEV1/FVC], restrictive [TLC], or diffusion [DLCOcorr]) and selected blood biomarkers of lung injury (TGF-β1, PDGF-A, PDGF-B, SP-A, SP-D; Appendix Table A4, online only).
DISCUSSION
The growing population of childhood cancer survivors has brought to the forefront several questions related to the modality, frequency, and duration of screening for therapy-related late effects. Recent cross-sectional screening studies6,7 have found pulmonary dysfunction to be the most prevalent complication in long-term childhood cancer survivors. However, it is not known whether these abnormalities are associated with symptoms or poor HRQL or whether pulmonary function continues to decline over time. Furthermore, the utility of blood biomarkers of lung injury for surveillance is not established. In this study, comprehensive profiling of childhood cancer survivors at risk for pulmonary dysfunction revealed increased odds of having symptomatic lung disease when compared with controls and a significant association between pulmonary dysfunction and worse HRQOL. Decline in lung function over time was largely a result of changes in diffusion capacity; the odds of decline in pulmonary function were greater than four-fold in females treated with pulmonary-toxic therapy and were twenty-four–fold among cancer survivors treated with higher (> 20 Gy) radiation dose.
Previous studies have reported wide-ranging (20% to 100%) prevalence for pulmonary dysfunction in childhood cancer survivors,2 attributed in part to differences in screening strategies used by each study. In this study, screening for pulmonary dysfunction was limited to survivors at risk according to COG LTFU Guidelines. Recent studies6,7 that used the same screening criteria reported a higher prevalence of pulmonary dysfunction (> 65%) than that found in this study (45%). However, it is important to note that these studies were more inclusive (all levels of severity were included); comparable definition of pulmonary dysfunction would have yielded an overall prevalence of 62% in this study. The more stringent criteria used in this study yielded a prevalence that was comparable (44%) to that reported by Mulder et al8 who used the same criteria for pulmonary dysfunction in childhood cancer survivors.
Compared with healthy controls, childhood cancer survivors had five times the odds of diffusion capacity abnormality, seven times the odds of restrictive lung disease, and five times the odds of reporting pulmonary symptoms, which highlights the substantial burden of pulmonary disease in this population. Conversely, we found no association between candidate blood markers of lung injury and indices of pulmonary dysfunction. This lack of association may be the result of a combination of both the timing of the assessment and reliance on biomarkers of acute lung injury included in the study.
Restrictive lung disease in childhood cancer survivors is characterized by reduced lung volumes as a result of either reduction in lung parenchyma or changes to the chest wall that may restrict lung parenchymal growth.1,2 These changes are likely a result of exposure to chest radiation at a young age, resulting in disturbance in normal growth and development,1,2 and the findings from this study support this phenomenon. However, little is known regarding the impact of these changes on HRQOL, and little is known regarding the trajectory of lung function over time. In this study, there were no differences in self-reported symptoms or HRQOL between survivors with and without restrictive lung disease. Moreover, the vast majority (93%) of the survivors without restrictive disease at baseline retained intact pulmonary function, indicating that new restrictive changes are unlikely to develop in a young adult (median age, 32 years) population more than 15 years after completion of therapy.
Diffusion capacity abnormality can result from radiation involving the lung parenchyma; exposure to bleomycin, busulfan, and nitrosoureas; or as a complication of GVHD.1,2,24 We found a dose-dependent association with radiation exposure, and female survivors had nearly four times the odds of diffusion capacity abnormality when compared with males, independent of radiation exposure. Survivors with diffusion capacity abnormality were significantly more likely to report respiratory symptoms and poor physical functioning as well as low energy and increased fatigue when compared with those with normal diffusion. Importantly, one in four survivors with intact diffusion capacity at baseline demonstrated a decline in function over time, and nearly half reported respiratory symptoms at t2, which emphasizes the importance of longitudinal follow-up that includes PFT measures of diffusion capacity.
The results of our studies, unlike those of previous studies,2,8,25,26 did not reveal an association between cumulative chemotherapy dose and diffusion capacity abnormality. This may be the result of exposure to relatively low doses of certain chemotherapy agents such as bleomycin (median, 60 IU/m2) in the study population (high risk,2,8,27 typically defined as > 300 mg/m2), and the small proportion of individuals (12.3%) treated with busulfan and/or nitrosoureas. It is important to note that only patients deemed to be at risk according to the COG LTFU Guidelines were screened. Thus, this study did not investigate associations with therapeutic exposures not consistently shown to cause long-term pulmonary toxicity. Furthermore, we did not capture detailed information on radiation dosimetry, including volume of the lungs irradiated as well as dose to parts of the lungs and chest wall. This limitation notwithstanding, it is important to note that in the community setting, health care providers caring for childhood cancer survivors typically do not have detailed information on lung dosimetry, relying instead on dose delivered to a treatment field to determine screening practices. The approach used in this study aligned with the approaches of other more recent studies7,8 that evaluated pulmonary outcomes in long-term childhood cancer survivors. With regard to our finding that females treated with pulmonary-toxic therapies were more likely to have diffusion capacity abnormality when compared with males, there is a large body of evidence supporting female predisposition to several health-related complications (cardiomyopathy, metabolic syndrome, osteonecrosis, hypothyroidism) among childhood cancer survivors28; the underlying cause(s) of this increased risk have not been uniformly elucidated.28 To the best of our knowledge, this study is the first to identify the association between female sex and pulmonary dysfunction. Further investigation is needed to validate these findings and to explore the pathogenesis of these differences.
In summary, comprehensive profiling of childhood cancer survivors who received potentially pulmonary-toxic therapy identified a high risk of symptomatic moderate-to-severe pulmonary dysfunction several years after completion of therapy. Moreover, certain subsets of patients continue to be at risk for declining pulmonary function over time, highlighting the need for continued vigilance beyond the recommended baseline screening visit. These findings may facilitate the development of targeted screening approaches for patients at high risk for progressive pulmonary disease such as diffusion capacity abnormality, setting the stage for the development of therapeutic29,30 or lifestyle interventions31,32 to improve pulmonary function in survivors at highest risk for symptomatic respiratory comorbidities.
Supplementary Material
Appendix
Table A1.
Patient and Treatment Characteristics and Prevalence of Abnormal PFTs at Baseline for Study Participants and Nonparticipants
| Characteristic | Participants (n = 121) |
Nonparticipants (n = 34) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Male sex | 61 | 50.4 | 22 | 64.7 | .14 |
| Race/ethnicity | |||||
| Non-Hispanic white | 45 | 37.2 | 18 | 52.9 | .25 |
| Hispanic | 59 | 48.8 | 13 | 38.2 | |
| Other | 17 | 14.0 | 3 | 8.8 | |
| Hematologic diagnosis | 88 | 72.7 | 25 | 75.8 | .78 |
| Age at diagnosis, years | |||||
| Median | 16.5 | 16.9 | .90 | ||
| Range | 0.2-21.9 | 0.5-21.7 | |||
| Cardiomyopathy | 8 | 6.6 | 4 | 11.8 | .32 |
| Treatment details | |||||
| Bleomycin | .80 | ||||
| Median | 60 | 60 | |||
| Range | 30-360 | 16-170 | |||
| Any | 42 | 34.7 | 14 | 41.2 | .49 |
| Busulfan | |||||
| Median | 436 | 1,178 | .52 | ||
| Range | 115-1,102 | 436-1,920 | |||
| Any | 15 | 12.4 | 2 | 5.9 | .28 |
| BCNU/CCNU | |||||
| Median | 450 | 525 | .81 | ||
| Range | 225-987 | 450-600 | |||
| Any | 12 | 9.9 | 2 | 5.9 | .47 |
| Radiotherapy, Gy | |||||
| Median | 13.2 | 21.2 | .91 | ||
| Range | 2-76 | 10-40 | |||
| None | 32 | 26.4 | 11 | 32.4 | .78 |
| ≤ 20 | 60 | 49.6 | 15 | 44.1 | |
| > 20 | 29 | 24.0 | 8 | 23.5 | |
| Lobectomy, metastectomy, wedge resection | 7 | 5.8 | 2 | 5.9 | .98 |
| HCT | |||||
| None | 57 | 47.1 | 16 | 47.1 | .58 |
| Autologous | 20 | 16.5 | 8 | 23.5 | |
| Allogeneic | 44 | 36.4 | 10 | 29.4 | |
| Age at baseline PFT, years | .43 | ||||
| Median | 27.16 | 29.8 | |||
| Range | 10.4-54.9 | 11.4-45.4 | |||
| Time since diagnosis to baseline PFT, years | |||||
| Median | 12.1 | 15.3 | .30 | ||
| Range | 4.3-36.1 | 5.2-36.5 | |||
| Lung function | |||||
| Obstructive lung disease, FEV1/VC < 0.7 and FEV1 < 80% | 6 | 5.0 | 3 | 8.8 | .40 |
| Restrictive lung disease, TLC < 75% predicted and FEV1 ≥ 80% | 26 | 21.5 | 7 | 20.6 | .91 |
| Diffusion capacity abnormality, DLCOcorr and/or DLCO/VA < 75% predicted | 32 | 26.4 | 10 | 23.4 | .73 |
Abbreviations: BCNU, carmustine; CCNU, chloroethylcyclohexylnitrosourea; DLCOcorr, diffusing capacity of the lungs for carbon monoxide (corrected for hemoglobin content, age, and sex); DLCO/VA, diffusing capacity of the lungs for carbon monoxide divided by volume of air; FEV1, forced expiratory volume in 1 second; HCT, hematopoietic cell transplantation; PFT, pulmonary function test; TLC, total lung capacity; VC, ventilator capacity.
Table A2.
Self-Reported Pulmonary Symptoms in Cancer Survivors With or Without Restrictive Lung Disease
| Self-Reported Symptoms* | Restrictive Lung Disease (n = 29) |
No Restrictive Lung Disease (n = 92) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Are you troubled by shortness of breath when hurrying on ground level or walking up a slight hill? | 10 | 34.5 | 23 | 25.0 | .32 |
| Do you notice shortness of breath walking with other people of your own age on level ground? | 6 | 20.7 | 17 | 18.5 | .79 |
| Do you have to stop for breath when walking at your own pace on level ground? | 4 | 13.8 | 6 | 6.5 | .22 |
| Are you short of breath when washing or dressing? | 1 | 3.4 | 8 | 8.7 | .35 |
| Are you short of breath at rest? | 1 | 3.4 | 4 | 4.3 | .83 |
| Symptomatic† | 7 | 24.1 | 19 | 20.7 | .69 |
As assessed from the Medical Research Council (MRC) Dyspnea Questionnaire.
Defined as having responded “yes” to any two of the MRC Dyspnea Questionnaire items.
Table A3.
Self-Reported Pulmonary Symptoms in Cancer Survivors With or Without Diffusion Abnormalities
| Self-Reported Symptoms* | Diffusion Capacity Abnormality (n = 42) |
No Diffusion Capacity Abnormality (n = 79) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Are you troubled by shortness of breath when hurrying on ground level or walking up a slight hill? | 19 | 45.2 | 14 | 17.7 | < .01 |
| Do you notice shortness of breath walking with other people of your own age on level ground? | 12 | 28.6 | 11 | 13.9 | .05 |
| Do you have to stop for breath when walking at your own pace on level ground? | 5 | 11.9 | 5 | 6.3 | .29 |
| Are you short of breath when washing or dressing? | 4 | 9.5 | 5 | 6.3 | .52 |
| Are you short of breath at rest? | 2 | 4.8 | 3 | 3.8 | .80 |
| Symptomatic† | 15 | 35.7 | 11 | 13.9 | .01 |
As assessed from the Medical Research Council (MRC) Dyspnea Questionnaire.
Defined as having responded “yes” to any two of the MRC Dyspnea Questionnaire items.
Table A4.
Pearson Correlation Between Blood Biomarkers and Indices of Obstructive (FEV1/FVC), Restrictive (TLC), and Diffusion (DLCO) Abnormalities
| Variable | TGF-β1 | P | PDGF-A | P | PDGF-B | P | SP-A | P | SP-D | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer survivors and controls (n = 163) | ||||||||||
| FEV1/FVC | –0.07 | .35 | –0.02 | .83 | –0.05 | .56 | 0.09 | .23 | 0.03 | .71 |
| TLC | –0.16 | .04 | –0.09 | .24 | –0.07 | .35 | 0.03 | .73 | 0.03 | .67 |
| DLCOcorr | –0.16 | .04 | –0.07 | .38 | –0.08 | .30 | 0.11 | .18 | 0.08 | .32 |
| Cancer survivors (n = 121) | ||||||||||
| FEV1/FVC | –0.11 | .22 | –0.05 | .62 | –0.09 | .31 | 0.15 | .11 | 0.06 | .53 |
| TLC | –0.06 | .51 | –0.06 | .50 | 0.01 | .89 | –0.08 | .40 | –0.05 | .61 |
| DLCOcorr | –0.08 | .38 | 0.03 | .76 | 0.03 | .76 | 0.02 | .87 | 0.03 | .77 |
Abbreviations: DLCO, diffusing capacity of the lungs for carbon monoxide; DLCOcorr, DLCO corrected for hemoglobin content, age, and sex; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PDGF-A/B, platelet-derived growth factor A/B; SP-A/D, surfactant proteins A and D; TGF-β1, tumor growth factor beta1; TLC, total lung capacity.
Fig A1.
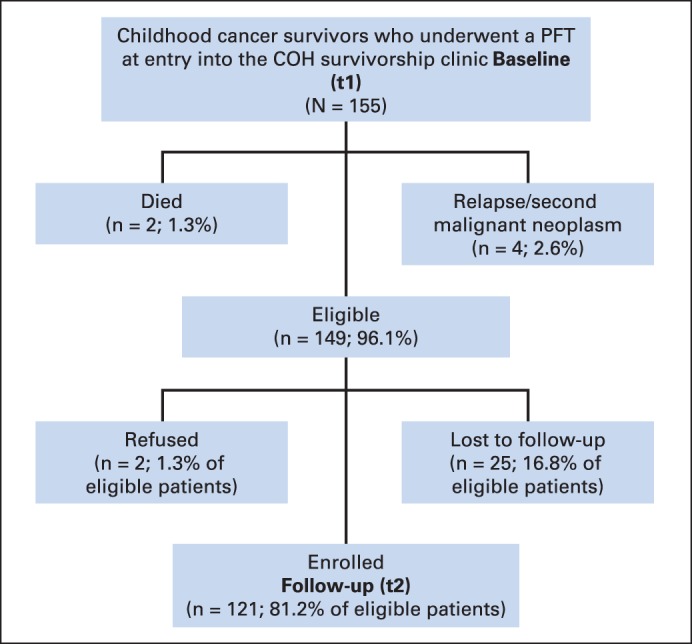
Study flow diagram demonstrating recruitment of the 155 childhood cancer survivors who underwent a pulmonary function test (PFT) at entry into the City of Hope (COH) survivorship clinic.
Footnotes
Supported by Grant No. 2 K12 CA001727-14 from the National Cancer Institute, National Institutes of Health, and by the STOP Cancer Foundation.
Presented in part at the European Symposium on Late Complications after Childhood Cancer, Edinburgh, UK, September 15-16, 2014.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Saro H. Armenian, Smita Bhatia
Financial support: Saro H. Armenian, Smita Bhatia
Administrative support: Saro H. Armenian, Wendy Landier, Liton Francisco, Claudia Herrera, Smita Bhatia
Provision of study materials or patients: Saro H. Armenian, Natt Supab, Karla Wilson
Collection and assembly of data: Saro H. Armenian, Wendy Landier, Liton Francisco, Claudia Herrera, George Mills, Aida Siyahian, Natt Supab, Karla Wilson, Julie A. Wolfson, David Horak
Data analysis and interpretation: Saro H. Armenian, Julie A. Wolfson, David Horak, Smita Bhatia
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Long-Term Pulmonary Function in Survivors of Childhood Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Saro H. Armenian
No relationship to disclose
Wendy Landier
Research Funding: Merck, Sharpe & Dohme (Inst)
Liton Francisco
No relationship to disclose
Claudia Herrera
No relationship to disclose
George Mills
No relationship to disclose
Aida Siyahian
No relationship to disclose
Natt Supab
No relationship to disclose
Karla Wilson
No relationship to disclose
Julie A. Wolfson
No relationship to disclose
David Horak
No relationship to disclose
Smita Bhatia
No relationship to disclose
REFERENCES
- 1.Liles A, Blatt J, Morris D, et al. Monitoring pulmonary complications in long-term childhood cancer survivors: Guidelines for the primary care physician. Cleve Clin J Med. 2008;75:531–539. doi: 10.3949/ccjm.75.7.531. [DOI] [PubMed] [Google Scholar]
- 2.Huang TT, Hudson MM, Stokes DC, et al. Pulmonary outcomes in survivors of childhood cancer: A systematic review. Chest. 2011;140:881–901. doi: 10.1378/chest.10-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2002;95:2431–2441. doi: 10.1002/cncr.10978. [DOI] [PubMed] [Google Scholar]
- 6.Landier W, Armenian SH, Lee J, et al. Yield of screening for long-term complications using the Children's Oncology Group Long-Term Follow-Up Guidelines. J Clin Oncol. 2012;30:4401–4408. doi: 10.1200/JCO.2012.43.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulder RL, Thönissen NM, van der Pal HJ, et al. Pulmonary function impairment measured by pulmonary function tests in long-term survivors of childhood cancer. Thorax. 2011;66:1065–1071. doi: 10.1136/thoraxjnl-2011-200618. [DOI] [PubMed] [Google Scholar]
- 9.Huang TT, Chen Y, Dietz AC, et al. Pulmonary outcomes in survivors of childhood central nervous system malignancies: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:319–325. doi: 10.1002/pbc.24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenney ME, Faragher EB, Jones PH, et al. Lung function and exercise capacity in survivors of childhood leukaemia. Med Pediatr Oncol. 1995;24:222–230. doi: 10.1002/mpo.2950240403. [DOI] [PubMed] [Google Scholar]
- 11.van den Blink B, Wijsenbeek MS, Hoogsteden HC. Serum biomarkers in idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. 2010;23:515–520. doi: 10.1016/j.pupt.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Kong FM, Ao X, Wang L, et al. The use of blood biomarkers to predict radiation lung toxicity: A potential strategy to individualize thoracic radiation therapy. Cancer Control. 2008;15:140–150. doi: 10.1177/107327480801500206. [DOI] [PubMed] [Google Scholar]
- 13.Children's Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, 2014. www.survivorshipguidelines.org. [Google Scholar]
- 14.Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 16.Brusasco V, Crapo R, Viegi G. Coming together: The ATS/ERS consensus on clinical pulmonary function testing. Eur Respir J. 2005;26:1–2. doi: 10.1183/09031936.05.00034205. [DOI] [PubMed] [Google Scholar]
- 17.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 18.Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 19.The Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management, and Prevention of COPD, 2008. http://www.goldcopd.org/uploads/users/files/GOLD_AtAGlance_2015_Feb18.pdf. [Google Scholar]
- 20.National Cancer Institute. Common Terminology Criteria for Adverse Events, version 3.0, 2003. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. [Google Scholar]
- 21.Viegi G, Pedreschi M, Pistelli F, et al. Prevalence of airways obstruction in a general population: European Respiratory Society vs American Thoracic Society definition. Chest. 2000;117:339S–345S. doi: 10.1378/chest.117.5_suppl_2.339s. [DOI] [PubMed] [Google Scholar]
- 22.Hyland RH, Krastins IR, Aspin N, et al. Effect of body position on carbon monoxide diffusing capacity in asymptomatic smokers and nonsmokers. Am Rev Respir Dis. 1978;117:1045–1053. doi: 10.1164/arrd.1978.117.6.1045. [DOI] [PubMed] [Google Scholar]
- 23.Shibata Y, Abe S, Inoue S, et al. Relationship between plasma fibrinogen levels and pulmonary function in the Japanese population: The Takahata study. Int J Med Sci. 2013;10:1530–1536. doi: 10.7150/ijms.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skinner R, Kaplan R, Nathan PC. Renal and pulmonary late effects of cancer therapy. Semin Oncol. 2013;40:757–773. doi: 10.1053/j.seminoncol.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Bossi G, Cerveri I, Volpini E, et al. Long-term pulmonary sequelae after treatment of childhood Hodgkin's disease. Ann Oncol. 1997;8:19–24. [PubMed] [Google Scholar]
- 26.Venkatramani R, Kamath S, Wong K, et al. Correlation of clinical and dosimetric factors with adverse pulmonary outcomes in children after lung irradiation. Int J Radiat Oncol Biol Phys. 2013;86:942–948. doi: 10.1016/j.ijrobp.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 27.Yahalom J, Portlock CS. Long-term cardiac and pulmonary complications of cancer therapy. Hematol Oncol Clin North Am. 2008;22:305–318. doi: 10.1016/j.hoc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong GT, Sklar CA, Hudson MM, et al. Long-term health status among survivors of childhood cancer: Does sex matter? J Clin Oncol. 2007;25:4477–4489. doi: 10.1200/JCO.2007.11.2003. [DOI] [PubMed] [Google Scholar]
- 29.Anscher MS. Targeting the TGF-beta1 pathway to prevent normal tissue injury after cancer therapy. Oncologist. 2010;15:350–359. doi: 10.1634/theoncologist.2009-S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 31.Stubblefield MD, Schmitz KH, Ness KK. Physical functioning and rehabilitation for the cancer survivor. Semin Oncol. 2013;40:784–795. doi: 10.1053/j.seminoncol.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Huang TT, Ness KK. Exercise interventions in children with cancer: A review. Int J Pediatr. 2011;2011:461512. doi: 10.1155/2011/461512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.