Anxiety disorders and clinical depression are the two most common psychiatric disorders. The lifetime risk for a major depressive episode is 17.1%, and the lifetime risk for any anxiety disorder is 24.9% (1). Moreover, these two disorders are co-morbid at a much greater rate than expected by chance. Patients with both Major Depressive Disorder (MDD) and any anxiety disorder have more severe depressive symptoms than those with “pure” depression. They also follow a more protracted course and are less responsive to treatment. This co-morbidity suggests that a common biological substrate mediates these two classes of stress-related disorders. In this commentary, we summarize the previous as well as the most recent evidence (2) for the hypothesis that fibroblast growth factor-2 (FGF2) is a key molecular regulator of anxiety and depression and that it may be critical in understanding their co-morbidity and addressing the associated treatment resistance.
Direct evidence for the involvement of the FGF system in MDD first emerged from postmortem studies. Our group, the Pritzker Neuropsychiatric Disorders Research Consortium, was the first to describe that FGF2 was downregulated in frontal cortical areas of individuals who had suffered from MDD when compared to controls (3). Others subsequently described downregulation of FGF2 in the hippocampus of MDDs. Moreover, we found that patients who had received antidepressant treatment showed less dysregulation of FGF2 than unmedicated MDD subjects (3), suggesting that antidepressants may correct the imbalance. This was consistent with findings in rodents by Mallei et al. showing that acute and chronic antidepressant treatment induced FGF2 expression in the cortex and hippocampus (4).
We then carried out animal studies to ascertain the functional significance of these changes in FGF2. Using social defeat in the rat as an animal model of depression, we demonstrated that, as in the case of human depression, the FGF system was altered. Repeated social stress reduced the expression of FGF2 and one of its receptors, FGFR1, in several fields of the hippocampus (5). In separate studies, we showed that FGF2 is itself an antidepressant when administered intracerebroventricularly to rats either acutely or chronically (6). Together, this body of work strongly implicates a decrease in the level of FGF2 in major depression, suggesting that FGF2 may be an endogenous antidepressant and that classical antidepressants may exert their action, at least in part, by inducing FGF2 expression.
The potential role of FGF2 in regulating anxiety behavior was first indicated by the Gomez-Pinilla group demonstrating that FGF2 expression increased in the hippocampus in response to the anxiolytic diazepam. Given the co-morbidity between anxiety and depression, we asked whether FGF2 may also be anxiolytic in its own right. We showed that peripherally administered FGF2 was anxiolytic following chronic administration and was particularly effective in rats selectively bred for greater anxiety-like behavior (7). Moreover, these effects were accompanied by changes in the survival of both neurons and astrocytes in the dentate gyrus, with the survival of glia predominating.
More recent work has directly implicated endogenous hippocampal FGF2 in controlling basal levels of anxiety. Thus, the higher the level of endogenous FGF2 in the hippocampus, the less the animal is prone to anxiety-like behavior (8). Furthermore, knocking down endogenous FGF2 in the hippocampus (dentate gyrus and CA3) was sufficient to induce anxiety-like behavior. It therefore appears that FGF2 may also be an endogenous anxiolytic, and a decrease in this growth factor, as was observed in the brains of severely depressed individuals, may contribute to their increased vulnerability for anxiety disorders.
The most recent line of work by Duman’s group enhances this body of knowledge about the role of FGF2 in affective behavior in several important directions. Elsayed et al. relied on a different animal model of depression in yet another species to extend our understanding of FGF2 function in responses to chronic stress and the mode of action of antidepressants (2). They used as their model 14 days of chronic unpredictable stress (CUS) in mice. This model has proven to be of relevance to human depression, and like repeated social defeat, CUS produced a significant decrease in FGFR1 expression (2). Importantly, the authors showed that peripheral FGF2 could prevent the depression-like behavior following chronic unpredictable stress, demonstrating its antidepressant power in the face of significant challenge. Since the investigators used mice and CUS, their findings validate the therapeutic effect of FGF2 in a different species and a different model of depression-like behavior.
Arguably the most important contribution of this article is that FGF signaling appears to be necessary for the behavioral effects of antidepressants. Blocking FGF signaling with SU5402 was sufficient to abolish the behavioral effects of two different classes of antidepressants. This strongly suggests that antidepressants require the FGF system to be intact in order to exert their effects on depression-like behavior. However, the use of SU5402 is also a limitation of this study because this drug also has effects on VEGF receptors. Thus, it is difficult to determine whether FGF signaling or some combination of FGF and VEGF signaling is required for the antidepressant effects.
FGF2 dysregulation was initially identified in the prefrontal cortex in MDDs. However, follow-up studies in animals focused on the hippocampus. Elsayed et al. brought the role of FGF2 in depression-like behavior back to the prefrontal cortex (2). Thus, infusion of FGF2 into the prefrontal cortex acted as an antidepressant, demonstrating another site of FGF2 actions. The authors went on to show that FGF2 increased oligodendrocyte “survival” in the prefrontal cortex, and this effect was associated with decreased depression-like behavior (2). Moreover, FGF2 prevented the inhibition of glial proliferation following CUS. In addition, the proliferating effect of fluoxetine was blocked by SU5402. Together, these data point to a key role of FGF2 in regulating glial proliferation in the prefrontal cortex and underscore the role of that brain region in the control of anxiety-like and depression-like behavior. Since Perez et al. had shown that FGF2 increases the survival of neurons and astrocytes in the hippocampus, an effect associated with decreased anxiety-like behavior (7), the relative role of FGF2 in the cortex and the hippocampus and in the control of glial and neuronal survival remains to be explored.
The use of the CUS model as a modulator of FGF2 function is interesting. It should be noted that acute stress can increase FGF2 expression, and this may be construed as a protective mechanism against ensuing anxiety and depression. However, this appears self-limiting as chronic stress can decrease FGF2 levels (1, 4). This begs the question: how do the long-term effects of FGF2 differ from the short-term effects? Are there, in fact, multiple domains of FGF2 actions ranging from immediate, to those on the order of days (as described in the current work), to developmental and long lasting (see for example (9)). Dissecting the organizational effects of FGF2 during development, to the remodeling effects during adulthood, to possible immediate signaling effects in the context of affective behavior, represents an important set of next steps if we are to capitalize on these findings for translational applications.
Taken together, the body of work on FGF2 as an antidepressant and anxiolytic is in agreement with the allostatic model proposed by Salmaso & Vaccarino (10). Individuals vulnerable to depression and/or anxiety due to environmental or genetic factors benefit from increasing the levels of FGF2. This work also suggests that having low levels of endogenous FGF2 may not only predispose one to affective disorders but may also interfere with the efficacy of antidepressants, since antidepressants require this growth factor to exert their beneficial effects. Moreover, chronic stress, and presumably depressive episodes, by further amplifying the FGF2 and FGFR deficits, may further interfere with antidepressant responsiveness. This would then suggest that enhancing FGF2 signaling, even transiently, might promote responsiveness to antidepressants.
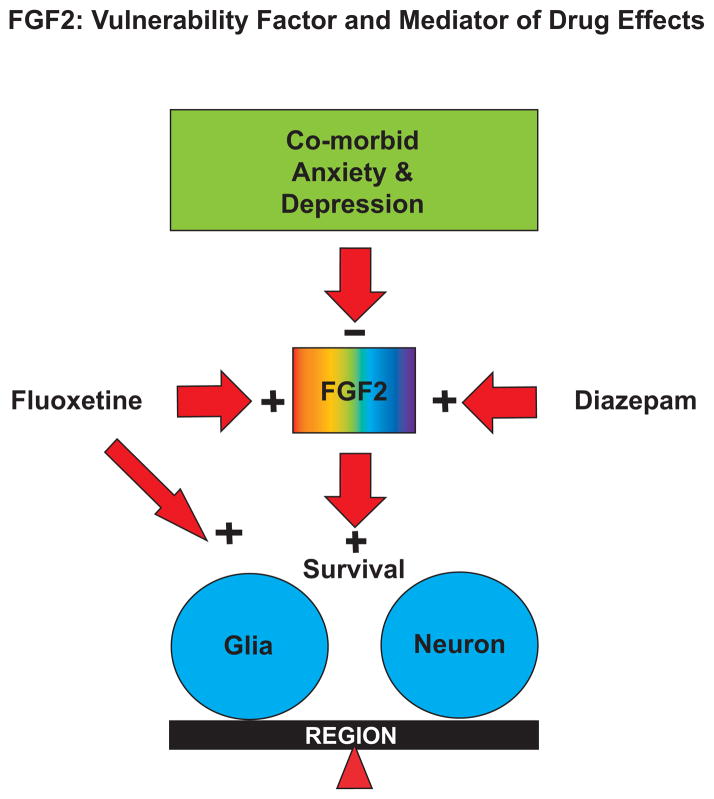
While MDD and anxiety disorders can occur separately, their co-morbidity is often associated with more severe illness and greater resistance to treatment. Since FGF2 is reduced in animal models of both depression-like and anxiety-like behavior, a model can be proposed whereby low FGF2 may be a predisposing factor in the co-morbidity between these two mood disorders in humans (see Figure 1). As indicated, drugs that reduce depressive and anxious symptoms would also increase FGF2 levels in the brain, and enhancing FGF2 signaling may accelerate the action of antidepressant and anxiolytic drugs. Thus, the relative roles of glial or neuronal survival, and the integration of the activities of the hippocampus, prefrontal cortex and other brain regions in these processes are worthy of further analyses.
Figure 1. Fibroblast Growth Factor-2 (FGF2): Vulnerability Factor and Mediator of Drug Effects.
FGF2 levels are low in animal models of depression and anxiety, and in postmortem brains of humans with a history of severe MDD. Therefore, FGF2 may be a co-morbidity factor that responds to anxiolytic and antidepressant treatments. FGF2 can also lead to increased survival of glial cells in the hippocampus and prefrontal cortex. The balance between the roles of neurons and glia, and the interplay between different brain regions in the regulation of anxiety and depression remain to be elucidated.
The series of studies leading up to and including the work of Elsayed et al (1) underscore the power of a “reverse translation” discovery-driven approach, whereby the use of genome-wide approaches led to discoveries first made in human brains then validated in animal models. As we increase our knowledge of the mechanisms of action of FGF2 in controlling affective behavior, we will enhance our understanding of the pathophysiology of mood and anxiety disorders, and identify novel targets and biomarkers for treatment.
Acknowledgments
This work was supported by NIMH Conte Center Grant P50 MH60398, NIDA P01 DA021633, The Office of Naval Research (ONR) Grants N00014-09-1-0598 and N00014-12-1-0366, the Pritzker Neuropsychiatric Disorders Research Consortium Fund LLC (http://www.pritzkerneuropsych.org), NCRR Grant UL1RR024986 and the Rachel Upjohn Clinical Scholars Award to CT.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Financial Disclosures
This work was supported by the Pritzker Neuropsychiatric Disorders Research Fund L.L.C. The authors are members of the Pritzker Neuropsychiatric Disorders Research Consortium, which is supported by the Pritzker Neuropsychiatric Disorders Research Fund L.L.C. A shared intellectual property agreement exists between this philanthropic fund and the University of Michigan, Stanford University, the Weill Medical College of Cornell University, the University of California at Irvine, and the HudsonAlpha Institute for Biotechnology to encourage the development of appropriate findings for research and clinical applications.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 2.Elsayed M, Banasr M, Duric V, Fournier NM, Licznerski P, Duman RS. Antidepressant Effects of Fibroblast Growth Factor-2 in Behavioral and Cellular Models of Depression. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, Lopez JF, Thompson RC, Meng F, Stead JD, Walsh DM, Myers RM, Bunney WE, Watson SJ, Jones EG, Akil H. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci U S A. 2004;101(43):15506–11. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallei A, Shi B, Mocchetti I. Antidepressant treatments induce the expression of basic fibroblast growth factor in cortical and hippocampal neurons. Mol Pharmacol. 2002;61(5):1017–24. doi: 10.1124/mol.61.5.1017. [DOI] [PubMed] [Google Scholar]
- 5.Turner CA, Calvo N, Frost DO, Akil H, Watson SJ. The fibroblast growth factor system is downregulated following social defeat. Neurosci Lett. 2008;430(2):147–50. doi: 10.1016/j.neulet.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner CA, Gula EL, Taylor LP, Watson SJ, Akil H. Antidepressant-like effects of intracerebroventricular FGF2 in rats. Brain Res. 2008;1224:63–8. doi: 10.1016/j.brainres.2008.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29(19):6379–87. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eren-Kocak E, Turner CA, Watson SJ, Akil H. Short-hairpin RNA silencing of endogenous fibroblast growth factor 2 in rat hippocampus increases anxiety behavior. Biol Psychiatry. 2011;69(6):534–40. doi: 10.1016/j.biopsych.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner CA, Clinton SM, Thompson RC, Watson SJ, Jr, Akil H. Fibroblast growth factor-2 (FGF2) augmentation early in life alters hippocampal development and rescues the anxiety phenotype in vulnerable animals. Proc Natl Acad Sci U S A. 2011;108(19):8021–5. doi: 10.1073/pnas.1103732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salmaso N, Vaccarino FM. Toward a novel endogenous anxiolytic factor, fibroblast growth factor 2. Biol Psychiatry. 2011;69(6):508–9. doi: 10.1016/j.biopsych.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]