Abstract
Purpose
Cixutumumab, formerly IMC-A12, is a recombinant human monoclonal immunoglobulin G1 antibody that targets insulin-like growth factor I receptor (IGF-IR). Cixutumumab was synergistic with castration in a hormone-sensitive prostate cancer xenograft model.
Patients and Methods
Patients with new metastatic prostate cancer were randomly assigned within 30 days of initiating androgen deprivation (AD) to cixutumumab added to a luteinizing hormone–releasing hormone agonist with bicalutamide versus AD alone. With 180 patients and one-sided alpha of 0.10, there would be 90% power to detect an absolute 20% difference in undetectable prostate-specific antigen (PSA; ≤ 0.2 ng/mL) rate at 28 weeks (relative risk, 1.44); this end point was previously strongly correlated with survival. Secondary end points included the proportion of patients with PSA > 4.0 ng/mL, safety and tolerability, circulating tumor cell (CTC) levels, and seven plasma IGF-IR biomarkers. Fisher's exact test was used for the primary end point, and extended Mantel-Haenszel χ2 test was used for three PSA response categories.
Results
The trial accrued 210 eligible patients (105 randomly assigned to each arm). Patient characteristics were similar in both arms. Undetectable PSA rate was 42 (40.0%) of 105 for cixutumumab plus AD and 34 (32.3%) of 105 for AD alone (relative risk, 1.24; one-sided P = .16). Lower baseline CTCs (0 v 1 to 4 v ≥ 5/7.5 mL whole blood) were associated with higher rate of PSA response (three categories; P = .036) in 39 evaluable patients. IGF-IR biomarkers were not correlated with PSA outcome, and cixutumumab did not significantly change these biomarker levels.
Conclusion
Cixutumumab plus AD did not significantly increase the undetectable PSA rate in men with new metastatic hormone-sensitive prostate cancer. CTCs at baseline may carry prognostic value.
INTRODUCTION
Type I insulin-like growth factor receptor (IGF-IR) activation is initiated by binding to multiple ligands, including IGF-I, IGF-II, and insulin, and leads to downstream signaling via phosphatidylinositol 3-kinase/protein kinase B and mitogen-activated protein kinase pathways.1 Dysregulated IGF-IR signaling is implicated in the development and progression of multiple malignancies.2 In prostate cancer, IGF-IR signaling stimulates nuclear translocation of androgen receptor (AR) and subsequent AR-mediated signaling in the absence of androgens.3 Preclinical studies have indicated that cixutumumab, a fully humanized monoclonal antibody (mAb) that induces IGF-IR internalization, leads to apoptosis and G1 cell-cycle arrest in hormone-sensitive prostate cancer (HSPC) xenografts but only G2 arrest in castration-resistant prostate cancer (CRPC) murine models.4
In early-phase clinical trials, targeting IGF-IR in patients with prostate cancer has demonstrated both biologic and clinical activity. In an open-label phase II trial, cixutumumab showed a 29% ≥ 6-month radiographic stabilization rate for patients with metastatic CRPC.5,6 In a neoadjuvant trial, cixutumumab combined with androgen deprivation (AD) showed pharmacodynamic effect, with significant increases in serum growth hormone (GH), IGF-1, IGF-II, IGF binding protein (BP) –III, c-peptide, and insulin and decreases in IGFBP-I.7 In a neoadjuvant trial using another mAb against IGF-IR—figitumumab—monotherapy induced ≥ 25% and ≥ 50% prostate-specific antigen (PSA) declines in 94% and 31% of patients, respectively.8
The randomized SWOG (Southwest Oncology Group) 9346 trial of intermittent versus continuous AD for men with metastatic HSPC demonstrated that absolute PSA value after 7 months was strongly associated with overall survival.9 Therefore, this large randomized phase II trial used that end point to determine if addition of cixutumumab to AD in men with metastatic HSPC would be superior to AD alone in increasing the undetectable PSA (≤ 0.2 ng/mL) rate after 28 weeks.
PATIENTS AND METHODS
This was a multicenter, randomized phase II trial designed and conducted within SWOG, approved by the Cancer Therapy Evaluation Program of the National Cancer Institute and by the independent institutional review board of each participating center. All patients provided informed written consent. An independent data and safety monitoring committee monitored this trial from activation until final reporting.
Patients
Eligible patients had pathologic confirmation of prostate cancer, PSA ≥ 5 ng/mL, and at least one radiographically detectable metastasis (eg, if pelvic lymph node, could not be amenable to irradiation). Patients were required to have Zubrod performance status of 0 to 2 or 3, if resulting from bone pain only.
Prior remote AD was allowed only if received in the neoadjuvant, concurrent, and/or adjuvant settings and > 2 years had elapsed from completion of therapy. Prior AD for metastatic disease was allowed if first luteinizing hormone–releasing hormone (LHRH) agonist injection was within 30 days of enrollment. LHRH antagonists and bilateral orchiectomy were not allowed. Only patients who had not started AD before enrollment (early-induction group) were eligible for prospective blood biomarker collection.
Key exclusion criteria included prior receipt of cytotoxic chemotherapy or any agent directly inhibiting IGF or IGF-IR. Other exclusions included known brain metastasis, any other cancer in the last 5 years, HIV requiring antiretroviral therapy, symptomatic congestive heart failure, or known left ventricular ejection fraction ≥ 10% below lower limit of normal. Patients were also excluded if leukocyte count was < 3,000/mcL, absolute neutrophil count < 1,500/mcL, hemoglobin < 9 g/dL, platelets < 100,000/mcL, total bilirubin > 1.5× institutional upper limit of normal (ULN; unless documented Gilbert's disease), AST or ALT > 3× institutional ULN (or > 5× if liver metastasis present), creatinine > 2× institutional ULN, hemoglobin A1C > 7%, fasting glucose ≥ 160 mg/dL, international normalized ratio > 1.5, or partial thromboplastin time > 5 seconds above institutional ULN. Any patient treated with radiation therapy, radiopharmaceuticals, major surgery, or biologic therapeutics within 28 days was excluded from the trial.
Treatment
Patients were randomly assigned at a 1:1 ratio to arm one (oral bicalutamide daily with LHRH agonist [AD] plus cixutumumab 10 mg/kg intravenously over 1 hour every 2 weeks for seven cycles [each cycle = two treatments in 28 days]) or arm two (bicalutamide with LHRH agonist [AD]). PSA, CBC, total bilirubin, AST, ALT, and creatinine were assessed every 4 weeks. Fasting serum glucose was obtained every 2 weeks before each cixutumumab treatment for those in arm one and every 4 weeks for those in arm two.
For assessment of adverse events, the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) were used. Cixutumumab was held for grade 3 to 4 hyperglycemia, defined by symptoms and/or fasting glucose ≥ 300 mg/mL. Cixutumumab was resumed with dose reduction to 8 mg/kg if the patient became asymptomatic and fasting glucose remained consistently < 220 mg/mL with a stable dose of insulin and/or oral diabetic agents. A second dose reduction to 6 mg/kg was allowed, if necessary. Missed doses of cixutumumab were omitted, and dose re-escalation was not allowed after reduction for toxicity.
Patients continued with protocol treatment until completion of seven cycles (28 weeks), early disease progression, unacceptable toxicity, or patient desire to withdraw from the trial. Early progression was defined as progression by symptoms, imaging, or development of castration resistance, defined as two serial PSA rises during protocol treatment. In addition, if protocol treatment was held for > 4 weeks, the patient was removed from the trial. Survival assessments once protocol treatment ended were to occur every 6 months for the first 2 years and then annually until 5 years after registration or death.
End Points
The primary end point was the undetectable PSA rate (≤ 0.2 ng/mL) after seven cycles (28 weeks) of protocol treatment. Secondary end points included safety and tolerability, proportion of patients with PSA > 4 ng/mL after seven cycles, whole-blood Cellsearch (Veridex, Raritan, NJ) circulating tumor cell (CTC) assessment, and plasma (sodium heparin tubes) IGF-IR biomarkers (RayBiotech, Norcross, GA), including C-peptide, IGFBP-I, IGFBP-III, IGF-I, IGF-II, growth hormone, and insulin. Laboratory staff were blinded to participant trial arm, and all IGF-IR biomarkers were run in duplicate. Only early-induction patients were eligible for biomarker measurements at baseline and week 12. Assessment of prostate cancer–associated microRNAs10 and validation of the overall survival prognostic PSA model from SWOG 93469 were additional secondary end points to be analyzed and reported separately.
Statistical Considerations
The accrual goal was 180 eligible patients, with an additional 10% (n = 198) to account for possible ineligibility. For the primary end point, a 45% undetectable PSA ≤ 0.2 ng/mL rate at 28 weeks was assumed for control arm two with AD, based on data from SWOG 9346.9 Using a one-sided type I error rate of 0.10, we had 90% statistical power to detect an absolute difference of 20% in the undetectable PSA rate with the addition of cixutumumab using Fisher's exact test. An intention-to-treat approach was used in analysis of the primary end point. An interim futility analysis was to be conducted when half the enrolled patients were evaluated for 28-week PSA response. The study was to be terminated if the undetectable PSA response rate relative risk (RR) of 1.44 was deemed to be highly unlikely (P < .005).
Patients without a PSA value at the completion of seven cycles (28 weeks) of protocol treatment were assumed to not have achieved PSA ≤ 4 ng/mL. One-sided Fisher's exact test was used to test whether the undetectable PSA rate at 28 weeks was greater in the experimental arm. Extended Mantel-Haenszel χ2 test was used to evaluate the association of treatment arm with the three PSA response categories (≤ 0.2 v > 0.2 to ≤ 4.0 v > 4.0 ng/mL), accounting for the ordinality of the response.
A univariable logistic regression model (generalized logits) was used to assess whether risk factors known to be associated with survival in this patient population were also correlated with PSA response at 28 weeks. A multivariable logistic regression model (generalized logits) was fit to all factors found to be statistically significant (P < .05) in the univariable setting.
Correlative studies were exploratory in nature because of limited sample size. An extended Mantel-Haenszel correlation statistic was used to evaluate the association between baseline CTC count and 28-week PSA response category. Kruskal-Wallis test was used to assess the association between baseline IGF-IR–related biomarkers and PSA response. Wilcoxon rank sum test was used to assess the association between the distribution of change from baseline to week 12 in measures of IGF-IR–related biomarker and the two treatment arms. Because of limited sample size, these exploratory analyses were limited to generating hypotheses for further investigation in larger cohorts; there was only sufficient power to detect strong relationships. No adjustments for multiple comparisons were made. All analyses were conducted using SAS software (version 9.3; SAS Institute, Cary, NC).
RESULTS
Study Population
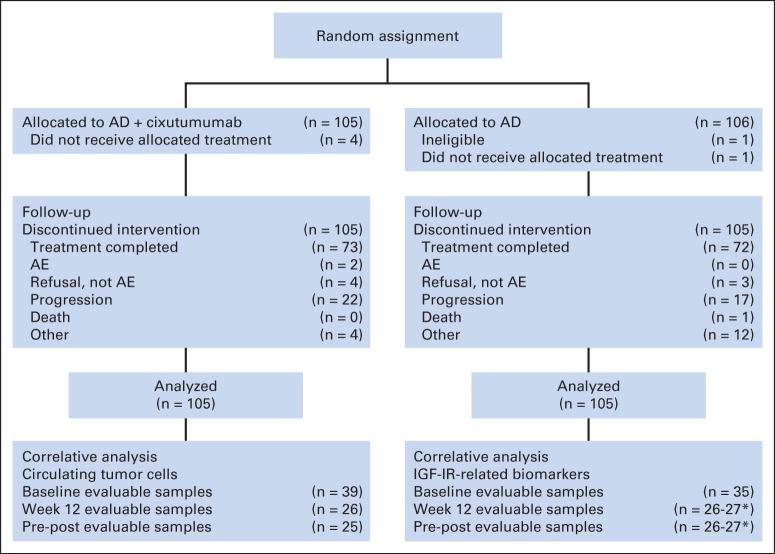
Between February 3, 2011, and December 1, 2012, 211 patients (arm one [AD plus cixutumumab], n = 105; arm two [AD alone], n = 106) were randomly assigned within SWOG institutions (Fig 1). Only one patient (from arm two) was ineligible because of lack of a demonstrable radiographic metastasis. Therefore, the intention-to-treat population included 210 patients. Baseline demographics and disease characteristics were generally balanced between treatment groups (Table 1).
Fig 1.
CONSORT diagram. AD, androgen deprivation; AE, adverse event; IGF-1R, insulin-like growth factor I receptor. (*) For individual IGF-IR–related biomarker.
Table 1.
Eligible Patient Baseline Demographic and Clinical Characteristics
| Characteristic | AD Plus Cixutumumab (n = 105) |
AD Alone (n = 105) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 65 | 66 | ||
| IQ range | 60-72 | 58-73 | ||
| PSA, ng/mL | ||||
| Median | 31 | 37 | ||
| IQ range | 12-74 | 10-200 | ||
| Weight, kg* | ||||
| Median | 90 | 86 | ||
| IQ range | 60-101 | 77-98 | ||
| BMI, kg/m2 | ||||
| < 18.5 (underweight) | 1 | 0.9 | 2 | 1.9 |
| 18.5 to 24.9 (normal weight) | 21 | 20.0 | 29 | 27.6 |
| 25 to 29.9 (overweight) | 36 | 34.3 | 39 | 37.1 |
| ≥ 30 (obese) | 43 | 40.9 | 33 | 31.4 |
| Unknown | 4 | 3.8 | 2 | 1.9 |
| Gleason score† | ||||
| < 7 | 8 | 7.6 | 6 | 5.7 |
| 7 | 29 | 27.6 | 11 | 10.4 |
| ≥ 7 | 63 | 60.0 | 82 | 78.0 |
| Race | ||||
| Black | 4 | 3.8 | 10 | 9.5 |
| White | 94 | 89.5 | 88 | 83.8 |
| Other | 7 | 6.7 | 7 | 6.7 |
| Zubrod performance score | ||||
| 0 | 62 | 59.0 | 65 | 61.9 |
| 1 | 41 | 39.0 | 38 | 36.1 |
| 2 | 2 | 1.9 | 2 | 1.9 |
| Site of metastasis | ||||
| Lymph node only | 15 | 14.3 | 9 | 8.6 |
| Bone only | 56 | 53.3 | 63 | 60.0 |
| Lymph node and bone | 19 | 18.1 | 17 | 16.2 |
| Visceral | 15 | 14.3 | 16 | 15.2 |
| Bone pain | 28 | 26.6 | 35 | 33.3 |
| Early-induction AD | 59 | 56.1 | 65 | 61.9 |
Abbreviations: AD, androgen deprivation; BMI, body mass index; IQ, interquartile; PSA, prostate-specific antigen.
Missing weight, n = 6.
Gleason score missing, n = 11.
Efficacy
A formal interim analysis of the alternative hypothesis provided no evidence that the trial should close early, and the data and safety monitoring committee recommended the trial proceed to completion. The undetectable PSA rate after 28 weeks of trial therapy was 42 (40.0%) of 105 for those receiving AD plus cixutumumab and 34 (32.3%) of 105 for those receiving AD alone (RR, 1.24; one-sided P = .16). A prespecified secondary end point was rate of PSA > 4 ng/mL, which was 46 (43.8%) of 105 for those receiving AD plus cixutumumab and 56 (53.3%) of 105 for those receiving AD alone (RR, 0.82; one-sided P = .11). When considering the aggregate PSA response categories of PSA ≤ 0.2, > 0.2 to ≤ 4.0, and > 4.0 ng/mL, there was no statistically significant difference between the two arms (P = .17; Table 2). Given the mechanism of action of cixutumumab, an exploratory analysis classified patients by body mass index categories and found no difference between treatment arms in likelihood of achieving the PSA response categories (data not shown). The proportion of patients who became castration resistant before completing seven cycles (28 weeks) of protocol treatment was 22 (20.9%) of 105 for those receiving AD plus cixutumumab and 17 (16.2%) of 105 for those receiving AD alone (RR, 1.29; two-sided P = .37).
Table 2.
PSA End Points by Treatment Arm
| Treatment Arm | PSA (ng/mL) |
Total |
||||||
|---|---|---|---|---|---|---|---|---|
| ≤ 0.2* |
> 0.2 to ≤ 4.0 |
> 4.0† |
||||||
| No. | % | No. | % | No. | % | No. | % | |
| AD plus cixutumumab | 42 | 40.0 | 17 | 16.2 | 46 | 43.8 | 105 | 100.0 |
| AD alone | 34 | 32.4 | 15 | 14.3 | 56 | 53.3 | 105 | 100.0 |
| P | .16‡ | .11‡ | .17§ | |||||
Abbreviations: AD, androgen deprivation; PSA, prostate-specific antigen.
Primary end point analysis comparing PSA ≤ 0.2 ng/mL between treatment arms.
Secondary end point analysis comparing PSA > 4.0 ng/mL between treatment arms.
Fisher's exact test.
Extended Mantel-Haenszel test using integer scores for columns.
As a supplemental analysis, we evaluated the likelihood of multiple patient characteristics being associated with undetectable PSA or normalized PSA > 0.2 to ≤ 4.0 ng/mL versus PSA > 4.0 ng/mL (Table 3). These evaluations included treatment arm, age, log PSA at baseline, Gleason score, performance status, presence or absence of bone pain, presence or absence of visceral metastasis, and body mass index. Those characteristics reaching levels of significance in the univariable models were brought forth with treatment arm into the multivariable model (Table 3). The addition of cixutumumab did not achieve statistical significance in either the univariable or multivariable model, although age, log PSA at baseline, and bone pain all retained significance in both models.
Table 3.
Association of Treatment Arm and Baseline Characteristics With PSA Response
| PSA Comparison (ng/mL) | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Treatment (AD plus cixutumumab v AD) | ||||||
| ≤ 0.2 v > 4.0 | 1.50 | 0.83 to 2.73 | .18 | 1.40 | 0.70 to 2.78 | .34 |
| > 0.2 to ≤ 4.0 v > 4.0 | 1.38 | 0.62 to 3.06 | .43 | 1.48 | 0.63 to 3.44 | .37 |
| Age, years* | ||||||
| ≤ 0.2 v > 4.0 | 1.15 | 1.01 to 1.35 | .04 | 1.21 | 1.02 to 1.44 | .03 |
| > 0.2 to ≤ 4.0 v > 4.0 | 1.05 | 0.87 to 1.27 | .62 | 1.07 | 0.87 to 1.31 | .55 |
| Log PSA at baseline | ||||||
| ≤ 0.2 v > 4.0 | 0.67 | 0.55 to 0.81 | < .001 | 0.67 | 0.54 to 0.83 | < .001 |
| > 0.2 to ≤ 4.0 v > 4.0 | 0.85 | 0.68 to 1.06 | .15 | 0.83 | 0.65 to 1.06 | .14 |
| Gleason score (> 7 v < 7) | ||||||
| ≤ 0.2 v > 4.0 | 0.47 | 0.14 to 1.58 | .22 | |||
| > 0.2 to ≤ 4.0 v > 4.0 | 0.74 | 0.14 to 4.10 | .73 | |||
| Performance status | ||||||
| ≤ 0.2 v > 4.0 | 0.79 | 0.45 to 1.39 | .42 | |||
| > 0.2 to ≤ 4.0 v > 4.0 | 0.57 | 0.25 to 1.24 | .17 | |||
| Bone pain (no v yes) | ||||||
| ≤ 0.2 v > 4.0 | 6.17 | 2.83 to 13.44 | < .001 | 5.09 | 2.23 to 11.60 | < .001 |
| > 0.2 to ≤ 4.0 v > 4.0 | 4.08 | 1.54 to 10.84 | .005 | 3.63 | 1.35 to 9.79 | .01 |
| Site of metastasis (nonvisceral v visceral) | ||||||
| ≤ 0.2 v > 4.0 | 1.32 | 0.57 to 3.07 | .52 | |||
| > 0.2 to ≤ 4.0 v > 4.0 | 1.40 | 0.44 to 4.51 | .57 | |||
| BMI (obese v normal weight) | ||||||
| ≤ 0.2 v > 4.0 | 2.05 | 0.93 to 4.52 | .07 | |||
| > 0.2 to ≤ 4.0 v > 4.0 | 2.09 | 0.70 to 6.27 | .19 | |||
Abbreviations: AD, androgen deprivation; BMI, body mass index; OR, odds ratio; PSA, prostate-specific antigen.
Five-year increment in age.
Safety
Cixutumumab was generally well tolerated, with minimal increase in adverse events when added to AD. Table 4 summarizes adverse events occurring in ≥ 10% of patients attributed by local investigator to be related or possibly related to study treatment. There were no grade 4 or 5 adverse events. There were few grade 3 adverse events, and most notable was hyperglycemia. To eliminate any potential bias, we compared patients who developed any-grade hyperglycemia regardless of drug attribution treated with AD plus cixutumumab with patients treated with AD alone. Patients treated with AD plus cixutumumab were 2.36× more likely to develop hyperglycemia than patients treated with AD alone (RR, 2.36; P < .001). No association was found between development of hyperglycemia and PSA response in patients receiving AD plus cixutumumab (Fisher's exact P = .15). Dose holds and reductions for patients receiving cixutumumab occurred in 19 (18.1%) and 8 (7.6%) patients, respectively, and 5 (4.8%) patients experienced both. Only 2 (1.9%) patients receiving cixutumumab withdrew early from the trial for unacceptable toxicity.
Table 4.
Treatment-Related AEs Experienced by ≥ 10% of Patients in Either Treatment Arm
| AE | AD Plus Cixutumumab (n = 101) |
AD Alone (n = 104) |
||||
|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | |
| ALT increased | 13 | 1 | 1 | 9 | 0 | 0 |
| AST increased | 11 | 1 | 1 | 10 | 0 | 0 |
| Anemia | 26 | 4 | 1 | 16 | 0 | 1 |
| Creatinine increased | 2 | 2 | 0 | 1 | 0 | 0 |
| Dry skin | 14 | 0 | 0 | 0 | 0 | 0 |
| Dysgeusia | 10 | 1 | 0 | 0 | 0 | 0 |
| Erectile dysfunction | 2 | 1 | 1 | 7 | 4 | 0 |
| Fatigue | 40 | 16 | 0 | 26 | 7 | 0 |
| Hot flashes | 31 | 5 | 1 | 43 | 13 | 1 |
| Hyperglycemia | 28 | 15 | 8 | 8 | 0 | 0 |
| Hypertension | 6 | 7 | 2 | 4 | 7 | 2 |
| Myalgia | 13 | 0 | 0 | 1 | 0 | 0 |
| Nausea | 9 | 2 | 1 | 1 | 2 | 0 |
| Neutrophil count decreased | 10 | 1 | 0 | 1 | 1 | 0 |
| Platelet count decreased | 23 | 1 | 0 | 2 | 0 | 0 |
Abbreviations: AD, androgen deprivation; AE, adverse event.
Correlative studies
Baseline CTC samples were received for 50 patients. However, only 39 patients had evaluable CTC levels: one patient was ineligible for the trial, six initiated AD before samples were obtained, and four were not assay evaluable. At baseline, 16 (41%) of 39 had a CTC count of 0. There was association between stratified baseline CTC level with the three PSA categories at 28 weeks (P = .04; Table 5). At week 12, CTC samples were received for 41 patients; however, only 26 patients had evaluable samples: one was from the ineligible patient, six initiated AD before samples were obtained, and eight were not assay evaluable. In sum, 25 patients had baseline and 12-week treatment CTC samples evaluable for response to treatment. Only one patient had an increase in CTC count from baseline, whereas all other patients had counts that declined or stayed stable, challenging the feasibility of a treatment response association analysis between CTCs and PSA.
Table 5.
Association of Baseline CTC Level With PSA Response Categories
| Baseline CTC Count (per 7.5 mL whole blood) | PSA (ng/mL) |
Total (n = 39) |
||||||
|---|---|---|---|---|---|---|---|---|
| ≤ 0.2 |
> 0.2 to ≤ 4.0 |
> 4.0 |
||||||
| No. | % | No. | % | No. | % | No. | % | |
| 0 | 12 | 75.0 | 0 | 0.0 | 4 | 25.0 | 16 | 41.0 |
| 1 to 4 | 5 | 55.6 | 2 | 22.2 | 2 | 22.2 | 9 | 23.1 |
| ≥ 5 | 4 | 28.6 | 3 | 21.4 | 7 | 50.0 | 14 | 35.9 |
NOTE. Association between baseline CTC count and PSA response categories at 28 weeks (P = .036) from extended Mantel-Haenszel (correlation) statistic.
Abbreviations: CTC, circulating tumor cell; PSA, prostate-specific antigen.
Specimens were obtained in 43 patients at baseline for the seven plasma biomarkers hypothesized to have a pharmacodynamic relationship to IGF-IR inhibition. However, only 35 patients were evaluable: one was ineligible for the trial, six started AD before sample acquisition, and one was not assay evaluable. Table 6 summarizes the associations of these baseline biomarker levels with 28-week stratified PSA response; none of these biomarkers had a statistically significant association with PSA response. At week 12, these biomarkers had been obtained for 27 patients. All specimens were evaluable for percent change in response to treatment from baseline. None of these biomarkers demonstrated a statistically significant difference in change between the treatment arms, although the analysis was limited by sample size (Appendix Table A1, online only).
Table 6.
Correlation of IGF-IR Biomarkers With PSA Response Categories
| Biomarker Measure | PSA (ng/mL) |
P* | |||||
|---|---|---|---|---|---|---|---|
| ≤ 0.2 (n = 19) |
> 0.2 to ≤ 4.0 (n = 4) |
> 4.0 (n = 12) |
|||||
| Median | Range | Median | Range | Median | Range | ||
| C-peptide | 15 | 125.2 | 32 | 68.6 | 18 | 29.5 | .77 |
| IGFBP-I | 1,583 | 11,293.8 | 7,177 | 14,173.8 | 1,756 | 87,213.0 | .27 |
| IGFBP-III | 73,934 | 90,660.8 | 64,237 | 396,021.7 | 63,478 | 65,506.7 | .12 |
| IGF-I | 0.7 | 22.2 | 0.5 | 22.2 | 4 | 21.3 | .47 |
| IGF-II | 129 | 1,087.2 | 164 | 1,296.9 | 120 | 161.7 | .30 |
| GH | 50 | 337.6 | 34 | 45.7 | 117 | 818.6 | .06 |
| Insulin | 9 | 119.3 | 8 | 81.2 | 8 | 34.0 | .51 |
Abbreviations: BP, binding protein; GH, growth hormone; IGF, insulin-like growth factor; IGF-IR, insulin-like growth factor I receptor; PSA, prostate-specific antigen.
Two-sided Kruskal-Wallis test.
DISCUSSION
In this study, the only randomized controlled trial to our knowledge of cixutumumab in men with HSPC, cixutumumab was well tolerated, with hyperglycemia as the sole notable adverse event. However, cixutumumab did not improve the undetectable PSA rate after 28 weeks of treatment. Although these results fail to confirm preclinical evidence supporting the combination of castration with IGF-IR inhibition by cixutumumab, it is possible that the primary end point of undetectable PSA at 28 weeks does not fully capture cixutumumab efficacy. In previous preclinical xenograft studies, time to castration resistance was significantly improved, yet PSA nadir was not different when cixutumumab was added to castration.11 Time to castration resistance would be a challenging and unvalidated end point for this patient population, where early readout of efficacy is highly desirable. For that reason, the short, 28-week PSA end point was purposefully chosen, because it was established from a large prospective randomized trial, and the intention was for an early go or no-go signal to determine if a large, randomized phase III trial would be warranted.9 A major advantage for incorporating this early end point was avoiding commitment of resources and large patient numbers to a potentially inactive therapy.
It is possible that previous xenograft studies did not adequately model human disease, an important consideration in translational oncology. There were a number of differences between the xenograft studies and this clinical trial; for instance, an immunocompromised host mouse was used, the administration of cixutumumab was intraperitoneal rather than intravenous, and the xenograft only expressed human IGF-IR on the implanted tumor cells rather than in most cells of the body. The latter issue could have led to accumulation of cixutumumab on the surface of the xenograft tumor, with potential for murine immune response leading to antitumor effect. In the future, xenograft studies of this sort should consider adding a control group where an antibody against another cell surface protein ascertains whether that alone is capable of generating antitumor effect.
LuCap 35 was the original xenograft used in preclinical studies, and characteristics of this model may not represent the patient population studied in our clinical trial.12 First, LuCap 35 was derived from a patient who had received treatment with castration, flutamide, and diethylstilbestrol, whereas our trial patient population was essentially systemic-treatment naive. LuCap 35 also harbors amplification of AR. Because IGF-IR inhibition decreases AR signaling and nuclear localization, cixutumumab might work better in AR-amplified patient populations. Therefore, given patient and tumor heterogeneity, it would be prudent in future testing of novel therapeutics to study multiple xenografts rather than rely on one model.
The correlative studies in this trial had a limited number of patients contributing blood-based biomarker specimens. Of 210 randomly assigned patients, 86 (41%) had already started LHRH therapy at time of random assignment and were in the late-induction group. Although flexibility was necessary to facilitate trial accrual, no late-induction patients were eligible for correlative science studies. In addition, cooperative group trial accruals occur at many sites where biospecimen acquisition may not be commonplace. The limited sample size may have especially affected the IGF-IR–related biomarker results and may have been a major barrier to proving the pharmacodynamic effect of cixutumumab in this trial. Given the biomarker findings, it is difficult to firmly conclude that the intended molecular target was inhibited. Future trials of this sort may benefit from metastatic tissue biopsies to ascertain baseline biologic characteristics and determine if the molecular target is expressed, if the therapeutic agent reaches the intended target, and if that interaction leads to the anticipated antitumor effect.
Baseline stratified CTC count was associated with stratified PSA response at 28 weeks, although our sample size was limited to 39 patients. Regulatory approval of CTCs in prostate cancer was supported by studies in metastatic CRPC,13 whereas CTCs in HSPC have only been explored in a limited number of studies.14,15 Although baseline CTC count may have prognostic value, it is noteworthy that 41% of patients who donated biospecimens had a CTC count of 0 at baseline.
Although the primary findings from this randomized controlled trial were negative, rationale exists for other combinations with cixutumumab, such as docetaxel16 or mammalian target of rapamycin inhibitors like temsirolimus.17 It is unlikely that another large randomized controlled trial with cixutumumab or another IGF-IR mAb will be explored in prostate cancer, given the experience to date.18 In addition, the ECOG (Eastern Cooperative Oncology Group) 3805 trial showed a significant overall survival benefit with addition of docetaxel to AD for patients with metastatic HSPC.19
Patients in our trial will continue to be observed for overall survival outcomes, and a prespecified secondary end point allows for validation of the undetectable PSA correlation with overall survival. This will be of great interest, because results from SWOG 9346 were reported before the era of at least five new survival-prolonging therapies in metastatic CRPC, all only commercially available since 2010.20 Should the prognostic value of the undetectable PSA model be validated in our trial, future testing of novel therapeutics in the metastatic HSPC setting will be facilitated with this earlier readout.
Supplementary Material
Acknowledgment
We thank Hung-Ming Lam and Valeri Vasioukhin for critical comments on the manuscript.
Glossary Terms
- androgen deprivation therapy (ADT):
treatment that suppresses or blocks the production or action of male hormones.
- androgen receptor:
a DNA-binding and hormone-activated transcription factor important to the development and progression of prostate cancer. Its primary ligand is dihydrotestosterone. In later-stage (castration-resistant) prostate cancer, oncogenic alterations such as androgen receptor overexpression allow the androgen receptor to continue signaling despite undetectable, or castrate, levels of serum testosterone.
- circulating tumor cells:
demonstration of isolated tumor cell circulation/dissemination in the peripheral blood.
- IGF-1R (insulin-like growth factor-1 receptor):
a tyrosine kinase receptor that protects several cell types from apoptotic injuries by way of the activation of PI3K, Akt/PKB, and phosphorylation of BAD (leading to its inactivation). IGF-1R also mediates regulation of angiogenic factors in tumor cells.
- metastatic castration-resistant prostate cancer (mCRPC):
progressive disease despite surgical castration or ongoing use of gonadotropin-releasing hormone agonists with confirmed castrate levels of testosterone.
- prostate-specific antigen (PSA):
a protein produced by cells of the prostate gland. The blood level of prostate-specific antigen (PSA) is used as a tumor marker for men who may be suspected of having prostate cancer. Most physicians consider 0 to 4.0 ng/mL to be the normal range. Levels of 4 to 10 and 10 to 20 ng/mL are considered slightly and moderately elevated, respectively. PSA levels have to be complemented with other tests to make a firm diagnosis of prostate cancer.
- xenograft:
host graft from a species that is not related to the recipient.
Appendix
Table A1.
IGF-IR Biomarker Percent Change From Baseline to Week 12
| Biomarker Measure (% Δ) | AD Plus Cixutumumab (n = 18) |
AD Alone (n = 9) |
P* | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| C-peptide† | 12 | 376.9 | −17 | 560.0 | .67 |
| IGFBP-I† | −24 | 117.5 | 33 | 622.3 | .29 |
| IGFBP-III† | 11 | 216.5 | −4 | 77.4 | .15 |
| IGF-I | 4 | 2,137.7 | 4 | 222.1 | .98 |
| IGF-II | −2 | 172.7 | −10 | 112.8 | .21 |
| GH† | 77 | 1,672.0 | −23 | 536.2 | .53 |
| Insulin | −2 | 239.4 | −1 | 128.8 | .82 |
Abbreviations: AD, androgen deprivation; BP, binding protein; GH, growth hormone; IGF, insulin-like growth factor; IGF-IR, insulin-like growth factor I receptor.
Two-sided Wilcoxon rank sum test.
n = 17.
Footnotes
Supported in part by National Cancer Institute, National Institutes of Health, Cooperative Group Grants No. CA180888, CA180819, CA180818, CA180828, CA46368, CA180801, CA180835, CA35421, CA180834, CA142559, CA35281, CA35090, CA37981, CA45807, CA46282, CA180846, CA180830, CA35431, CA58416, CA63848, CA63844, CA12644, CA11083, CA35178, CA67575, CA 45808, Pacific Northwest SPORE 2 P50 CA097186-06, P01 CA85859, P01 CA163227, and 5K12CA086913-13; by ImClone Systems (subsidiary of Eli Lilly); and by Veridex (Janssen Diagnostics/Johnson & Johnson).
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Presented at the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01120236.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Evan Y. Yu, Celestia S. Higano, Nicholas J. Vogelzang, Steve R. Plymate, Maha Hussain, Catherine M. Tangen, Ian M. Thompson
Financial support: Evan Y. Yu
Administrative support: Evan Y. Yu, David I. Quinn, Ian M. Thompson
Provision of study materials or patients: Evan Y. Yu, Celestia S. Higano, Neeraj Agarwal, Sumanta K. Pal, Elisabeth I. Heath, Shilpa Gupta, Kim N. Chi, Nicholas J. Vogelzang, David I. Quinn, Maha Hussain
Collection and assembly of data: Evan Y. Yu, Hongli Li, Ajjai Alva, Michael B. Lilly, Yoshio Inoue, David I. Quinn, Heather H. Cheng, Catherine M. Tangen
Data analysis and interpretation: Evan Y. Yu, Hongli Li, Celestia S. Higano, Neeraj Agarwal, Sumanta K. Pal, Elisabeth I. Heath, Elaine T. Lam, Shilpa Gupta, Kim N. Chi, David I. Quinn, Heather H. Cheng, Steve R. Plymate, Catherine M. Tangen, Ian M. Thompson
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
SWOG S0925: A Randomized Phase II Study of Androgen Deprivation Combined With Cixutumumab Versus Androgen Deprivation Alone in Patients With New Metastatic Hormone-Sensitive Prostate Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Evan Y. Yu
Honoraria: Amgen, Bayer, Dendreon, Janssen Pharmaceuticals, Medivation, sanofi-aventis
Consulting or Advisory Role: Amgen, Dendreon, Janssen Pharmaceuticals, Medivation, sanofi-aventis
Research Funding: Agensys (Inst), Astellas Pharma (Inst), Bristol-Myers Squibb (Inst), Dendreon (Inst), GTx (Inst), ImClone Systems (Inst), Janssen Pharmaceuticals (Inst), Oncogenex (Inst), Genentech/Roche (Inst)
Travel, Accommodations, Expenses: Dendreon, Janssen Pharmaceuticals, Medivation, Amgen, sanofi-aventis
Hongli Li
No relationship to disclose
Celestia S. Higano
Employment: Cell Therapeutics (I)
Leadership: Cell Therapeutics (I)
Stock or Other Ownership: Cell Therapeutics (I)
Consulting or Advisory Role: Dendreon, Bayer, Medivation, Novartis, BHR Pharma, Ferring, Johnson & Johnson, AbbVie, Genentech, Astellas, Pfizer, Foreseeacer Pharmaceuticals, Oncology Trials Insights, Orion Pharma, sanofi-aventis, Tolmar Pharmaceuticals, Exelixis, Chiltern International, Aragon Pharmaceuticals, Astellas Pharma
Research Funding: Algeta/Bayer (Inst), Aragon Pharmaceuticals (Inst), AstraZeneca (Inst), Dendreon (Inst), Genentech (Inst), Medivation (Inst), Millennium Pharmaceuticals (Inst), sanofi-aventis (Inst), TEVA Pharmaceuticals Industries (Inst), Exelixis (Inst), Emergent BioSolutions (Inst), Oncogenex (Inst), Novartis (Inst), ImClone Systems (Inst)
Travel, Accommodations, Expenses: AbbVie, Aragon Pharmaceuticals, Astellas Pharma, Bayer, Chiltern International, Dendreon, Exelixis, Genentech, Johnson & Johnson, Medivation, Novartis, Orion Pharma, Pfizer, sanofi-aventis, TEVA Pharmaceuticals Industries
Neeraj Agarwal
Research Funding: Amgen (Inst), Bayer (Inst), Bristol-Myers Squibb (Inst), GlaxoSmithKline (Inst), MedImmune (Inst), Takeda Pharmaceuticals (Inst), Pfizer (Inst), TRACON Pharma (Inst), Active Biotech (Inst), Exelixis (Inst), Prometheus (Inst), Bavarian Nordic (Inst), Medivation (Inst), Novartis (Inst)
Sumanta K. Pal
Honoraria: Novartis, Medivation
Consulting or Advisory Role: Pfizer, Novartis, AVEO Pharmaceuticals, Myriad Genetics
Research Funding: Medivation
Ajjai Alva
No relationship to disclose
Elisabeth I. Heath
Honoraria: Bayer Diagnostics, Dendreon
Research Funding: Tokai (Inst), Seattle Genetics (Inst), Agensys (Inst), Dendreon (Inst), Genentech/Roche (Inst), Millennium Pharmaceuticals (Inst)
Elaine T. Lam
Research Funding: Exelixis (Inst), Janssen Pharmaceuticals (Inst), Astellas Pharma (Inst), Bayer (Inst)
Shilpa Gupta
No relationship to disclose
Michael B. Lilly
No relationship to disclose
Yoshio Inoue
No relationship to disclose
Kim N. Chi
Honoraria: Eli Lilly
Consulting or Advisory Role: Eli Lilly
Research Funding: Eli Lilly (Inst)
Nicholas J. Vogelzang
Employment: US Oncology
Stock or Other Ownership: Caris Life Sciences
Honoraria: DAVA Oncology, Mannkind, UpToDate, AbbVie, Bavarian Nordic, Endocyte
Consulting or Advisory Role: Janssen Biotech, Amgen, AVEO Pharmaceuticals, BIND Biosciences
Speakers' Bureau: Medivation, Dendreon, Bayer, Caris Life Sciences, Millennium Pharmaceuticals, sanofi-aventis, GlaxoSmithKline, Novartis, Pfizer
Research Funding: PAREXEL International (Inst), Progenics (Inst), Exelixis (Inst), US Oncology (Inst), Viamet Pharmaceuticals (Inst), Endocyte (Inst), GlaxoSmithKline (Inst), Merck (Inst), Genentech/Roche (Inst)
Travel, Accommodations, Expenses: Genentech/Roche, Celgene, US Oncology, Dendreon, Novartis, Pfizer, Bayer/Onyx Pharmaceuticals, Exelixis, Research to Practice, Johns Hopkins Greenberg Bladder Cancer Institute
David I. Quinn
Honoraria: Astellas Pharma, Bayer, Janssen Oncology, Medivation, Novartis, sanofi-aventis, Dendreon
Consulting or Advisory Role: Astellas Pharma, Bayer, Janssen Oncology, Medivation, Novartis, sanofi-aventis, Dendreon
Research Funding: Astellas Pharma, Bayer, Janssen Oncology, Medivation, Novartis, Dendreon, Millennium, sanofi-aventis
Expert Testimony: TEVA Pharmaceuticals Industries, Medivation
Heather H. Cheng
No relationship to disclose
Steve R. Plymate
Honoraria: ESSA Pharma, Astellas Pharma, Medvation
Consulting or Advisory Role: ESSA Pharma
Maha Hussain
Research Funding: EMD Serono (Inst), Eli Lilly/ImClone Systems (Inst), Genentech (Inst)
Catherine M. Tangen
No relationship to disclose
Ian M. Thompson
Consulting or Advisory Role: Exosome Diagnostics, Mag Force, OncocellMDX
Patents, Royalties, Other Intellectual Property: Involved in establishment of new company—NanoTX Therapeutics—to commercialize novel therapy for glioblastoma for our cancer center; on board of directors, which has intellectual property developed by our cancer center; several patents with colleagues involving novel biomarkers for cancer and two devices for sexual dysfunction and urinary incontinence (no revenues at this time; our university intellectual property office is working with industry to determine if these can be commercialized)
Travel, Accommodations, Expenses: Oncocell MDX
REFERENCES
- 1.Wu J, Yu E. Insulin-like growth factor receptor-1 (IGF-IR) as a target for prostate cancer therapy. Cancer Metastasis Rev. 2014;33:607–617. doi: 10.1007/s10555-013-9482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samani AA, Yakar S, LeRoith D, et al. The role of the IGF system in cancer growth and metastasis: Overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 3.Wu JD, Haugk K, Woodke L, et al. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J Cell Biochem. 2006;99:392–401. doi: 10.1002/jcb.20929. [DOI] [PubMed] [Google Scholar]
- 4.Wu JD, Odman A, Higgins LM, et al. In vivo effects of the human type I insulin-like growth factor receptor antibody A12 on androgen-dependent and androgen-independent xenograft human prostate tumors. Clin Cancer Res. 2005;11:3065–3074. doi: 10.1158/1078-0432.CCR-04-1586. [DOI] [PubMed] [Google Scholar]
- 5.Higano CS, Alumkal JJ, Ryan CJ, et al. A phase II study evaluating the efficacy and safety of single agent IMC A12, a monoclonal antibody, against the insulin-like growth factor-1 receptor, as monotherapy in patients with metastatic, asymptomatic castration-resistant prostate cancer. J Clin Oncol. 2009;27(suppl):269s. abstr 5142. [Google Scholar]
- 6.Higano CS, Alumkal JJ, Ryan CJ, et al. A phase II study of cixutumumab (IMC-A12), a monoclonal antibody against the insulin-like growth factor 1 receptor (IGF-IR), monotherapy in metastatic castration-resistant prostate cancer: Feasibility of every 3-week dosing and updated results. J Clin Oncol. 2010;(suppl):28. abstr 189. [Google Scholar]
- 7.Dean JP, Sprenger CC, Wan J, et al. Response of the insulin-like growth factor (IGF) system to IGF-IR inhibition and androgen deprivation in a neoadjuvant prostate cancer trial: Effects of obesity and androgen deprivation. J Clin Endocrinol Metab. 2013;98:E820–E828. doi: 10.1210/jc.2012-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi KN, Gleave ME, Fazli L, et al. A phase II pharmacodynamic study of preoperative figitumumab in patients with localized prostate cancer. Clin Cancer Res. 2012;18:3407–3413. doi: 10.1158/1078-0432.CCR-12-0482. [DOI] [PubMed] [Google Scholar]
- 9.Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: Data from Southwest Oncology Group trial 9346 (INT-0162) J Clin Oncol. 2006;24:3984–3990. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 10.Cheng HH, Mitchell PS, Kroh EM, et al. Circulating microRNA profiling identifies a subset of metastatic prostate cancer patients with evidence of cancer-associated hypoxia. PLoS One. 2013;8:e69239. doi: 10.1371/journal.pone.0069239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plymate SR, Haugk K, Coleman I, et al. An antibody targeting the type I insulin-like growth factor receptor enhances the castration-induced response in androgen-dependent prostate cancer. Clin Cancer Res. 2007;13:6429–6439. doi: 10.1158/1078-0432.CCR-07-0648. [DOI] [PubMed] [Google Scholar]
- 12.Corey E, Quinn JE, Buhler KR, et al. LuCaP 35: A new model of prostate cancer progression to androgen independence. Prostate. 2003;55:239–246. doi: 10.1002/pros.10198. [DOI] [PubMed] [Google Scholar]
- 13.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 14.Resel Folkersma L, San José Manso L, Galante Romo I, et al. Prognostic significance of circulating tumor cell count in patients with metastatic hormone-sensitive prostate cancer. Urology. 2012;80:1328–1332. doi: 10.1016/j.urology.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Goodman OB, Jr, Symanowski JT, Loudyi A, et al. Circulating tumor cells as a predictive biomarker in patients with hormone-sensitive prostate cancer. Clin Genitourin Cancer. 2011;9:31–38. doi: 10.1016/j.clgc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Wu JD, Haugk K, Coleman I, et al. Combined in vivo effect of A12, a type 1 insulin-like growth factor receptor antibody, and docetaxel against prostate cancer tumors. Clin Cancer Res. 2006;12:6153–6160. doi: 10.1158/1078-0432.CCR-06-0443. [DOI] [PubMed] [Google Scholar]
- 17.Rathkopf DE, Danila DC, Chudow JJ, et al. Anti-insulin-like growth factor-1 receptor (IGF-IR) monoclonal antibody cixutumumab plus mammalian target of rapamycin (mTOR) inhibitor temsirolimus in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(suppl):41s. abstr TPS242. [Google Scholar]
- 18.de Bono JS, Piulats JM, Pandha HS, et al. Phase II randomized study of figitumumab plus docetaxel and docetaxel alone with crossover for metastatic castration-resistant prostate cancer. Clin Cancer Res. 2014;20:1925–1934. doi: 10.1158/1078-0432.CCR-13-1869. [DOI] [PubMed] [Google Scholar]
- 19.Sweeney C, Chen YH, Carducci MA, et al. Impact on overall survival with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer: An ECOG-led phase III randomized trial. J Clin Oncol. 2014;32(suppl 15s):3s. abstr LBA2. [Google Scholar]
- 20.Cheng HH, Lin DW, Yu EY. Advanced clinical states in prostate cancer. Urol Clin North Am. 2012;39:561–571. doi: 10.1016/j.ucl.2012.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.