Abstract
Background
A shortcoming of the pediatric electrocardiogram (ECG) appears to be its inability to accurately detect left ventricular hypertrophy (LVH). This study prospectively assesses the usefulness of the pediatric ECG as a screening modality for LVH.
Methods
Concomitant echocardiograms and ECGs from a large cohort of children who were exposed to the human immunodeficiency virus (HIV; uninfected) and children who were infected with HIV were compared. By use of the values of Davignon et al, qualitative determination of LVH and quantitative criteria for LVH (RV6, SV1, RV6+SV1, QV6, and QIII >98% for age, R/SV1 <98% for age, and [−]TV6) were compared to body surface area adjusted for left ventricular (LV) mass z score. Results were then stratified according to weight and weight-for-height z scores. New age-adjusted predicted values were then constructed from children of a mixed race who were HIV-uninfected, ≤6 years old, and similarly assessed.
Results
The sensitivity rate was <20% for detecting increased LV mass, irrespective of HIV status; the specificity rate was 88% to 92%. The sensitivity rate of the individual criteria ranged from 0 to 35%; the specificity rate was 76% to 99%. Test sensitivities remained low when stratified by weight and weight-for-height z scores. Areas under the receiver operator characteristic curves were between 0.59 and 0.70, also suggesting poor accuracy of the ECG criteria. By use of new age-adjusted predicted values, the sensitivity rate decreased to <17%, and the specificity rate increased to 94% to 100%.
Conclusion
The ECG is a poor screening tool for identifying LVH in children. Sensitivity is not improved with revision of current criteria.
Noninvasive, cost-effective, and efficient, the electrocardiogram (ECG) is invaluable in the diagnosis and management of congenital and acquired heart disease. One of the major shortcomings of the ECG, however, appears to be its inability to accurately detect left ventricular hypertrophy (LVH) in children. Currently, the gold standard for LVH detection is a left ventricular (LV) mass >2 z-scores for body surface area (BSA), as defined by m-mode echocardiography. The purpose of this study was to prospectively assess the accuracy of the ECG in detecting LVH among participants in the multicenter Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection (P2C2 HIV) study. This is the first large-scale prospective study to address this issue in children of mixed race. It assesses the validity of the ECG as a screening tool in children without known congenital or rheumatic heart disease and takes into consideration constitutional variations. This study also revises currently accepted ECG criteria for determination of LVH in young children and compares these data with the criteria currently in use.
Methods
The multicenter P2C2 HIV study was designed to assess the incidence of heart and lung disease in children with vertically acquired human immunodeficiency virus (HIV) infection. Children were enrolled at 5 centers: Baylor College of Medicine/Texas Children’s Hospital; Children’s Hospital/Harvard Medical School; Mount Sinai School of Medicine; Columbia-Presbyterian Medical Center/Babies and Children’s Hospital; and the University of California, Los Angeles School of Medicine. Details of the study design have been previously described.1
Two cohorts of children were studied: the first group consisted of infants and children with documented HIV infection at the time of enrollment, and the second group consisted of infants born to mothers who were HIV-infected. Group I (the cohort of children known to be HIV-infected at enrollment) consisted of 205 children enrolled between May 1990 and April 1993; the median age at enrollment was 23 months (range 0.1–14.0 years). Group II (the neonatal cohort) consisted of 443 infants enrolled during fetal life and 168 infants enrolled in the first month of life who entered the study between May 1990 and January 1994. Follow-up continued through January 1997. Ninety-three infants in the neonatal group became HIV-infected (group IIa); 463 infants were HIV-uninfected (group IIb); 11 infants died in utero, and 44 infants had an indeterminate HIV status. Approximately half of the infants who were determined to be free of HIV infection were randomly selected to remain in the study as a control group.1 Most of the children were black (49.3%, 375/761), Hispanic (33.1%, 252/761), or white (12.8%, 97/761).
The initial intent of the P2C2 HIV study was to recruit a control sample of children from mothers who were HIV-negative and had a similar socioeconomic status, but this was not feasible. Instead, we included data from an external control sample consisting of 164 healthy infants and children without known cardiac disease who were of a similar age as the P2C2 HIV cohorts. These children represent a subset of patients previously reported by Khan et al2 whose ECGs and echocardiograms were performed on the same day.
Echocardiograms were performed every 4 to 6 months at the 5 clinical centers by use of a standardized protocol. M-mode strips were forwarded to Boston Children’s Hospital for centralized digitization. LV mass (in grams) was calculated by the method of Devereux et al.3 Normative values for LV mass by BSA were developed by use of data from 285 healthy children measured at the same central digitizing facility as the study patient data.4 To adjust for the changes in LV size associated with growth, z scores were calculated for all children by taking each LV mass measure, subtracting the BSA-appropriate mean, and dividing by 1 SD. Therefore, a z score of 0 represents a measurement equal to the normal mean value for the child’s BSA, whereas a z score of +2 represents a measurement 2 SD higher than average for BSA.
Twelve- or 15-lead ECGs were performed at enrollment and annually. ECGs were interpreted at each center on the basis of published normal ECG standards for infants and children by Davignon et al.5 Initially, through October 1993, actual amplitude measurements were recorded only for RV6 and SV1 and only when LVH was detected with the ECG. From November 1993 to January 1997, amplitude measurements for all ECG criteria reported by Davignon et al were recorded for all ECG studies. This report is limited to measurements of RV6, SV1, QV6, QIII, (−)TV6, RV6+SV1, and R/S V1. An ECG quality control review was performed in 1995 to evaluate agreement on the ECG LVH diagnosis and the reliability of amplitude measurements.6
To determine the diagnostic usefulness of the ECG in detecting LVH, 2 data analysis files were used. In the first, all echocardiograms and ECGs performed within 3 months of each other were compared. In the second, only a single ECG/echocardiogram data pair for each child was used. For children with an increased LV mass, the first data pair with documented echocardiographic evidence was selected; this was done to allow a maximal number of LVH comparisons. Otherwise, the first ECG/echocardiogram pair performed was used.
Statistical analyses
The cumulative incidence of ECG LVH was estimated by use of the Kaplan-Meier method. ECG LVH rates were compared between children who were HIV-infected and children who were HIV-uninfected by use of the log-rank test. Longitudinal analyses of RV6 and SV1 were performed with SAS Proc Mixed (SAS Institute, Cary, NC), which provided estimates and 95% CIs for the means according to HIV group and age. Rates of change for each ECG amplitude outcome were obtained by use of a mixed-effects model that specified that amplitude measurements follow a linear regression with age, with random slope and intercept for each child who was HIV-uninfected. A similar linear regression model was fit for LV mass versus RV6 and LV mass versus SV1 adjusted for age and BSA. The test sensitivity and specificity and standard errors for ECG LVH and amplitude criteria were estimated by the method described by Obuchowski.7 The predictive power of logistic regression models on the basis of each ECG criterion was summarized by constructing receiver operator characteristic curves and comparing the areas under these curves.
Quality control
A quality control review of 45 ECGs was conducted by the Clinical Coordinating Center in the fall of 1995.6 Each ECG was read by one central reviewer and compared with the original ECG. The observed and chance agreement rates for ECG LVH were 75.6% and 53.6%, respectively, with a κ statistic of 0.48 for the diagnosis of LVH by ECG, which is in the moderate agreement range.8 The amplitude measurements for RV6 and SV1 had intraclass correlation coefficients of 0.95 and 0.86, respectively, confirming agreement among the centers. The intraclass correlation coefficients for QV6, QIII, and (−)TV6 were similarly acceptable, at 0.81, 0.84, and 0.77, respectively.
Results
Incidence of ECG LVH
The incidence of ECG LVH increased with time in both cohorts. The 1-year cumulative incidence of ECG LVH was 14.1% for the infants who were HIV-infected and 11.0% for the infants who were HIV-uninfected. By the time the children were aged 4 years, the cumulative incidence of ECG LVH had increased to 36.2% and 32.5%, respectively. The ECG LVH rates over time were not statistically different (P = .21) between the groups. Among the older HIV-infected cohort, the prevalence of ECG LVH at the time of the first ECG was 12.9% (26/201), and the 4-year cumulative incidence of ECG LVH was 35.2%.
Longitudinal changes in ECG voltages
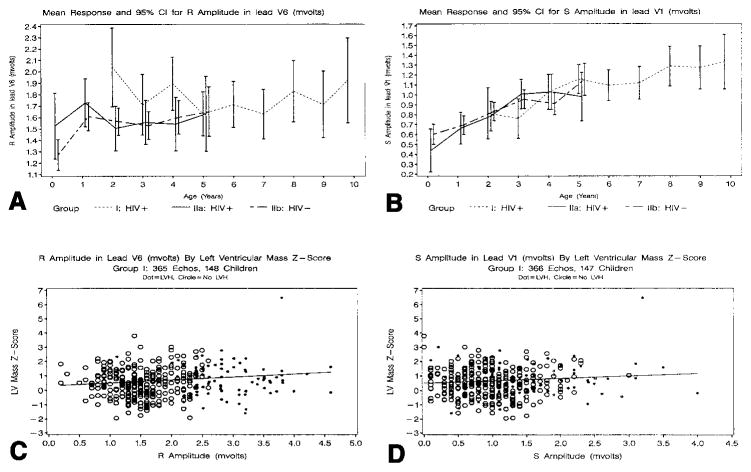
Figure 1, A and B, summarizes the data for RV6 and SV1 by age. In the neonatal cohort, there was an initial rise in mean RV6 between birth and 1 year of age and a very gradual increase in mean RV6 between ages 2 and 5 years, regardless of HIV status. In the older HIV-infected cohort, sparse data precluded an accurate estimation of the mean for RV6 and SV1 before age 2 years, but mean RV6 did not change substantially between ages 2 and 10 years. Mean SV1 increased linearly with age in both groups, irrespective of HIV status.
Figure 1.
Longitudinal changes in mean R amplitude in lead V6 (A) and mean S amplitude in lead V1 (B) according to HIV group and age. The vertical bars indicate the 95% CIs for the mean. A and B, RV6 and SV1, respectively, in mV for the neonatal cohort (n = 77 HIV-infected, group IIa; and n = 251 HIV-uninfected, group IIb) and for the older cohort (n = 106, group I). Scatterplots and rates of change for LV mass z scores versus RV6 (C) and LV mass z scores versus SV1 (D) for the older HIV-infected cohort (n = 148 and 147 children, respectively). Solid dots indicate LVH on ECG and open circles indicate no LVH on ECG.
LV mass correlated with ECG voltages
In the neonatal cohort, a significant linear relationship was not demonstrated between LV mass and RV6 after adjusting for BSA and age. The association between LV mass and SV1 was also weak. In the older HIV-infected cohort, however, there was a significant linear relationship between LV mass z score and RV6 (P = .006 for comparing the slope to zero) and between LV mass z score and SV1 (P = .03) after adjusting for BSA and age. Although these relationships were statistically significant, the associations were weak, as indicated in Figure 1, C and D.
ECG detection of LV hypertrophy: Results from the P2C2 cohort
Table I summarizes the LV mass and ECG LVH data. The sensitivity rate of the ECG as a means of detecting increased LV mass was poor, ranging from 18% to 20% for all P2C2 participants combined. A slightly higher sensitivity rate was noted among neonates (22%–40%) compared with older participants (14%–18%). The sensitivity rate for the external control subjects was 17%. Table II indicates similarly weak sensitivity rate estimates (≤35%) for the 7 individual ECG LVH voltage criteria among all P2C2 participants. Sensitivity rate estimates for ECG LVH remained low when calculated by race. Sensitivity rate estimates for RV6 and SV1 were low (<20%) for the external control subjects (data not shown).
Table I.
Diagnostic utility of ECG determination of LVH
| ECG/echo study pairs | ↑ LV mass, z score >2* (echo) | LVH† (ECG) | (Sensitivity ± SE) | (Specificity ± SE) | |
|---|---|---|---|---|---|
| All P2C2 children (n = 761‡) | |||||
| First pair | 663 | 57 (8.6%) | 56 (8.4%) | 10/57 (17.5 ± 5.0) | 560/606 (92.4 ± 1.1) |
| All pairs | 1688 | 71 (4.2%) | 205 (12.1%) | 14/71 (19.7 ± 4.4) | 1426/1617 (88.2 ± 1.1) |
| HIV+ (older cohort) | |||||
| First pair | 186 | 43 (23.1%) | 26 (14.0%) | 6/43 (14.0 ± 5.3) | 123/143 (86.0 ± 2.9) |
| All pairs | 601 | 56 (9.3%) | 91 (15.1%) | 10/56 (17.9 ± 4.6) | 464/545 (85.1 ± 2.3) |
| HIV+ (neonatal cohort) | |||||
| First pair | 84 | 5 (6.0%) | 13 (15.5%) | 2/5 (40.0 ± 21.9) | 68/79 (86.1 ± 3.9) |
| All pairs | 242 | 6 (2.5%) | 34 (14.0%) | 2/6 (33.3 ± 23.2) | 204/236 (86.4 ± 3.8) |
| HIV− (neonatal cohort) | |||||
| First pair | 393 | 9 (2.3%) | 17 (4.3%) | 2/9 (22.2 ± 13.9) | 369/384 (96.1 ± 1.0) |
| All pairs | 845 | 9 (1.1%) | 80 (9.5%) | 2/9 (22.2 ± 14.7) | 758/836 (90.7 ± 1.2) |
| External controls | |||||
| All data | 164 | 23 (14.0%) | 21 (12.8%) | 4/23 (17.4 ± 7.9) | 124/141 (87.9 ± 2.7) |
Gold standard for LVH: BSA-adjusted LV mass z score >2.
Diagnostic criteria for LVH included RV6 and/or SV1 exceeding the 98th percentile for age in absence of a biphasic QRS complex.
Ninety-eight of the 761 children never had an ECG performed within 3 months of an echocardiogram.
Table II.
Diagnostic utility of standard ECG amplitude criteria (Davignon et al*) for the determination of LVH in children on the basis of the first ECG/echo data pair
| ECG criteria | No. | ↑ LV mass, z score >2 (Echo) | LVH (ECG) | Sensitivity | Specificity |
|---|---|---|---|---|---|
| RV6 (%) | 443 | 34 (7.7) | 88 (19.9) | 12/34 (35.3) | 333/409 (81.4) |
| SV1 (%) | 442 | 34 (7.7) | 22 (5.0) | 5/34 (14.7) | 391/408 (95.8) |
| RV6 + SV1 (%) | 442 | 34 (7.7) | 43 (9.7) | 5/34 (14.7) | 370/408 (90.7) |
| QV6 (%) | 444 | 34 (7.7) | 107 (24.1) | 9/34 (26.5) | 312/410 (76.1) |
| QIII (%) | 417 | 27 (6.5) | 39 (9.4) | 0/27 (0.0) | 351/390 (90.0) |
| (−)TV6 (%) | 416 | 27 (6.5) | 5 (1.2) | 1/27 (3.7) | 385/389 (99.0) |
| R/S V1 (%) | 411 | 25 (6.1) | 4 (1.0) | 0/25 (0.0) | 382/386 (99.0) |
Amplitude data available on 67% (444/663) of children with qualitative assessment (present or absent) of ECG LVH.
RV6, SV1, RV6 + SV1, QV6 and QIII >98th percentile for age, R/S V1 <2nd percentile for age and (−) TV6 (negative).
LV posterior wall thickness was independently assessed. The sensitivity rate was no greater than that for LV mass, ranging from 16% to 18% for all participants combined, with equally poor sensitivity rates for each of the 7 individual voltage criteria (data not shown). The influence of pericardial effusion on ECG voltages was also considered. Adequate numbers, however, could not be obtained because the 5-year cumulative incidence of significant effusion (≥5 mm) was only 2.8% and 5.4%, respectively, in the older and neonatal HIV-infected cohorts.
Individual ECG criteria for LVH: Results from the P2C2 cohort
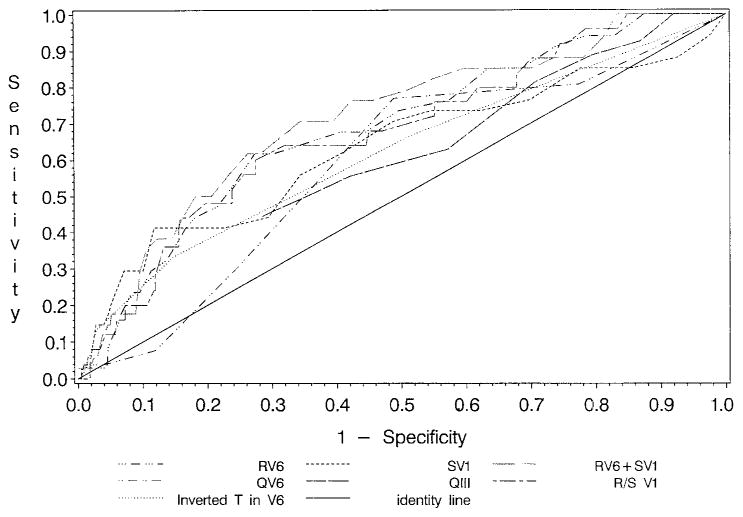
Receiver operator characteristic analyses were performed on each of the 7 criteria for ECG LVH. Results demonstrate that RV6 and SV1 have little usefulness in the identification of increased LV mass, with areas under the curve of 0.69 and 0.63, respectively. Cutoff points yielding the largest combined sensitivity (61.8, 73.5) and specificity (69.7, 45.3) were 1.87 mV for RV6 and 0.62 mV for SV1. Areas under the curve were also low for QV6, QIII, R/S V1, and (−)TV6 (0.59, 0.59, 0.68, and 0.62, respectively). The area under the curve was greatest at 0.71 for RV6+SV1. The identity line (area under the curve = 0.50) lies along the major diagonal where the true- and false-positive proportions are equal, and any ECG criterion can achieve this performance by chance alone. Both Figure 2 and Table II provide unimpressive estimates of sensitivity for all 7 ECG criteria as diagnostic tools for detecting echocardiographic LVH.
Figure 2.
Receiver operator characteristics curves for each LVH amplitude criterion. An receiver operator characteristic curve is a plot of the true-positive proportion (sensitivity) against the false-positive proportion (1−specificity) for various possible values for each ECG criterion. None of the ECG criteria provided strong discrimination of LVH on the basis of the echocardiogram LV mass z score.
ECG determination of LV hypertrophy: Influence of constitutional variables
The diagnostic usefulness of ECG LVH by baseline weight z scores and weight-for-height z scores was evaluated by use of the first ECG/echocardiogram data pair within 3 months of enrollment in group I. Sparse data in group II precluded a meaningful analysis, because few infants (0–3 months) had an increased LV mass. Test sensitivity rates remained low for ECG LVH among children with baseline weight z scores <−1 SD and for children with baseline weight z scores >−1 SD (data not shown). Low test sensitivities were also found when stratified by weight-for-height z scores.
New age-adjusted predicted values and prediction intervals for children uninfected by HIV
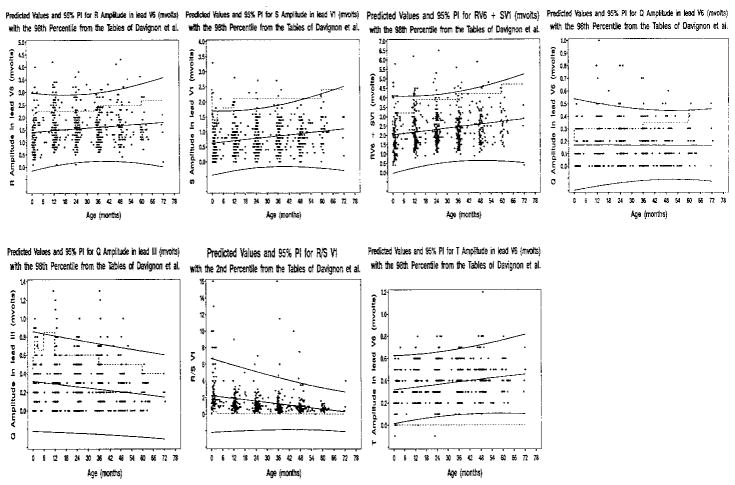
New age-adjusted predicted values with 95% prediction intervals for each of the 7 individual ECG criteria for LVH were established for children who were HIV-uninfected, between the ages of 0 and 6 years, and without increased LV mass (Figure 3). Results are plotted in Figure 3. RV6, SV1, (−)TV6, and RV6+SV1 increased linearly with age; R/SV1 and QIII decreased linearly with age; and QV6 showed no linear association with age. The 95% prediction intervals and 98th percentiles of Davignon et al5 were similar for SV1. The upper bound for the 95% prediction interval was higher than the 98th percentiles from Davignon’s tables for RV6, suggesting an over-representation of false-positive results with currently used criteria. By use of the new voltage criteria, estimates of sensitivity remained low (0–16%). The specificity rate increased to 94% to 100%.
Figure 3.
Scatterplots, rates of change versus age, and 95% prediction intervals for each of the 7 ECG criteria for LVH. The linear regression and 95% prediction intervals were calculated by using 250 children who were HIV-uninfected without increased LV mass and who were observed from birth. The dashed lines identify the 98th or 2nd percentiles from Davignon et al.4 The regression equations are available from the authors at keasle2@sph.emory.edu.
Discussion
Cardiovascular abnormalities including LV dilation, dilated cardiomyopathy, congestive heart failure, and arrhythmias have been described in children with HIV.4,6,9 Among these, LV dilation is the most common occurrence, and it may be related to anemia, afterload excess, and/or direct effects of the virus. The result is compensatory LVH, which results in a persistent elevation of LV peak wall stress.4 The increased incidence of increased LV mass among children who are HIV-infected makes them an ideal group for assessing LVH screening modalities. We compared the echocardiographic data in this cohort with corresponding ECG data to determine the usefulness of the ECG in assessing LV mass. Results of this study demonstrate conclusively that the ECG is a poor predictor of echocardiographic LVH.
Normal ECG standards for infants and children were initially established by Ziegler et al10 in 1951 and revised by Davignon et al5 in 1979 on the basis of measurements of 2141 Caucasian children aged 0 to 16 years in Quebec, Canada.5 These standards have been invaluable in the interpretation of pediatric ECGs; however, standards for the interpretation of ECG LVH have traditionally been less accurate than other voltage measurements.
In this study, among all 843 echocardiogram/ECG study pairs in infants and children who were HIV-infected, increased LV mass was present in 7.4% of the echocardiograms and ECG LVH was identified in 14.8% of the ECGs. Only 12 of the 62 children with abnormal echocardiograms, however, also had an abnormal ECG (sensitivity, 19.4%). More than 80% of the ECG results were interpreted as normal. The specificity rate was higher at 85.5%; nonetheless, in approximately 15% of ECGs, LVH was incorrectly identified in children with a normal LV mass. Results were similar for the 845 study pairs in the children who were HIV-uninfected, with a sensitivity rate of 22.2% and a specificity rate of 90.7%.
Data from this study are similar to those noted in an abstract by Khan et al.2 In their study, ECG and M-mode data from 500 consecutive children with same-day tests were assessed. Nine criteria (left axis deviation, RV6 >98%, SV1 >98%, R/SV1 <2%, QV6 = 0, QV6 >98%, QV6 >500 mV, [−]TV6, and abnormal T axis) were individually assessed. The sensitivity rate for each criterion ranged from 2% to 22%; the specificity rate ranged from 87% to 96%. Allowing for a positive finding among any of the 9 criteria to indicate LVH, the sensitivity rate increased to 56% and the specificity rate decreased to 51%. Requiring at least 4 positive criteria to constitute a positive test decreased the sensitivity rate to 6% and improved the specificity rate to 99%. An abstract from Urbina et al documents further supportive evidence from the Bogalusa Heart Study.11
Sensitivity of the ECG for detecting increased LV mass appears to be greater in children with pressure-or volume-loading conditions caused by congenital heart disease. Fogel et al compared ECGs with LV mass determined by echocardiography in 19 patients with severe aortic stenosis, 12 patients with hemodynamically significant ventricular septal defects, and 21 healthy control subjects.12 Voltages assessed for ECG LVH included SV1, RV6, SV1+RV6, QV6, and (−)TV6. The sensitivity rate for patients with aortic stenosis was 67% and that for patients with ventricular septal defects was 60%; no correlation was found between voltages and hemodynamic or anatomic data. Similar higher sensitivity rates have been noted in children with rheumatic heart disease, depicted in a study by Sastroasmoro et al.13 ECG evidence of LVH was noted in 49% of 84 children, and increased LV mass was found in 56%. The sensitivity rate of the ECG for detecting LVH was 68%, and the specificity rate was 76%.
The usefulness of the ECG in determining LVH has also been evaluated in children with hypertrophic cardiomyopathy. In a paper by Panza and Maron, 38 affected children ≥1 year old were reviewed. ECG voltages were assessed in 3 ways: the tallest R wave plus the deepest S wave in the standard leads; SV1 plus RV5 or RV6 (the greater of the 2 voltages); and SV1, SV2, RV5, or RV6 (the greater of the 4 voltages).14 Q waves and repolarization abnormalities were also assessed. Despite the evolution of LVH with echocardiography, ECG voltages did not reflect an increasing LV mass; findings were similar to those in our study. Of interest in the paper on hypertrophic cardiomyopathy, however, was a noted correlation between the initial ECG voltages and the extent of LVH on the most recent echocardiogram in the 7 patients in whom LVH developed de novo.
In this study, we revised the voltage criteria for the determination of LVH by using children who were HIV-uninfected, aged ≤6 years, and had healthy hearts (Figure 3). There are 2 important advantages to these revised data. The first is that normal cardiac structure and function were demonstrated echocardiographically, rather than by physical examination alone. The second advantage is the inclusion of children of black and Hispanic heritage, making these data more clinically applicable to patients who are ethnically diverse than the data in current use. When compared with the established criteria, upper ceilings as defined by 95% prediction intervals were consistently higher than the 98% prediction intervals of Davignon et al for all voltages except SV1. Higher ceilings may, in part, be explained by the inclusion of nonwhite children and would presumably suggest fewer false-positive diagnoses of LVH. To confirm this, we recalculated sensitivity and specificity rates for 6 voltage criteria for LVH with the revised data. As expected, fewer false-positives were noted, with an increased specificity rate >90% for each voltage criteria. Nonetheless, sensitivity also substantially decreased, with fewer true-positives also identified.
There are study limitations inherent to this study. The first is that the control cohort, although documented as HIV-uninfected, was unique in that all subjects had been HIV-exposed in utero. At this time, however, no evidence exists to suggest that these children cannot be considered as “normal” control subjects after infancy. The second limitation is that the control subjects used in revising the criteria of Davignon et al consisted only of preschool-aged children, which limits comparative age groups. Third, the control group in this study consisted of children of primarily black and Hispanic descent, reflecting a dissimilar racial composition. Finally, results should not be extrapolated to children with known cardiac disease and are intended to reflect the usefulness of the ECG solely as a screening tool.
Despite these limitations, this study conclusively determines that the ECG is a poor screening tool for the identification of LVH in children. By use of the criteria of Davignon et al, the sensitivity rate is <25%. Revising criteria to incorporate multiracial children improves only on specificity, rather than sensitivity. In conclusion, the inaccuracy of the ECG as a screening modality outweighs its inherent cost-effective appeal. When financially feasible, echocardiography alone is recommended for the identification of LVH in children.
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute (N01-HR-96037, NO1-HR-96038, NO1-HR-96039, NO1-HR-96040, NO1-HR-96041, NO1-HR-96042, NO1-HR-96043) and in part by the National Institutes of Health General Clinical Research Center Grants (RR-00188, RR-00533, RR-00071, RR-00645, RR-00865 and RR-00043).
Appendix
A partial list of participants in the P2C2 HIV study is listed, with principal investigators identified with an asterisk. A complete list of study participants can be found in reference 1.
National Heart, Lung, and Blood Institute
Hannah Peavy, MD (project officer), Anthony Kalica, PhD, Elaine Sloand, MD, George Sopko, MD, MPH, Margaret Wu, PhD.
Chairman, the Steering Committee
Robert Mellins, MD.
Clinical centers
Baylor College of Medicine, Houston, Tex: William Shearer, MD, PhD,* Nancy Ayres, MD, J. Timothy Bricker, MD, Arthur Garson, MD, Linda Davis, RN, BSN, Paula Feinman, Mary Beth Mauer, RN, BSN.
University of Texas: Debra Mooneyham, RN; Teresa Tonsberg, RN.
Children’s Hospital/Harvard Medical School, Boston, Mass: Steven Lipshultz, MD,* Steven Colan, MD, Lisa Hornberger, MD, Steven Sanders, MD, Marcy Schwartz, MD, Helen Donovan, Janice Hunter, MS, RN, Ellen McAuliffe, BSN, Nandini Moorthy, Patricia Ray, BS, Sonia Sharma, BS.
Boston Medical Center: Karen Lewis, RN, BSN.
Mount Sinai School of Medicine, New York: Meyer Kattan, MD,* Wyman Lai, MD, MPH, Diane Carp, MSN, RN, Donna Lewis, Sue Mone, MS.
Beth Israel Medical Center: Mary Anne Worth, RN.
Presbyterian Hospital in the City of New York/Columbia University, New York: Robert Mellins, MD,* Fred Bierman, MD (through 5/91),* Welton Gersony, MD, Jane Pitt, MD, Thomas Starc, MD, MPH, Anthony Brown, Margaret Challenger, Kimberly Geromanos, RN, MS, CNS.
University of California, Los Angeles School of Medicine, Los Angeles, Calif: Samuel Kaplan, MD,* Y. Al-Khatib, MD, Robin Doroshow, MD, Josephine Isabel-Jones, MD, Roberta Williams, MD, Helene Cohen, RN, PNP, Sharon Golden, RDMS, Karen Simandle, RDMS, Ah-Lin Wong, RDMS.
Children’s Hospital, Los Angeles, Calif: Arno Hohn, MD, Barry Marcus, MD, Audrey Gardner, BS, Toni Ziolkowski, RN.
LAC/USC: Lynn Fukushima, MSN, RN.
Clinical Coordinating Center
The Cleveland Clinic Foundation: Kirk A. Easley, MS,* Michael Kutner, PhD (through 12/99),* Mark Schluchter, PhD (through 04/98),* Johanna Goldfarb, MD, Douglas Moodie, MD, Cindy Chen, MS, Scott Husak, BS, Victoria Konig, ART, Sunil Rao, PhD, Amrik Shah, ScD, Susan Sunkle, BA, Weihong Zhang, MS.
Policy, Data, and Safety Monitoring Board
Henrique Rigatto, MD (Chairman), Edward B. Clark, MD, Robert B. Cotton, MD, Vijay V. Joshi, MD, Paul S. Levy, ScD, Norman S. Talner, MD, Patricia Taylor, PhD, Robert Tepper, MD, PhD, Janet Wittes, PhD, Robert H. Yolken, MD, Peter E. Vink, MD.
References
- 1.The P2C2 HIV Study Group. The Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted Human Immunodeficiency Virus (P2C2 HIV) infection study: design and methods. J Clin Epidemiol. 1996;49:1285–94. doi: 10.1016/s0895-4356(96)00230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan MN, Colan SD, Gamble W, et al. Diagnostic performance of electrocardiographic criteria for left ventricular hypertrophy in pediatric patients [abstract] Circulation. 1998;98:I-835. [Google Scholar]
- 3.Devereux RB, Alonso DR, Lutas EM. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 4.Lipshultz SD, Easley KA, Orav EJ, et al. Left ventricular structure and function in children infected with human immunodeficiency virus: the prospective P2C2 HIV multicenter study. Circulation. 1998;97:1246–56. doi: 10.1161/01.cir.97.13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davignon A, Rautaharju P, Boiselle E, et al. Normal ECG standards for infants and children. Pediatr Cardiol. 1979;1:123–52. [Google Scholar]
- 6.Saidi AS, Moodie DS, Garson A, Jr, et al. Electrocardiography and 24-hour electrocardiographic ambulatory recording (Holter monitor) studies in children infected with human immunodeficiency virus type 1. Pediatr Cardiol. 2000;21:189–96. doi: 10.1007/s002460010038. [DOI] [PubMed] [Google Scholar]
- 7.Obuchowski NA. On the comparisons of correlated proportions for clustered data. Stat Med. 1998;17:1495–507. doi: 10.1002/(sici)1097-0258(19980715)17:13<1495::aid-sim863>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 9.Starc TJ, Lipshultz SE, Kaplan S, et al. Cardiac complications in children with human immunodeficiency virus infection. Pediatrics. 1999;104:e14. doi: 10.1542/peds.104.2.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler RF. Electrocardiographic studies in normal infants and children. Philadelphia: Charles C. Thomas; 1951. [Google Scholar]
- 11.Urbina EM, Matlaga BR, Elkasabany A, et al. Diagnosis of left ventricular hypertrophy in healthy adolescents and young adults: comparision of electrocardiographic criteria to echocardiographic left ventricular mass. The Bogalusa Heart Study [abstract] Circulation. 1998;98:I-150. [Google Scholar]
- 12.Fogel MA, Lieb DR, Seliem MA. Validity of electrocardiographic criteria for left ventricular hypertrophy in children with pressure- or volume-loaded ventricles: comparison with echocardiographic left ventricular muscle mass. Pediatr Cardiol. 1995;16:261–9. doi: 10.1007/BF00798059. [DOI] [PubMed] [Google Scholar]
- 13.Sastroasmoro S, Madiyono B, Oesman IN. Sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy in children with rheumatic heart disease. Paediatr Indonesia. 1991;31:233–44. [PubMed] [Google Scholar]
- 14.Panza JA, Maron BJ. Relation of electrocardiographic abnormalities to evolving left ventricular hypertrophy in hypertrophic cardiomyopathy during childhood. Am J Cardiol. 1989;63:1258–65. doi: 10.1016/0002-9149(89)90187-2. [DOI] [PubMed] [Google Scholar]