Abstract
Although saliva endothelins are emerging as valuable noninvasive cardiovascular biomarkers, reports on the relationship between isoforms in saliva and plasma remain scarce. We measured endothelins in concurrent saliva and plasma samples (n = 30 males; age 18–63) by HPLC-fluorescence. Results revealed statistically significant positive correlations among all isoforms between saliva and plasma: big endothelin-1 (BET-1, 0.55 ± 0.27 versus 3.35 ± 1.28 pmol/mL; r = 0.38, p = 0.041), endothelin-1 (ET-1, 0.52 ± 0.21 versus 3.45 ± 1.28 pmol/mL; r = 0.53, p = 0.003), endothelin-2 (ET-2, 0.21 ± 0.07 versus 1.63 ± 0.66 pmol/mL; r = 0.51, p = 0.004), and endothelin-3 (ET-3, 0.39 ± 0.19 versus 2.32 ± 1.44 pmol/mL; r = 0.75, p < 0.001). Correlations of BET-1, ET-1, and ET-3 within each compartment were positive in both plasma (p < 0.05) and saliva (p ≤ 0.1), whereas ET-2 was not significantly correlated with other isoforms in either plasma or saliva. For all isoforms, concentrations varied on average fivefold between individuals (90th/10th percentiles); individuals with high plasma endothelin levels generally had high saliva endothelin levels. Our results reveal that salivary ET isoform profiles portray the plasmatic profiles and support the view of coordinated regulation of ET-1 and ET-3, but distinct regulatory pathways for ET-2.
1. Introduction
As a diagnostic fluid, saliva has several advantages over blood [1, 2]. Saliva is inexpensive and easier to collect, and sufficient volume can be obtained to allow performance of a variety of analyses. For patients, particularly children, the noninvasive sample collection reduces anxiety and discomfort and simplifies procurement of repeated samples for time-series analyses. Saliva contains a wide array of proteins and peptides that are responsive to pathological conditions [3]. Advanced instrumentation and refined analytical techniques have been successfully applied for discovering oncological [4], hormonal [5], immunological [6], and cardiovascular [7, 8] biomarkers that can be informative for early detection and assessment of progression of oral and systemic diseases.
Of particular interest, saliva is known to contain detectable levels of endothelins, an important risk marker for cardiovascular disease [9]. Endothelins are a family of potent vasoconstrictor peptides consisting of three distinct isoforms, endothelin-1 (ET-1), endothelin-2 (ET-2), and endothelin-3 (ET-3) coded by distinct genes [10]. The mature endothelins are produced through cleavage of the big endothelin (BET) precursors by endothelin-converting enzymes [11, 12]. Endothelin-1, the most studied isoform, has been implicated in several diseases, particularly in the progression of cardiovascular diseases [13, 14]. It has been known for two decades that ET-1, ET-2, and ET-3 are present in saliva [15, 16]. However, only recently have levels of saliva ET-1 been related to conditions such as chronic heart failure [17], upper gastrointestinal diseases [18], vibration-induced white finger [19], and oral cancer [20, 21]. The relationship between saliva and plasma ET-2 and ET-3 isoforms is comparatively less well understood. Because of the emerging significance of all three isoforms in health and disease, notably the role of ET-2 in the cardiovascular system, in ovulation, immunology, and cancer [22], we sought to extend the data on the relationships between the three endothelin isoforms in concurrent saliva and plasma samples.
2. Materials and Methods
2.1. Reagents
Ethylenediaminetetraacetic acid (EDTA), trifluoroacetic acid (TFA), phenylmethyl sulfonyl fluoride (PMSF), 3,4-dichloroisocoumarin, molecular weight cut-off filters (30 kDa), endothelin-1 (ET-1, human), endothelin-2 (ET-2, human), and endothelin-3 (ET-3, human) were obtained from Sigma Aldrich (Oakville, Ontario). Big endothelin-1 (BET-1, human) was obtained from Bachem Bioscience (American Inc., CA, USA). Acetonitrile, acetone, methanol, and hydrochloric acid were purchased from Sigma Aldrich (Oakville, Ontario). Amber glass vials and screwcaps with septa were purchased from Chromatographic Specialities (Brockville, Ontario). Deionized water was obtained from a Super-Q Plus high purity water system (Millipore Corporation, Bedford, MA). Compressed gaseous nitrogen was of UHP grade quality and was supplied by Matheson Gas Products (Whitby, Ontario).
2.2. Biological Samples
Anonymous, paired human plasma and saliva samples (from males) certified free of HIV, HEP-A, and HEP-B were purchased commercially (Innovative Research Inc., MI). Saliva samples were collected by spitting in a sterile cup, without stimulation. Both plasma and saliva samples were treated on site with PMSF (final, 1.7 mg/mL) and EDTA (final, 10 mg/mL) to stabilize endothelins [23], shipped to our laboratory in dry ice, and stored at −80°C until further use. Saliva samples containing phlegm or low volume of fluid were discarded. Thirty (30) sample pairs of good quality and in sufficient amounts for analysis were retained for this study.
2.3. Extraction of Endothelins from Plasma and Saliva
Plasma and saliva endothelins were extracted following Kumarathasan et al. [23]. Briefly, plasma (250 μL) and saliva (1 mL) samples were treated with ice-cold 3,4-dichloroisocumarin solution in isopropanol to prevent conversion of BET-1 to ET-1 during the sample processing. The samples were deproteinized with ice-cold acid-acetone mix (acetone : 1 N HCl : water, 40 : 1 : 5) and centrifuged at 9000 ×g for 10 min, and the supernatants obtained were concentrated by evaporation under nitrogen flow. Deproteinization and concentration stages were repeated once more to ensure the removal of abundant proteins. Samples were then loaded onto 30 kDa molecular weight cut-off filters (prewashed with deionised water) and centrifuged at 5000 ×g for 30 min. These filters were then washed with 50% methanol and centrifuged at 5000 ×g for 30 min. Filtrates were dried under nitrogen and reconstituted in 75 μL of 30% acetonitrile in 0.2% TFA/H2O for HPLC-fluorescence analysis.
2.4. HPLC-Fluorescence Analysis of Endothelins
The HPLC unit consisted of a Gilson solvent delivery system (Mandel Scientific, Guelph, ON), a Gilson autosampler (model 231 XL; Middleton, WI), a Supelcosil LC-318 reverse-phase column (25 cm length, 4.6 mm id, 5 μm particle size, and 300 Å pore dimension; Supelco, Oakville, ON), and a RF 551 model fluorescence detector (Shimadzu, Japan). Endothelins (20 μL injection volume) were separated using a gradient elution with acetonitrile/water mobile phase at a flow rate of 1 mL/min and detected at λ EX 280 nm and λ EM 340 nm [23]. Blanks were analyzed after each set of four samples in order to assess the extent of analyte carryover.
2.5. Statistical Analyses
Student's t-test or Mann-Whitney Rank Sum Test was carried out as appropriate using SigmaStat v 11.0 (SPSS Inc., Chicago, IL). Results are presented as mean ± standard deviation. Correlation between different endothelin isoforms in plasma and saliva was tested using Pearson's Product Moment correlation revealing p and r values. Statistical significance was accepted for α = 0.05.
3. Results and Discussion
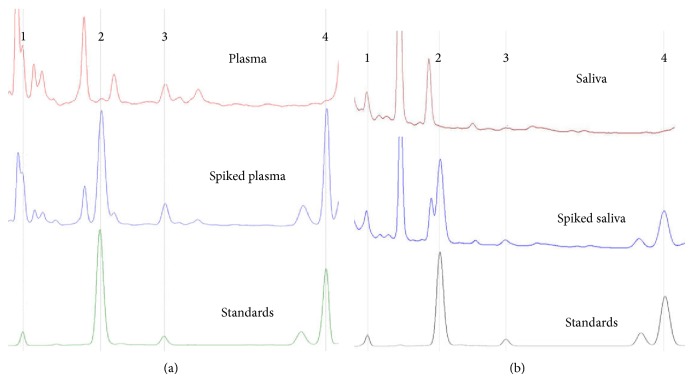
We used HPLC-fluorescence to measure simultaneously the isoforms BET-1, ET-1, ET-2, and ET-3 (Figures 1(a)-1(b)) in time-matched plasma and saliva sample pairs (N = 30) obtained from anonymous individuals (Table 1). Our results confirm and extend previous reports of the presence of endothelins in saliva [15, 16] and of a relationship between saliva and circulating endothelins [17–19], and the correlated measurements provide additional insight into the relationship of ET-2 to the other two isoforms [22]. The recoveries, analytical precision, and accuracy of the HPLC-fluorescence procedure [23] are comparable to values reported by Walczak et al. for HPLC with electrospray tandem mass spectrometry detection [24]: recoveries of endothelins from spiked plasma are between 60% (ET-2) and 97% (ET-1), depending on the endothelin isoforms; analytical precision is on the order of ±4% for replicate peptide standards; analytical accuracy is ±20% for replicate measurements of plasma samples; limit of detection is 0.2–0.5 pmol; and linearity is 1–100 pmol on the column (20 μL injection volume). Both methods detect the separated peptides directly (i.e., on the basis of autofluorescence of aromatic amino acids by HPLC [23, 25] or from mass ion fingerprints in MS/MS [24]), which is different from ELISA detection of immunoreactive endothelin in total plasma [26, 27]. For this reason, we have verified identities of analytes by pulling down of ET-1 and ET-3 with monoclonal antibodies during sample processing, confirming the correspondence between immunoreactive endothelins in plasma and the peptides measured by direct autofluorescence detection after separation on column [23].
Figure 1.
HPLC profiles of endothelin isoforms ET-3 (1), BET-1 (2), ET-1 (3), and ET-2 (4) in human (a) plasma and (b) saliva samples (including unspiked and spiked biological matrices along with endothelin standards).
Table 1.
Plasma and saliva endothelin levels (mean ± SD) in male subjects.
| BET-1 | ET-1 | ET-2 | ET-3 | Total | BET-1/ET-3 | |
|---|---|---|---|---|---|---|
| Plasma (pmol/mL) | ||||||
| All (30) | 3.35 ± 1.28 | 3.45 ± 1.28 | 1.63 ± 0.66 | 2.32 ± 1.44 | 10.8 ± 3.46 | 1.84 ± 1.00 |
| 18–40 years (19) | 3.39 ± 1.38 | 3.22 ± 1.43 | 1.56 ± 0.61 | 1.97 ± 1.21 | 10.1 ± 3.7 | 2.10 ± 1.07 |
| 41–63 years (11) | 3.29 ± 1.15 | 3.87 ± 0.89 | 1.77 ± 0.73 | 2.91 ± 1.67 | 11.8 ± 2.9 | 1.38 ± 0.70 |
| P = 0.039 | P = 0.043 | |||||
|
| ||||||
| Saliva (pmol/mL) | ||||||
| All (30) | 0.55 ± 0.27 | 0.52 ± 0.21 | 0.21 ± 0.07 | 0.39 ± 0.19 | 1.68 ± 0.53 | 1.61 ± 0.75 |
| 18–40 years (19) | 0.59 ± 0.32 | 0.56 ± 0.25 | 0.20 ± 0.07 | 0.37 ± 0.16 | 1.71 ± 0.64 | 1.68 ± 0.66 |
| 41–63 years (11) | 0.49 ± 0.12 | 0.47 ± 0.13 | 0.23 ± 0.09 | 0.43 ± 0.23 | 1.62 ± 0.27 | 1.48 ± 0.91 |
|
| ||||||
| Plasma/saliva | ||||||
| All (30) | 6.94 ± 3.30 | 7.05 ± 2.58 | 8.18 ± 3.16 | 6.15 ± 2.34 | 6.67 ± 1.79 | |
| 18–40 (19) | 6.86 ± 3.50 | 6.11 ± 2.16 | 8.16 ± 3.18 | 5.71 ± 2.73 | 6.30 ± 1.93 | |
| 41–63 (11) | 7.07 ± 3.10 | 8.67 ± 2.51 | 8.22 ± 3.28 | 6.89 ± 1.25 | 7.31 ± 1.36 | |
| P = 0.006 | ||||||
Note: P value for comparison between the two age groups (t-test or Mann-Whitney Rank Sum Test). The number of subjects is shown in parentheses.
It should be noted that the aim of our study was not to explore the physiological significance of variations of saliva and plasma endothelin levels, within and between individuals, and for this reason we have not requested any information on the health status, cardiovascular or buccal, of the donors. Salivary secretions and saliva volume will be affected by water and food uptake and by medication. These factors, together with the health status of subjects and sample quality, can contribute to the variance in observed saliva endothelin concentrations and relationship to plasma levels. Furthermore, sample collection procedures, sample preparation methods, matrix effects, and the analytical approach used are likely to impact the absolute values of estimates. Finally, because all samples analyzed for endothelin content in our small study were from male donors, our data do not address potential gender differences.
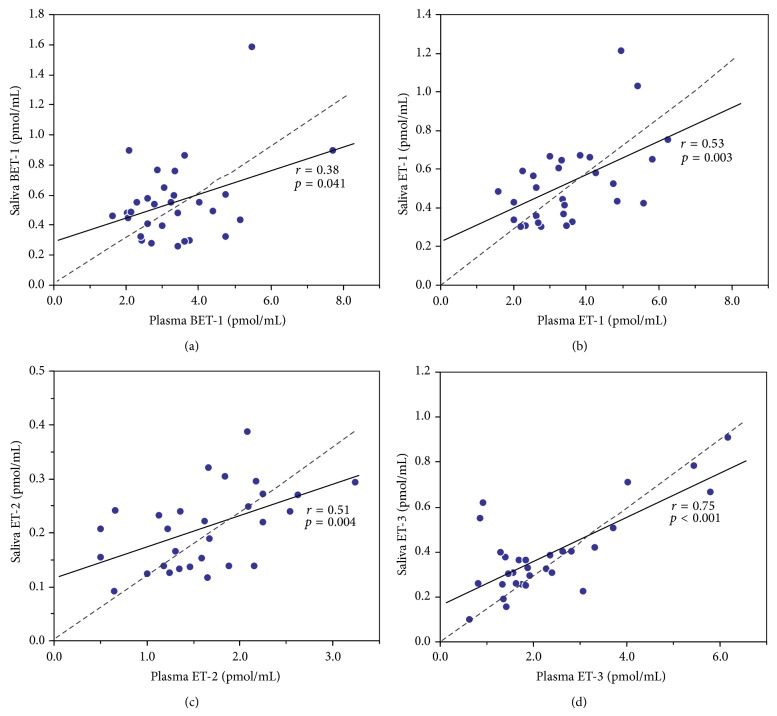
Notwithstanding those limitations, our observations reveal that levels of all endothelin isoforms were significantly correlated between the saliva and plasma matrices in the subjects studied: BET-1 (r = 0.38, p = 0.041), ET-1 (r = 0.53, p = 0.003), ET-2 (r = 0.51, p = 0.004), and ET-3 (r = 0.75, p < 0.001) (Table 1, Figure 2). Our results indicate a slight increase in the ratios of endothelin levels in plasma versus saliva with age of the subjects (ET-1, plasma/saliva ratio in 18–40 years versus 41–63 years, 6.11 ± 2.16 versus 8.67 ± 2.51, p = 0.006), which is potentially attributable to saliva osmolality changes with age [28, 29]. Interestingly, the relationship between osmolality changes and endothelin system has been previously reported [30]. Endothelin levels varied on average by fivefold between individuals, and this applied to all four isoforms measured, in plasma as well as saliva (90th/10th percentiles). In general, high BET-1 and ET-1 levels were predictive of high ET-3 levels within an individual (Table 2). Within plasma, a significant positive correlation was seen for BET-1 versus ET-1 (r = 0.39, p = 0.033), BET-1 versus ET-3 (r = 0.46, p = 0.011), and ET-1 versus ET-3 (r = 0.57, p = 0.001). Within saliva, a significant positive correlation was seen for BET-1 versus ET-1 (r = 0.75, p < 0.001). Correlations between BET-1 versus ET-3 (r = 0.31, p = 0.091) and ET-1 versus ET-3 (r = 0.27, p = 0.144) in saliva were not significant. There were no significant correlations between ET-2 and other isoforms in either plasma or saliva.
Figure 2.
Correlation between endothelin isoforms BET-1 (a), ET-1 (b), ET-2 (c), and ET-3 (d) in plasma and saliva of male subjects (n = 30) measured by HPLC-fluorescence. Assuming simple linearity, the linear regression (solid line) intercept on saliva axis is an estimate of salivary gland endothelin production (r and p values indicated). The linear regression forced through zero (dashed line) assumes diffusion of endothelins entirely from plasma.
Table 2.
Pearson product moment correlation (r-value) for endothelin isoforms in plasma (PL) and saliva (SL) of male subjects (n = 30).
| ET-1 PL | ET-2 PL | ET-3 PL | BET-1 SL | ET-1 SL | ET-2 SL | ET-3 SL | |
|---|---|---|---|---|---|---|---|
| BET-1 PL | 0.39 (0.033) | 0.27 (0.157) | 0.46 (0.011) | 0.38 (0.041) | 0.30 (0.110) | −0.11 (0.571) | 0.41 (0.024) |
| ET-1 PL | 0.25 (0.183) | 0.57 (0.001) | 0.31 (0.093) | 0.53 (0.003) | −0.22 (0.251) | 0.46 (0.011) | |
| ET-2 PL | 0.13 (0.511) | 0.01 (0.974) | 0.15 (0.434) | 0.51 (0.004) | 0.16 (0.406) | ||
| ET-3 PL | 0.17 (0.383) | 0.16 (0.409) | −0.29 (0.121) | 0.75 (<0.001) | |||
| BET-1 SL | 0.75 (<0.001) | −0.12 (0.534) | 0.31 (0.091) | ||||
| ET-1 SL | −0.13 (0.491) | 0.27 (0.144) | |||||
| ET-2 SL | −0.167 (0.378) |
Note: P values are given in parentheses.
The origin of saliva endothelins is not well established. Whole saliva contains secretions from the major parotid, submandibular and sublingual glands, the palate, buccal and labial mucosa, and so on [31]. Expression of preproET-1 and preproET-3 as well as the ETA and ETB receptors has been detected by RT-PCR analyses of submandibular glands of rats [32]. Endothelin-1 has also been detected in striated duct cells of human salivary glands by immunohistochemical analysis [33]. Therefore, it is plausible that endothelins are secretory products from salivary glands. However, serum molecules that are not part of the normal salivary secretory constituents, such as proteins, drugs, and hormones, can also reach saliva by passive diffusion [1, 2, 34–36]. We have measured six- to eightfold higher concentrations of endothelins in plasma by comparison to saliva, and hence the concentration gradient may result in diffusion of the peptides from the capillaries of the mucosa into the salivary fluid. If the intercept of the linear regressions of saliva versus plasma endothelin concentrations is taken as an estimate of the contribution by salivary glands, then at least half (BET-1, ET-1, and ET-2) to two-thirds (ET-3) of the salivary endothelins may originate from plasma (Figure 2).
Correlation among the different endothelin isoforms within individuals and the large variance of endothelin levels between individuals may be due to a number of factors, including common regulatory mechanisms, genetic polymorphisms, and physiological status. Correlation between BET-1 and ET-1 should be expected because of the direct precursor-product relationship [37]. Furthermore, endothelin converting enzyme-1, which cleaves BET-1 to ET-1, shares some regulatory elements with the preproET-1 gene, such that when preproET-1 is transcriptionally activated, ECE-1 tends to be activated as well [38]. In healthy individuals, ET-1 plays an important role in the modulation of vasomotor tone, in conjunction with nitric oxide, but also in regulating cellular proliferation and differentiation in tissues during growth, development, and repair [39, 40].
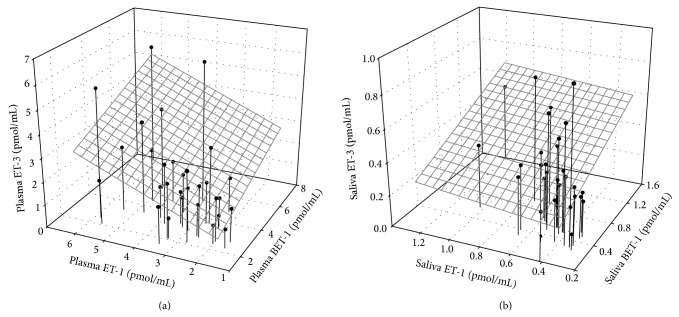
There is good evidence for coordinated regulation of ET-1 and ET-3. In individuals with high basal ET-1 production, activation of preproET-3 expression through a feedback control mechanism may compensate for the pressor effects of the high circulating levels of ET-1. Endothelin-3 stimulates nitric oxide synthase expression and NO production through binding to the ETB receptor [41], and increase of ET-1 with a decrease of ET-3, due to endothelial dysfunction, has been reported in patients with pulmonary arterial hypertension of various etiologies [42, 43]. It is also possible that high or low transcriptional activity of both preproET-1 and preproET-3 genes within an individual is determined by genetic polymorphism or epigenetic conditioning of transacting elements with common cis-acting regulatory sequences between the two genes, but this remains to be investigated. In our analyses, the strongest associations were seen between saliva ET-3 and plasma ET-3 (r = 0.75, p < 0.001) and between saliva BET-1 and saliva ET-1 (r = 0.75, p < 0.001). An overall relationship between ET-3 and BET-1 and its mature product ET-1 was observed in plasma and saliva but was stronger in plasma (Figure 3). The similar ratio of BET-1 to ET-1 in plasma (3.35 versus 3.45 pmol/mL) and saliva (0.55 versus 0.52 pmol/mL) suggests a relative stability of the peptides during sampling and sample processing. However, although saliva ET-2 correlated with plasma ET-2 (r = 0.51, p = 0.004), the poor correlation between ET-2 and the other endothelin isoforms, within plasma (positive, p > 0.05) and within saliva (negative, p > 0.05), is in line with the concept of ET-2 regulatory pathways distinct from those of ET-1 and ET-3 [22].
Figure 3.
Correlation between BET-1, ET-1, and ET-3 isoforms in plasma (a) and saliva (b) of male subjects.
In conclusion, our data confirm an overall positive correlation between plasmatic and salivary BET-1, ET-1, ET-2, and ET-3 levels and between the BET-1, ET-1, and ET-3 within each of the two compartments. The isoform ET-2 correlates poorly with ET-1 and ET-3, within saliva and within plasma. Salivary ET isoform profiles portray the plasmatic profiles and support the view of coordinated regulation of ET-1 and ET-3, but distinct regulatory pathways for ET-2.
Acknowledgments
The authors are grateful to Alain Filiatreault and Erica Blais for assistance with analyses and Josée Guénette for technical assistance. This work was supported by the Clean Air Regulatory Agenda, Health Canada.
Abbreviations
- ET:
Endothelin
- ECE:
Endothelin-converting enzyme
- EDTA:
Ethylenediaminetetraacetic acid
- HEP-A:
Hepatitis A
- HEP-B:
Hepatitis B
- HIV:
Human immunodeficiency virus
- PMSF:
Phenylmethylsulfonyl fluoride
- TFA:
Trifluoroacetic acid.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Kaufman E., Lamster I. B. The diagnostic applications of saliva—a review. Critical Reviews in Oral Biology and Medicine. 2002;13(2):197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 2.Streckfus C. F., Bigler L. R. Saliva as a diagnostic fluid. Oral Diseases. 2002;8(2):69–76. doi: 10.1034/j.1601-0825.2002.1o834.x. [DOI] [PubMed] [Google Scholar]
- 3.Segal A., Wong D. T. Salivary diagnostics: enhancing disease detection and making medicine better. European Journal of Dental Education. 2008;12(1):22–29. doi: 10.1111/j.1600-0579.2007.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Streckfus C., Bigler L., Dellinger T., Dai X., Kingman A., Thigpen J. T. The presence of soluble c-erbB-2 in saliva and serum among women with breast carcinoma: a preliminary study. Clinical Cancer Research. 2000;6(6):2363–2370. [PubMed] [Google Scholar]
- 5.Quissell D. O. Steroid hormone analysis in human saliva. Annals of the New York Academy of Sciences. 1993;694:143–145. doi: 10.1111/j.1749-6632.1993.tb18348.x. [DOI] [PubMed] [Google Scholar]
- 6.Hodinka R. L., Nagashunmugam T., Malamud D. Detection of human immunodeficiency virus antibodies in oral fluids. Clinical and Diagnostic Laboratory Immunology. 1998;5(4):419–426. doi: 10.1128/cdli.5.4.419-426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giannessi D., Maltinti M., del Ry S. Adiponectin circulating levels: a new emerging biomarker of cardiovascular risk. Pharmacological Research. 2007;56(6):459–467. doi: 10.1016/j.phrs.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Toda M., Tsukinoki R., Morimoto K. Measurement of salivary adiponectin levels. Acta Diabetologica. 2007;44(1):20–22. doi: 10.1007/s00592-007-0236-8. [DOI] [PubMed] [Google Scholar]
- 9.Luscher T. F., Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation. 2000;102(19):2434–2440. doi: 10.1161/01.cir.102.19.2434. [DOI] [PubMed] [Google Scholar]
- 10.Inoue A., Yanagisawa M., Kimura S., et al. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaw S., Hecker M., Vane J. R. The two-step conversion of big endothelin 1 to endothelin 1 and degradation of endothelin 1 by subcellular fractions from human polymorphonuclear leukocytes. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(15):6886–6890. doi: 10.1073/pnas.89.15.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanagisawa M. The endothelin system: a new target for therapeutic intervention. Circulation. 1994;89(3):1320–1322. doi: 10.1161/01.cir.89.3.1320. [DOI] [PubMed] [Google Scholar]
- 13.Galatius-Jensen S., Wroblewski H., Emmeluth C., Bie P., Haunsø S., Kastrup J. Plasma endothelin in congestive heart failure: a predictor of cardiac death? Journal of Cardiac Failure. 1996;2(2):71–76. doi: 10.1016/s1071-9164(96)80025-x. [DOI] [PubMed] [Google Scholar]
- 14.Wei C.-M., Lerman A., Rodeheffer R. J., et al. Endothelin in human congestive heart failure. Circulation. 1994;89(4):1580–1586. doi: 10.1161/01.cir.89.4.1580. [DOI] [PubMed] [Google Scholar]
- 15.Lam H.-C., Takahashi K., Ghatei M. A., Warrens A. N., Rees A. J., Bloom S. R. Immunoreactive endothelin in human plasma, urine, milk, and saliva. Journal of Cardiovascular Pharmacology. 1991;17(7):S390–S393. doi: 10.1097/00005344-199100177-00109. [DOI] [PubMed] [Google Scholar]
- 16.Lam H.-C., Takahashi K., Ghatei M. A., Suda K., Kanse S. M., Bloom S. R. Presence of immunoreactive endothelin in human saliva and rat parotid gland. Peptides. 1991;12(4):883–885. doi: 10.1016/0196-9781(91)90151-e. [DOI] [PubMed] [Google Scholar]
- 17.Denver R., Tzanidis A., Martin P., Krum H. Salivary endothelin concentrations in the assessment of chronic heart failure. The Lancet. 2000;355(9202):468–469. doi: 10.1016/s0140-6736(00)82019-x. [DOI] [PubMed] [Google Scholar]
- 18.Lam H.-C., Lo G.-H., Lee J.-K., et al. Salivary immunoreactive endothelin in patients with upper gastrointestinal diseases. Journal of Cardiovascular Pharmacology. 2004;44(1):S413–S417. doi: 10.1097/01.fjc.0000166288.87571.ae. [DOI] [PubMed] [Google Scholar]
- 19.Bovenzi M., D'Agostin F., Rui F., Ambrosi L., Zefferino R. Salivary endothelin and vascular disorders in vibration-exposed workers. Scandinavian Journal of Work, Environment and Health. 2008;34(2):133–141. doi: 10.5271/sjweh.1224. [DOI] [PubMed] [Google Scholar]
- 20.Pickering V., Jordan R. C. K., Schmidt B. L. Elevated salivary endothelin levels in oral cancer patients—a pilot study. Oral Oncology. 2007;43(1):37–41. doi: 10.1016/j.oraloncology.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Y.-S. L., Rees T., Jordan L., et al. Salivary endothelin-1 potential for detecting oral cancer in patients with oral lichen planus or oral cancer in remission. Oral Oncology. 2011;47(12):1122–1126. doi: 10.1016/j.oraloncology.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling L., Maguire J. J., Davenport A. P. Endothelin-2, the forgotten isoform: emerging role in the cardiovascular system, ovarian development, immunology and cancer. British Journal of Pharmacology. 2013;168(2):283–295. doi: 10.1111/j.1476-5381.2011.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumarathasan P., Goegan P., Vincent R. An automated high-performance liquid chromatography fluorescence method for the analyses of endothelins in plasma samples. Analytical Biochemistry. 2001;299(1):37–44. doi: 10.1006/abio.2001.5362. [DOI] [PubMed] [Google Scholar]
- 24.Walczak M., Fedorowicz A., Chłopicki S., Szymura-Oleksiak J. Determination of endothelin-1 in rats using a high-performance liquid chromatography coupled to electrospray tandem mass spectrometry. Talanta. 2010;82(2):710–718. doi: 10.1016/j.talanta.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 25.Vincent R., Kumarathasan P., Goegan P., et al. Inhalation toxicology of urban ambient particulate matter: acute cardiovascular effects in rats. Research Report (Health Effects Institute) 2001;104:5–54. [PubMed] [Google Scholar]
- 26.Bouthillier L., Vincent R., Goegan P., et al. Acute effects of inhaled urban particles and ozone: lung morphology, macrophage activity, and plasma endothelin-1. The American Journal of Pathology. 1998;153(6):1873–1884. doi: 10.1016/s0002-9440(10)65701-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calderón-Garcidueñas L., Vincent R., Mora-Tiscareño A., et al. Elevated plasma endothelin-1 and pulmonary arterial pressure in children exposed to air pollution. Environmental Health Perspectives. 2007;115(8):1248–1253. doi: 10.1289/ehp.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chauncey H. H., Feller R. P., Kapur K. K. Longitudinal age-related changes in human parotid saliva composition. Journal of Dental Research. 1987;66(2):599–602. doi: 10.1177/00220345870660024101. [DOI] [PubMed] [Google Scholar]
- 29.Höld K. M., de Boer D., Zuidema J., Maes R. A. A. Saliva as an analytical tool in toxicology. International Journal of Drug Testing. 1995;1(1):1–36. [Google Scholar]
- 30.Vernace M. A., Mento P. F., Maita M. E., et al. Osmolar regulation of endothelin signaling in rat renal medullary interstitial cells. Journal of Clinical Investigation. 1995;96(1):183–191. doi: 10.1172/JCI118019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaffe T., Cooper-White J., Beyerlein P., Kostner K., Punyadeera C. Diagnostic potential of saliva: current state and future applications. Clinical Chemistry. 2011;57(5):675–687. doi: 10.1373/clinchem.2010.153767. [DOI] [PubMed] [Google Scholar]
- 32.Ventimiglia M. S., Rodriguez M. R., Morales V. P., et al. Endothelins participate in the central and peripheral regulation of submandibular gland secretion in the rat. American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 2011;300(1):R109–R120. doi: 10.1152/ajpregu.00041.2010. [DOI] [PubMed] [Google Scholar]
- 33.Nakamizo M., Pawankar R., Ohkubo K. Presence of endothelin-1 in human salivary glands and tumors. Nippon Ika Daigaku Zasshi. 1998;65(6):471–477. doi: 10.1272/jnms1923.65.471. [DOI] [PubMed] [Google Scholar]
- 34.Aps J. K. M., Martens L. C. Review: the physiology of saliva and transfer of drugs into saliva. Forensic Science International. 2005;150(2-3):119–131. doi: 10.1016/j.forsciint.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 35.Toker A., Aribas A., Yerlikaya F. H., Tasyurek E., Akbuğa K. Serum and saliva levels of ischemia-modified albumin in patients with acute myocardial infarction. Journal of Clinical Laboratory Analysis. 2013;27(2):99–104. doi: 10.1002/jcla.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiappin S., Antonelli G., Gatti R., De Palo E. F. Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clinica Chimica Acta. 2007;383(1-2):30–40. doi: 10.1016/j.cca.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Stow L. R., Jacobs M. E., Wingo C. S., Cain B. D. Endothelin-1 gene regulation. The FASEB Journal. 2011;25(1):16–28. doi: 10.1096/fj.10-161612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomson E., Kumarathasan P., Vincent R. Pulmonary expression of preproET-1 and preproET-3 mRNAs is altered reciprocally in rats after inhalation of air pollutants. Experimental Biology and Medicine. 2006;231(6):979–984. [PubMed] [Google Scholar]
- 39.Shah R. Endothelins in health and disease. European Journal of Internal Medicine. 2007;18(4):272–282. doi: 10.1016/j.ejim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Glassberg M. K., Ergul A., Wanner A., Puett D. Endothelin-1 promotes mitogenesis in airway smooth muscle cells. The American Journal of Respiratory Cell and Molecular Biology. 1994;10(3):316–321. doi: 10.1165/ajrcmb.10.3.7509612. [DOI] [PubMed] [Google Scholar]
- 41.Hirata Y., Emori T., Eguchi S., et al. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. Journal of Clinical Investigation. 1993;91(4):1367–1373. doi: 10.1172/jci116338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montani D., Souza R., Binkert C., et al. Endothelin-1/endothelin-3 ratio: a potential prognostic factor of pulmonary arterial hypertension. Chest. 2007;131(1):101–108. doi: 10.1378/chest.06-0682. [DOI] [PubMed] [Google Scholar]
- 43.Tsiakalos A., Hatzis G., Moyssakis I., Karatzaferis A., Ziakas P. D., Tzelepis G. E. Portopulmonary hypertension and serum endothelin levels in hospitalized patients with cirrhosis. Hepatobiliary & Pancreatic Diseases International. 2011;10(4):393–398. doi: 10.1016/s1499-3872(11)60066-0. [DOI] [PubMed] [Google Scholar]