Abstract
Objective
To examine the expression of ADAMTS-7 during the progression of osteoarthritis (OA), defining its role in the pathogenesis of OA, and elucidating the molecular events involved.
Methods
ADAMTS-7 expression in cartilage of a rat OA model was assayed using immunohistochemistry. Cartilage-specific ADAMTS-7 transgenic mice and ADAMTS-7 small interfering (si)RNA knockdown mice were generated and used to analyse OA progression in both spontaneous and surgically induced OA models. Cartilage degradation and OA was evaluated using Safranin-O staining, immunohistochemistry, ELISA and western blotting. In addition, mRNA expression of tumour necrosis factor (TNF)-α and metalloproteinases known to be involved in cartilage degeneration in OA was analysed. Furthermore, the transactivation of ADAMTS-7 by TNF-α and its downstream NF-κB signalling was measured using reporter gene assay.
Results
ADAMTS-7 expression was elevated during disease progression in the surgically induced rat OA model. Targeted overexpression of ADAMTS-7 in chondrocytes led to chondrodysplasia characterised by short-limbed dwarfism and a delay in endochondral ossification in ‘young mice’ and a spontaneous OA-like phenotype in ‘aged’ mice. In addition, overexpression of ADAMTS-7 led to exaggerated breakdown of cartilage and accelerated OA progression, while knockdown of ADAMTS-7 attenuated degradation of cartilage matrix and protected against OA development, in surgically induced OA models. ADAMTS-7 upregulated TNF-α and metalloproteinases associated with OA; in addition, TNF-α induced ADAMTS-7 through NF-κB signalling.
Conclusions
ADAMTS-7 and TNF-α form a positive feedback loop in the regulation of cartilage degradation and OA progression, making them potential molecular targets for prevention and treatment of joint degenerative diseases, including OA.
INTRODUCTION
Osteoarthritis (OA) is the most prevalent form of arthritis in the USA, characterised by synovitis, cartilage degeneration and osteophyte formation.1 The destruction of the extracellular matrix of articular cartilage and bone in arthritic joints is thought to be mediated by excessive proteolytic activity.2 Theoretically, inhibition of degradation enzymes can slow down or block disease progression. The isolation and characterisation of cartilage degradation enzymes is therefore of great interest from both a pathophysiological and a therapeutic standpoint.
The ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family consists of secreted zinc metalloproteinases with a precisely ordered modular organisation that includes at least one thrombospondin type I repeat.3 It is known that some members of the ADAMTS family degrade aggrecan; ADAMTS-5 plays a primary role in aggrecan loss in murine arthritis.4,5 ADAMTS-7, another member of the ADAMTS family, is known to be expressed in musculoskeletal tissues, including bone, cartilage, synovium and ligament.6 It appears to be a direct target of parathyroid hormone-related protein (PTHrP) and negatively mediates chondrocyte differentiation.7 The interaction and importance of ADAMTS-7 and PTH signalling in chondrogenesis are also supported by a report that downregulation of PTHrP caused by a t(8;12)(q13;p11.2) translocation leads to brachydactyly type E, and PTHrP and ADAMTS-7 are downregulated in differentiated fibroblasts from patients with this condition into chondrogenic cells.8 Furthermore, ADAMTS-7 associates with and inactivates progranulin (PGRN), a chondroprotective growth factor.7 Moreover, it has recently been reported that ADAMTS-7 also negatively regulates endplate cartilage differentiation.9
ADAMTS-7 has been found to associate with and degrade cartilage oligomeric matrix protein (COMP), which is a prominent non-collagenous component of cartilage, and it may play a key role in the pathogenesis of arthritis7,10,11 and atherosclerosis. 7 It is significantly increased in arthritic cartilage and synovium compared with healthy controls, 11,12 and an increase in fragments of COMP has been detected in the cartilage, synovial fluid and serum of patients with arthritis.13 ADAMTS-7 was also involved in cytokine-induced COMP degradation in cartilage explants.14 In addition, dramatic increases in expression of ADAMTS-7 and levels of COMP fragments were detected during degeneration of rat intervertebral disc.15
ADAMTS-7 was recently identified as a novel locus for coronary atherosclerosis.16–18 It was found to mediate vascular remodelling via association with and degradation of COMP in a rat model.19 The association between variation at the ADAMTS-7 locus and susceptibility to coronary artery disease was further strengthened by a very recent report that rs3825807, a non-synonymous single-nucleotide polymorphism leading to a Ser-to-Pro substitution in the prodomain of ADAMTS-7, has an effect on ADAMTS-7 maturation, COMP cleavage and vascular smooth muscle cells migration, with the variant associated with protection from atherosclerosis.20
The role of ADAMTS-7 in cartilage degradation and OA progression in vivo, however, remains unknown. Herein we took advantage of several OA models generated from various genetically modified animals to define the role of ADAMTS-7 in the progression of OA as well as the molecular mechanisms involved.
MATERIALS AND METHODS
Generation of cartilage-specific ADAMTS-7 transgenic (TG) mice and ADAMTS-7 knockdown (KD) mice
All animal studies were performed in accordance with institutional guidelines and approval by the Institutional Animal Care and Use Committee of New York University (IACUC Approval No 09112-02). Detailed information on plasmid construction and generation and genotyping of these genetically modified mice is provided in online supplementary materials and methods.
Aging-associated and surgically induced OA models
All animals were provided with water and food ad libitum throughout these studies. For the aging-associated model of OA, wild-type (WT) and ADAMTS-7 TG mice were kept up to the age of 8 months and were followed for spontaneous development of OA. For the surgically induced OA model, we back-crossed the TG mice in FVB/n background with C57/BL6 mice for six generations to reduce the FVB/n background, and destabilisation of medial meniscus (DMM) surgery was performed in indicated mice.
Sandwich ELISA for COMP
Serum concentration of COMP was analysed by our new sandwich ELISA.21 The detailed method for this ELISA is available in online supplementary materials and methods.
Cartilage explant cultures
Briefly, mouse femoral head cartilage from either WT or TG mice was dissected into pieces of diameter ~1 mm of 1–2 mm thickness. The cartilage was dispensed into tissue-culture flasks and incubated overnight in the indicated medium. After 3 days of culture, the supernatants were harvested for COMP degradation analysis by western blotting, and RNA was extracted from the cartilage samples for real-time reverse transcriptase (RT)-PCR.
For details, please see online supplementary materials and methods.
RESULTS
Increased expression of ADAMTS-7 in the progression of surgically induced OA
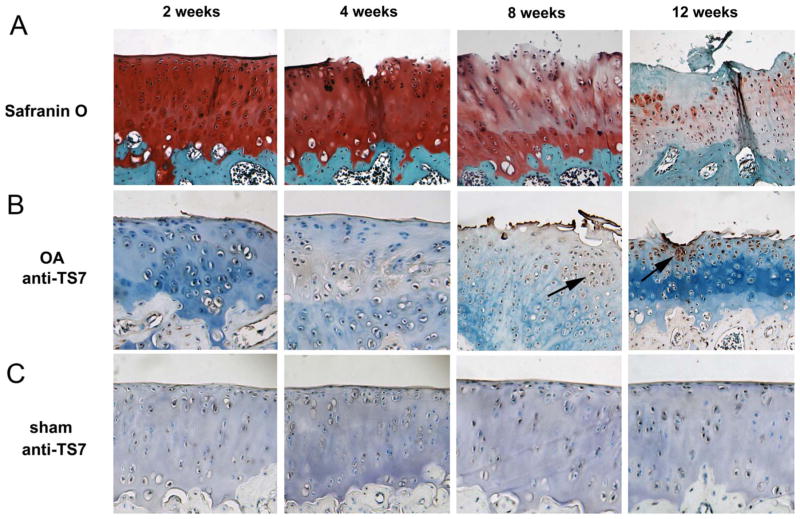
We have previously reported that ADAMTS-7 associated with and degraded COMP and that its level was elevated in the cartilage and synovium of patients with both OA and rheumatoid arthritis.6,22 To determine whether the expression of this metalloproteinase was altered in the course of OA progression, we used a surgical OA rat model, with anterior cruciate ligament transection and partial medial meniscectomy,23 then killed them at the indicated time points for histological analysis. Immunohistochemistry results revealed that ADAMTS-7 was markedly upregulated after OA progression; the highest level was observed at 12 weeks (figure 1). In addition, we observed a strong signal for ADAMTS-7 in the superficial and middle zones. Histological analysis of the Safranin-O-stained section is shown for articular degradation in this model.
Figure 1.
ADAMTS-7 is upregulated in a surgically induced rat osteoarthritis (OA) model. (A) Safranin-O staining of femur articular cartilage in the operated knees. Samples from rat knee cartilage at different time points after surgery, as indicated, were harvested, and histopathological studies were performed. (B) Expression of ADAMTS-7 during OA development, assayed by immunohistochemistry. Femur cartilage sections were stained with anti-ADAMTS-7 serum (brown) and counterstained with methyl green. Arrows indicate the signal. (C) ADAMTS-7 expression in sham-operated knee femur cartilage, examined by immunohistochemistry with anti-ADAMTS-7 serum. Representative sections are shown. Original magnification ×40.
Targeted overexpression of ADAMTS-7 in chondrocytes leads to chondrodysplasia
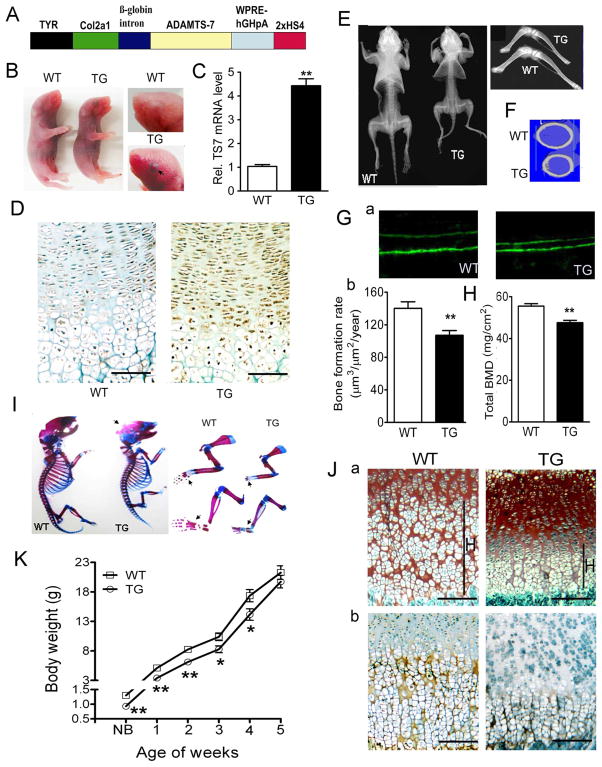
To study the role of ADAMTS-7 in cartilage and arthritis in vivo, we generated TG mice overexpressing ADAMTS-7 under the control of a well-characterised type II collagen (ColII) promoter24,25 (figure 2A). Expression of the transgene could be observed from the black eye colour (figure 2B), and was confirmed using quantitative real-time RT-PCR of tail mRNA (figure 2C) and immunohistochemistry of ADAMTS-7 in the growth plate of 3-week-old TG mice (figure 2D). Although Col2a1-TS7 mice are viable, they are smaller. Representative X-ray images of whole animals and hind limbs at 3 weeks revealed notable reductions in skeletal length and bone volume of the TG mice (figure 2E). Micro-CT of slices across the mid-femur showed a smaller diameter in TG mice (figure 2F). Moreover, the rate of bone formation indicated by the distance between the two consecutive labels was significantly decreased in TG mice (figure 2G). A dual-energy X-ray absorptiometry scan indicated significantly lower bone mineral density in TG mice at the age of 3 weeks compared with WT mice (figure 2H). Alcian blue/Alizarin red whole-mount staining revealed that overexpression of ADAMTS-7 delayed mineralisation in the TG mice, characterised by less red staining in the calvaria, forelimb and hind limb (figure 2I). Safranin-O staining for the growth plates of newborn mice displayed a significantly reduced hypertrophic zone in the TG mice (figure 2J, a). Immunohistochemistry of type X collagen (ColX) demonstrated that it was reduced in TG mice, suggesting delayed chondrocyte hypertrophy in the growth plate (figure 2J, b). A body weight curve from newborn to 4 weeks of age was plotted to monitor the growth of TG mice (figure 2K). The average weight of TG mice was significantly lower than that of their WT littermates. However, these TG mice had reached normal size at the age of 5 weeks, and no defects in cartilage, bone and joint were observed after 5 weeks (data not shown).
Figure 2.
Targeted overexpression of ADAMTS-7 in chondrocytes leads to short-limbed dwarfism and a delay in endochondral ossification in young mice. (A) Diagram of Col2a1-ADAMTS-7 transgenic (TG) construct. Full-length human ADAMTS-7 cDNA was cloned into the 6.0 kb chondrocyte-specific promoter in a coat colour vector. TYR, tyrosinase minigene; WPRE, woodchuck hepatitis virus post-transcriptional regulatory element; HS4, chicken β-globin insulator. (B) Smaller size of newborn Col2a1-TG founders compared with wild-type (WT) littermate controls (left panel). High magnification of black eye in the transgenic mouse indicates the strong transgene expression (right panel). The arrow indicates the black eye in TG mouse. (C) Real-time RT-PCR analysis of ADAMTS-7 mRNA expression from newborn TG mice and WT control. Values are mean ±SEM from three independent founders per genotype. (D) Increased ADAMTS-7 expression in the newborn TG mouse growth plate detected by immunohistochemistry. (E) Representative pictures of whole body from WT and TG mice at 3 weeks old, assayed by X-ray. (F) The coronal section of middle femurs in 3-week-old WT and TG mice, assayed by micro-CT. (G) Calcein double labelling in 4-week-old mice. (a) The bone formation rate represented by the distance between two green labels (original magnification ×40); (b) bone formation rate was compared between WT and TG mice (n=5 in each group). (H) Dual-energy X-ray absorptiometry scan analysis of total bone mineral density (BMD) in WT and TG mice at the age of 3 weeks (n=6 in each group). (I) Skeletal preparation with Alcian blue/Alizarin red staining of newborn WT and TG mice. Representative pictures are shown as whole-mount view (left), and high magnification of forelimbs and hind limbs (right). Black arrows indicate the mineralisation site of the bone. (J) Delayed hypertrophy in TG mice. (a) Safranin-O staining of the tibial growth plates in 1-week-old mice; H, hypertrophic zone. (b) Hypertrophic chondrocytes-specific marker type X collagen was examined by immunohistochemistry in WT and TG mice. Brown signal indicates the location of collagen X in the matrix. (K) Body weight curve of WT and TG mice from newborn to 5 weeks old (n=6 for each group). Data are means ±SEM. *p<0.05, **p<0.01 between WT and TG mice. Bars=100 μm.
‘Aged’ TG mice develop an OA-like phenotype
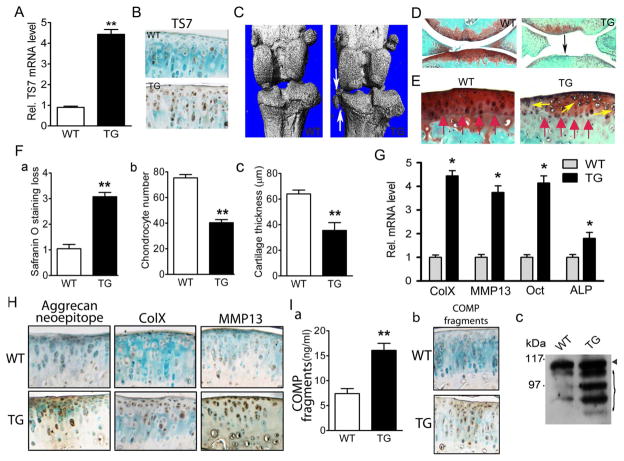
We first examined the level of ADAMTS-7 in 8-month-old TG lines, and found that ADAMTS-7 mRNA measured using real-time RT-PCR of tail showed an ~4.5-fold increase in the TG mice (figure 3A), and overexpression of ADAMTS-7 protein in the articular cartilage of TG mice was also revealed using immunohistochemistry (figure 3B). Interestingly, we observed OA-like phenotypes in 8-month-old TG mice (figure 3). Micro-CT of the hind knees revealed osteophyte formation and ectopic subchondral sclerosis in aged TG mice (figure 3C). Safranin-O/fast green staining showed remarkable loss of smooth cartilage surface and proteoglycan in the articular cartilage of the TG mice (figure 3D). High-power photography revealed clear chondrocyte clustering and migration of the irregular tide mark to the superficial zone (figure 3E). Quantification of the OA-like pathological changes revealed that loss of proteoglycan, the number of chondrocytes per defined cartilage area in the femur cartilage section, and articular cartilage thickness were all significantly altered in the TG mice compared with the WT controls (figure 3F). In addition, marker genes specific for chondrocyte hypertrophy, including ColX and matrix metalloproteinase (MMP)13 were significantly upregulated (figure 3G). Furthermore, immunostaining for the neoepitope of aggrecan 374ARGSV, which indicated degradation of aggrecan, showed increased aggrecan degradation in the cartilage of the TG mice (figure 3H, left panel). Moreover, immunostaining for ColX and MMP13 revealed elevated levels of these two molecules in the articular chondrocytes, mainly located in the superficial and middle zone, of the TG mice (figure 3H, middle and right panels). In addition, ELISA was performed for cleavage products of ColII, and 8-month-old TG mice displayed significantly higher levels of ColII cleavage (see online supplementary figure S1). The specificity of antibodies against ADAMTS-7, ColX and MMP13 was examined using the corresponding pre-immune serum (for ADAMTS-7) or control IgGs (for ColX and MMP13) (see online supplementary figure S2).
Figure 3.
Eight-month-old ADAMTS-7 transgenic (TG) mice spontaneously developed a mild osteoarthritis (OA)-like phenotype. (A) Relative level of ADAMTS-7 transgene in 8-month-old adult mice, assayed by real-time reverse transcriptase (RT)-PCR. Normalised values were calibrated against the wild-type (WT), given the value of 1 (n=6 in each group). (B) Immunohistochemical analysis of ADAMTS-7 expression in articular cartilage of 8-month-old mice. (C) Micro-CT images of knee joints from WT and TG mice. Osteophyte formation is indicated by white arrows. (D) Safranin O staining of knee joint articular cartilage. Significant proteoglycan loss in TG mice is indicated (black arrow). (E) High-magnification pictures of Safranin-O staining showing tidemark shift (red arrows) and chondrocyte cluster formation (yellow arrows) in aged transgenic mice. (F) Comparison of OA severity between WT and TG mice in aging-associated model, as assessed by Safranin-O staining loss score (a), chondrocyte number (b) and articular cartilage layer thickness (c) in the tibia. n=6 for each group. (G) Transcript levels of mature chondrocyte marker genes for collagen X (ColX), matrix metalloproteinase 13 (MMP13), osteocalcin (Oct), alkaline phosphatase (ALP) from WT and TG articular cartilage were measured by real-time RT-PCR. The units are arbitrary, and the normalised values were calibrated against the control, here given the value of 1. Each real-time RT-PCR was performed in triplicate. (H) Immunostaining for aggrecan degradation neoepitope (left), ColX (middle) and MMP13 (right) in articular cartilage from WT and TG mice. (I) Analyses of COMP degradation fragments in WT and TG mice. (a) Serum levels of COMP fragments in WT and TG mice, assayed by COMP sandwich ELISA using a fragment-preferred COMP monoclonal antibody21 (n=6 for each group). (b) Higher levels of COMP fragments in TG cartilage, assayed by immunohistochemistry with a fragment-preferred COMP monoclonal antibody. (c) Femoral head articular cartilage from WT and TG mice was cultured for 3 days, and conditioned medium was precipitated by 100% trichloroacetic acid, then subjected to 10% reducing sodium dodecyl sulphate/polyacrylamide gel electrophoresis and detected by a polyclonal COMP antibody.6 The arrowhead indicates the intact COMP, and the bracket indicates the degraded COMP fragments. Values are normalised mean±SEM. *p<0.05, **p<0.01 versus WT mice. Original magnification ×100 in (B), (E), (H) and (I) and ×40 in (D).
ADAMTS-7 is known to be able to bind and cleave COMP in vitro.6 To test whether COMP degradation was changed in TG mice, we used a sandwich ELISA that was designed to detect degraded COMP fragments in serum,21 and found that serum COMP degradation fragments were significantly elevated in TG mice (figure 3I, a). Increased COMP degradation in TG mice was also revealed using immunohistochemistry with a fragment-preferred monoclonal COMP antibody which was raised against recombinant mouse COMP type III domain (figure 3I, b). It can be noted that increased COMP fragments were primarily localised in the superficial and middle zone of cartilage, which is similar to the expression pattern of ADAMTS-7 in OA cartilage (figure 1). Moreover, a western blotting assay using the conditioned medium obtained from articular cartilage explant culture with a polyclonal antibody against COMP demonstrated much higher levels of COMP fragments in the conditioned medium of TG mice (figure 3I, c).
Overexpression of ADAMTS-7 in chondrocytes led to exaggerated OA development and progression in a surgically induced OA model
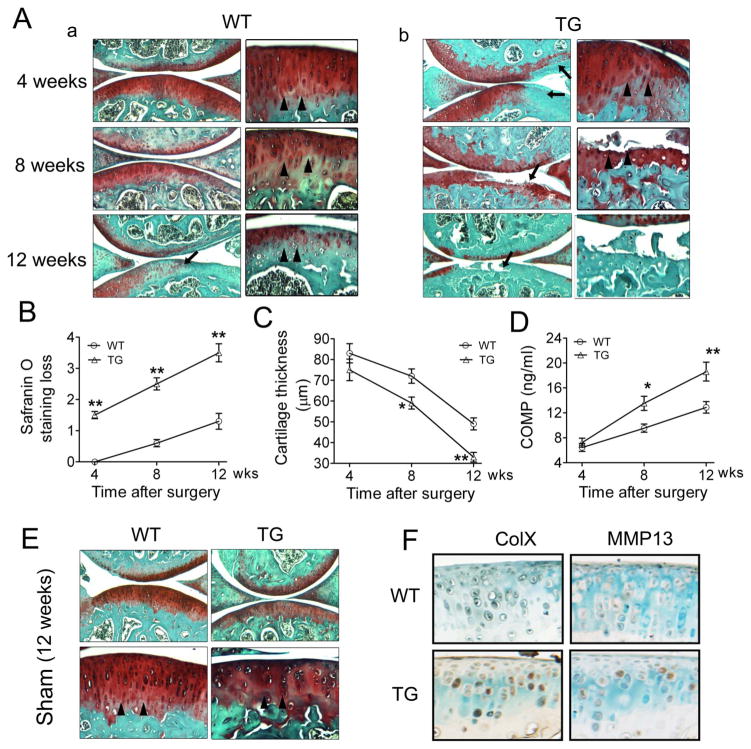
To further establish the role of ADAMTS-7 in OA onset and progression, we used a DMM surgical model. A moderate loss of proteoglycan was observed 4 weeks after DMM in the TG group, significant destruction of cartilage structure could be observed at 8 weeks, and even more proteoglycan loss and cartilage degradation was displayed at 12 weeks (figure 4A, b), while in the WT mice, minor proteoglycan loss could be seen until 8 weeks, and cartilage destruction was hardly visible even at 12 weeks (figure 4A, a). High magnification revealed that the tide mark shifted to the surface of the cartilage in the process of OA. TG mice exhibited a faster shift of the tide mark and a more irregular tide mark, and had almost completely lost the tide mark at 12 weeks (figure 4A). In addition, quantification of the OA pathological changes showed significant proteoglycan loss and cartilage destruction (as indicated by thinner cartilage thickness) at each time point in TG mice (figure 4B, C). As expected, the serum levels of COMP degradation fragments were also significantly higher at the 8- and 12-week time points in the TG mice (figure 4D). In the sham-operated knee joints of the contralateral side, articular cartilage showed a smooth surface, evenly stained with Safranin-O 12 weeks after surgery, and no significant difference could be observed between WT and TG mice (figure 4E). Expression of ColX and MMP13 in articular cartilage was assayed by immunohistochemistry 4 weeks after surgery, and revealed that ColX and MMP13 were nearly undetectable in WT mice, whereas significantly elevated levels of both molecules were observed in chondrocytes in the superficial and middle zone of cartilage in TG mice (figure 4F). These results suggest that ADAMTS-7 TG mice are more susceptible to surgery, leading to more severe changes in the articular cartilage and accelerated OA progression.
Figure 4.
Overexpression of ADAMTS-7 led to accelerated osteoarthritis (OA) progression in a surgically induced model. (A) Histological analysis of knee joints in wild-type (WT) (a) and transgenic (TG) (b) mice at various time points after surgery. The surgical model was induced in 8-week-old WT and TG mice by transecting the right knee medial meniscotibial ligament (WT, n=6; TG, n=8 for each time point). Representative low (left, original magnification ×40) and high (right, original magnification ×100) magnifications of articular cartilage are presented. Arrows indicate the Safranin-O staining loss, and arrowheads indicate the tidemark. (B) OA severity in the course of OA development, assessed by Safranin-O staining loss score. (C) OA severity in the course of OA development, assayed by cartilage thickness measurement. (D) Serum levels of COMP fragments in the OA progression, measured by ELISA. (E) Safranin-O staining of sham-operated knee joints 12 weeks after surgery. Representative low (top, ×40) and high (bottom, ×100) magnifications are presented. (F) Immunohistochemistry for collagen X (ColX) and matrix metalloproteinase (MMP13) in the WT and TG mouse articular cartilage 4 weeks after surgery (original magnification ×100). Values are normalised mean±SEM. *p<0.05, **p<0.01 versus WT mice.
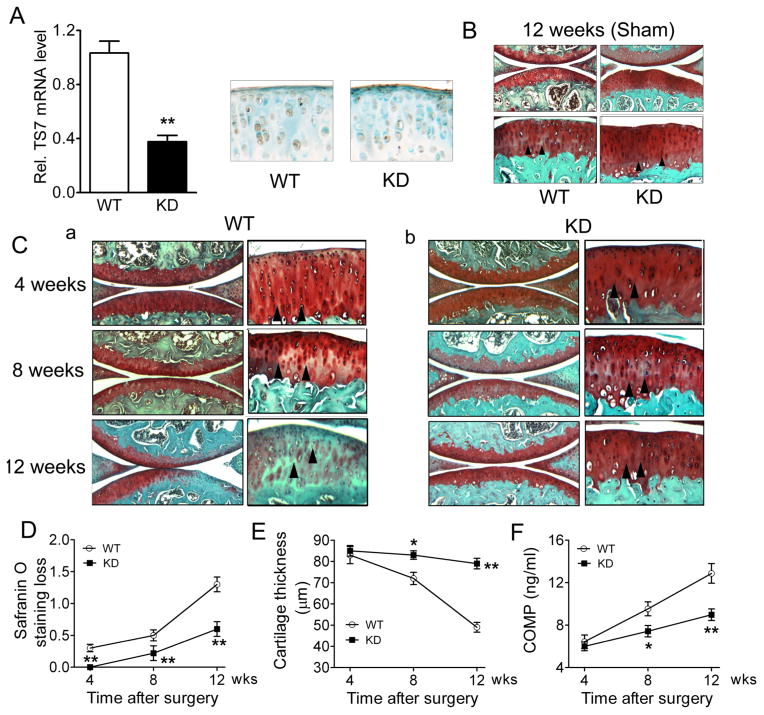
Knockdown of ADAMTS-7 protected against OA development in the DMM model
We further confirmed the detrimental role of ADAMTS-7 in surgically induced OA by using ADAMTS-7 conditional KD mice. Reduced expression of ADAMTS-7 in the articular cartilage of KD mice was verified using real-time RT-PCR and immunostaining of ADAMTS-7 (figure 5A). The ADAMTS-7 KD mice did not show any apparent abnormalities in skeletal development (data not shown). Both sham operation and DMM were performed in WT and KD mice, and knee joints were collected 4, 8 and 12 weeks after surgery. In both WT and KD groups, sham-operated knee joints remained healthy after 12 weeks, and no obvious OA phenotype was observed (figure 5B). In the DMM group, WT mice showed severe proteoglycan loss at 12 weeks (figure 5C, a), while KD mice did not show any significant signs of OA development over the time course (figure 5C, b). Quantification analysis demonstrated that proteoglycan loss (figure 5D) and cartilage destruction (as indicated by thinner cartilage thickness, figure 5E) were significantly less in KD mice than in WT mice at each time point. In addition, serum levels of COMP fragments were significantly lower in KD mice (figure 5F). Collectively, these data indicate that suppression of ADAMTS-7 expression prevents surgically induced OA progression.
Figure 5.
ADAMTS-7 knockdown (KD) mice displayed attenuated osteoarthritis (OA) development in surgically-induced model. (A) The relative level of ADAMTS-7 in 8-week-old KD mice, assayed by real-time RT-PCR. Normalised values were calibrated against the wild-type (WT), given the value of 1. (B) Immunohistochemistry analysis for ADAMTS-7 expression in the articular cartilage from both 8-week-old WT and KD mice. (C) Safranin-O staining of sham-operated knee joints 12 weeks after surgery. Representative low (top, ×40) and high (bottom, ×100) magnifications are presented. (D) Histological analysis of knee joints in WT (a) and KD (b) mice at various time points, as indicated, after surgery. Low (left, original magnification ×40) and high (right, original magnification ×100) magnification of articular cartilage are presented. Arrowheads indicate tidemark. OA severity was quantified by Safranin-O staining loss score (E) and cartilage thickness measurement (F). (G) Serum levels of COMP fragments were measured by sandwich ELISA in the course of OA development. Values are the normalised mean±SEM, n=5 and n=8 for WT and KD, respectively. *p<0.05, **p<0.01 versus WT mice.
ADAMTS-7 forms a positive regulatory loop with tumour necrosis factor (TNF)-α in cartilage and OA
Inflammatory cytokines, including TNF-α, are known to induce ADAMTS-7 expression in articular cartilage explant culture.14 To further explore the transcription regulation of ADAMTS-7 by TNF-α, a search for transcription factor consensus sequences (MatInspector, Genomatix) was performed, and three NF-κB-binding sites at the hADAMTS-7 5′ regulatory region were identified (see online supplementary figure S3A). Chromatin immunoprecipitation assay with p65 antibody demonstrated NF-κB recruitment to the ADAMTS-7 promoter region (see online supplementary figure S3B). Next we generated a series of ADAMTS-7-specific reporter constructs bearing the fragments of the 5′ flanking region of ADAMTS-7 ranging from −99/+61 to −1702/+61. Analysis of reporter gene activity indicated that the minimal promoter lies between −416 and +61. Activity increased greatly in deletions from −735 to −416, indicating the presence of a transcription-suppressive element within this region (see online supplementary figure S3C). Co-transfection of the ADAMTS-7 reporter constructs with an expression plasmid encoding NF-κB p65 showed significantly increased transcription activity, in which −416 TS7-luc showed the greatest increase in reporter gene activity (see online supplementary figure S3D left panel). As expected, TNF-α also activated ADAMTS-7 reporter constructs in chondrocytes (see online supplementary figure S3D right panel). Interestingly, TNF-α-mediated transactivation of ADAMTS-7 reporter genes was dose-dependently inhibited by Bay 11-7082, an NF-κB-specific inhibitor that blocks phosphorylation and subsequent degradation of IκB (see online supplementary figure S3E). These results clearly indicate that TNF-α induces ADAMTS-7 by activating the NF-κB pathway. To determine whether direct binding of NF-κB to its binding sites in the 5′-flanking region of the ADAMTS-7 gene is required for TNF-α activation of ADAMTS-7 reporter constructs, several point mutation reporter constructs of −1702 TS7-luc were generated (see online supplementary figure S3F). Mutations in the first and second NF-κB-binding sites did not show any obvious effects on TNF-α-induced transactivation of the ADAMTS-7-specific reporter gene. In contrast, mutations in the third NF-κB-binding site, which is closest to the transcription start site, gave rise to dramatically reduced reporter gene activity (see online supplementary figure S3G), suggesting that the most important NF-κB-binding site responding to TNF-α is located in this region. To further verify the role of NF-κB-specific sequences in driving ADAMTS-7 expression, the NF-κB-binding sequence of the −416-TS7-luc plasmid was mutated (see online supplementary figure S3H). The mutated reporter gene dramatically reduced luciferase activity induced by TNF-α, which further confirms that the NF-κB-specific site is responsible for the TNF-α-induced ADAMTS-7 expression.
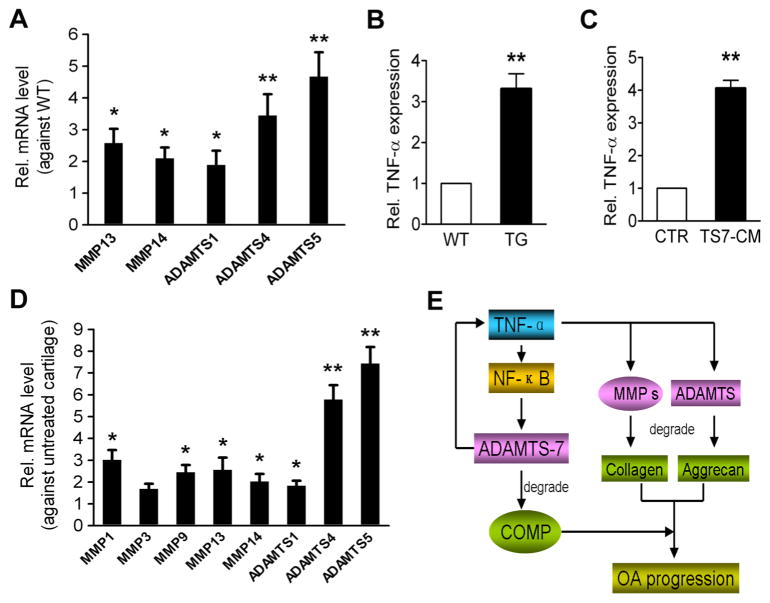
The detrimental role of ADAMTS-7 in cartilage degeneration and OA progression observed in this study prompted us to investigate whether overexpression of ADAMTS-7 affected the expressions of other MMPs and other ADAMTS family members, which are known to play important roles in cartilage degeneration in OA. Real-time RT-PCR results showed that the levels of MMP13, MMP14, ADAMTS-1, ADAMTS-4 and ADAMTS-5 in cartilage of 8-month-old TG mouse were significantly elevated (figure 6A). Intriguingly, TNF-α was also dramatically elevated in TG mice (figure 6B), suggesting that elevated levels of metalloproteinases seen in ADAMTS TG mice may be due, at least in part, to the upregulation of TNF-α by ADAMTS-7. In addition, upregulation of TNF-α and the metalloproteinases involved in OA, such as MMP1, MMP3, MMP9, MMP13, MMP14, ADAMTS-1, ADAMTS-4 and ADAMTS-5, by ADAMTS-7 was also confirmed using ex vivo culture of cartilage explants treated with control or ADAMTS-7-conditioned medium collected from HEK-293 EBNA ADAMTS-7 stable cell lines (figure 6C, D).
Figure 6.
ADAMTS-7 upregulates tumour necrosis factor (TNF)-α and metalloproteinases in cartilage. (A) Levels of matrix metalloproteinase (MMP) 13, MMP14, ADAMTS-1, ADAMTS-4 and ADAMTS-5 mRNA in articular cartilage from 8-month-old wild-type (WT) and transgenic (TG) mice, assayed by real-time RT-PCR. (B) RNA level of TNF-α in articular cartilage from 8-month-old WT and TG mice, assayed by real-time RT-PCR. The normalised values were calibrated against the control, given the value of 1. (C) ADAMTS-7 upregulates TNF-α levels in cartilage explants. Femoral head cartilage from 2-month-old WT mice was harvested, and cartilage explants were cultured for 48 h with either Dulbecco’s modified Eagle’s medium only (CTR; n=5) or ADAMTS-7-conditioned medium (TS7-CM; n=6) collected from HEK-293 EBNA stable cell lines transfected with a construct encoding ADAMTS-7, followed by real-time RT-PCR. (D) Expression levels of MMP1, MMP3, MMP9, MMP13, MMP14, ADAMTS-1, ADAMTS-4 and ADAMTS-5 mRNA in cartilage explants stimulated by ADAMTS-7, assayed by real-time RT-PCR. Normalised values were calibrated against the untreated controls, given the value of 1. (E) A proposed model for the role and regulation of ADAMTS-7 in the development of osteoarthritis. Each real-time RT-PCR was carried out in triplicate. All the data are presented as mean±SEM, *p <0.05, **p<0.01.
DISCUSSION
Excessive enzymatic activity of some proteases is thought to be associated with OA. The ADAMTS family is known to play an important role in the pathogenesis of OA.26 We have previously reported that ADAMTS-7 directly associates with and degrades COMP in vitro, and that its level is elevated in arthritic patients.6 In the present study, we first examined the expression pattern of ADAMTS-7 in OA and found that ADAMTS-7 expression was upregulated in cartilage during OA progression. Interestingly, the expression of ADAMTS-7 was relatively late in OA development (figure 1). One possible explanation is that, like many of the molecules we measure in OA, the presence of ADAMTS-7 is a late effect of cartilage damage. Next, we generated cartilage-specific TG mice. It has been reported that cartilage-specific overexpression of metalloproteinase results in cartilage degradation.27 Intriguingly, the TG mice presented dwarf-like status during the early stages of life, which was consistent with our previous finding that ADAMTS-7 is involved in chondrocyte differentiation and endochondral ossification.7 However, these TG mice reached a normal size and did not display any defects of skeletal development after 5 weeks of age, which may suggest the presence of compensatory mechanisms during postnatal development.
One of the risk factors for OA development is aging.28 We found that aged ADAMTS-7 TG mice displayed a significantly more severe OA phenotype than their WT littermates. Intriguingly, there was no significant difference in cartilage between WT and TG groups even 12 weeks after sham operation (figure 4E). In addition, no significant difference in body size was observed at 5 weeks or later between these two genotypes (figure 2K). These findings indicate that early developmental problems may not account for the exaggerated cartilage degeneration seen in aged TG mice. Time-controlled overexpression of ADAMTS-7 using inducible TG mice is needed to further determine the possible effect of development issues on OA development. In this study, we observed spontaneously developed OA in ‘aged’ TG mice, characterised by exaggerated loss of proteoglycan, destruction of cartilage structure, and osteophyte formation. In addition, levels of hypertrophic marker genes for chondrocytes, ColX and MMP13, as well as the degradation of ColII, aggrecan and COMP, were significantly higher in TG than WT mice.
ADAMTS-7 overexpression reduced ColX levels in the growth plate of 3-week-old TG mice, whereas elevated ColX levels were found in articular cartilage of aged TG mice. Inhibition of chondrocyte differentiation and hypertrophy by ADAMTS-7 overexpression in the growth plate at 3 weeks (figure 2) is probably due to its inactivation of the chondrogenic growth factor, PGRN, known to stimulate chondrocyte hypertrophy. 7 In contrast, elevated ColX expression seen in OA articular cartilage in aged ADAMTS-7 TG mice (figure 3J) may result from upregulated TNF-α and metalloproteinases, such as MMP13, which are known to induce articular chondrocyte hypertrophy and apoptosis. Surgically induced OA models, in particular the DMM model, are commonly used for OA research. However, there are limitations to this model, as it has been reported that DMM-induced OA severity is sex-dependent, and male mice sustain a more severe arthritis phenotype than female mice.29 Moreover, arthritis severity is closely associated with the strain of mouse, which impairs the application of the DMM operation in some insensitive strains.30 Despite the limitations of this model in exactly mimicking degenerative arthritis in humans, the DMM operation is well accepted because of its good reproducibility and slower disease progression.31 We established the DMM model using various genetically modified ADAMTS-7 mice, and demonstrated that overexpression of ADAMTS-7 exaggerated, while KD of ADAMTS-7 attenuated, the severity of the OA, suggesting that altered ADAMTS-7 expression may be associated with OA development.
COMP is a prominent non-collagenous component of extracellular matrix in cartilage, and is important for chondrocyte function and cartilage integrity.32,33 TG mice exhibited exaggerated degradation of COMP in OA progression, and accelerated onset of arthritis in OA models. This implies that overexpression of ADAMTS-7 led to increased degradation of COMP, which may subsequently weaken the stability of other matrix proteins, including aggrecan and ColII, and make them susceptible to degradation by proteases such as ADAMTS-5 and MMP13. This finding, together with the recent reports that ADAMTS-7 functions as a key regulator of arthrosclerosis mainly by degrading COMP,20 suggests that the role of ADAMTS-7 is mediated by its protease function in both the circulatory system and cartilage.19,34
TNF-α has been reported to play a critical role in cartilage destruction in arthritis.35–37 TNF-α enhances expression of MMPs and ADAMTS, and these increased proteases in turn cause exaggerated degradation of cartilage matrix, including ColII and aggrecan, which promotes progression of OA. TNF-α-mediated activation of the NF-κB signalling pathway is known to play an important role in the pathogenesis of OA.38 We also demonstrated that TNF-α-mediated upregulation of ADAMTS-7 is primarily through the NF-κB signalling pathway. Intriguingly, it appears that ADAMTS-7 and TNF-α form a positive feedback regulatory loop in cartilage, since ADAMTS-7 also upregulated TNF-α both in vitro and in vivo. On the basis of the present study and the literature, a model is proposed to illustrate the role and regulation of ADAMTS-7 in OA development (figure 6E). ADAMTS-7 plays a detrimental role in the progression of OA through multiple pathways. First, it directly associates with and degrades COMP, which may lead to the susceptibility of other cartilage matrix proteins, including ColII and aggrecan, to degradation. Second, it enhances expression of TNF-α, which in turn induces the expression of cartilage-degrading proteases and loss of cartilage. It is interesting to note that TNF-α upregulates ADAMTS-7 levels through the NF-κB pathway, thus forming a positive feedback loop with ADAMTS-7 in mediating cartilage degradation in OA.
In summary, this study not only provides insights into the actions of the ADAMTS-7 metalloproteinase and the inflammatory cytokine TNF-α, as well as their interactions in cartilage and degenerative arthritis, but may also lead to the development of novel therapeutic interventions for degenerative diseases by recruiting their inhibitors or derivatives.
Supplementary Material
Acknowledgments
We thank Dr Mary B Goldring for providing C28I2 chondrocytes. This work was supported partly by NIH research grants R01AR062207, R01AR061484, R56AI100901, K01AR053210, and a Disease Targeted Research Grant from Rheumatology Research Foundation (to C-JL).
Funding NIH research grants and a Disease Targeted Research Grant from the Rheumatology Research Foundation.
Footnotes
Contributors All authors made substantial contributions to the design, execution and reporting of the study, and approved the final version of the manuscript.
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/annrheumdis-2013-203561).
References
- 1.Herndon JH, Davidson SM, Apazidis A. Recent socioeconomic trends in orthopaedic practice. J Bone Joint Surg Am. 2001;83-A:1097–105. doi: 10.2106/00004623-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Salzet M. Leech thrombin inhibitors. Curr Pharm Des. 2002;8:493–503. doi: 10.2174/1381612023395664. [DOI] [PubMed] [Google Scholar]
- 3.Hurskainen TL, Hirohata S, Seldin MF, et al. ADAM-TS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM- TS family. J Biol Chem. 1999;274:25555–63. doi: 10.1074/jbc.274.36.25555. [DOI] [PubMed] [Google Scholar]
- 4.Glasson SS, Askew R, Sheppard B, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–48. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 5.Stanton H, Rogerson FM, East CJ, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–52. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 6.Liu CJ, Kong W, Ilalov K, et al. ADAMTS-7: a metalloproteinase that directly binds to and degrades cartilage oligomeric matrix protein. FASEB J. 2006;20:988–90. doi: 10.1096/fj.05-3877fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai XH, Wang DW, Kong L, et al. ADAMTS-7, a direct target of PTHrP, adversely regulates endochondral bone growth by associating with and inactivating GEP growth factor. Mol Cell Biol. 2009;29:4201–19. doi: 10.1128/MCB.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maass PG, Wirth J, Aydin A, et al. A cis-regulatory site downregulates PTHLH in translocation t(8;12)(q13;p11. 2) and leads to Brachydactyly Type E. Hum Mol Genet. 2010;19:848–60. doi: 10.1093/hmg/ddp553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q, Huang M, Wang X, et al. Negative effects of ADAMTS-7 and ADAMTS-12 on endplate cartilage differentiation. J Orthop Res. 2012;30:1238–43. doi: 10.1002/jor.22069. [DOI] [PubMed] [Google Scholar]
- 10.Guo F, Lai Y, Tian Q, et al. Granulin-epithelin precursor binds directly to ADAMTS-7 and ADAMTS-12 and inhibits their degradation of cartilage oligomeric matrix protein. Arthritis Rheum. 2010;62:2023–36. doi: 10.1002/art.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balsitis S, Dick F, Dyson N, et al. Critical roles for non-pRb targets of human papillomavirus type 16 E7 in cervical carcinogenesis. Cancer Res. 2006;66:9393–400. doi: 10.1158/0008-5472.CAN-06-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choubey D, Snoddy J, Chaturvedi V, et al. Interferons as gene activators. Indications for repeated gene duplication during the evolution of a cluster of interferon-activatable genes on murine chromosome 1. J Biol Chem. 1989;264:17182–89. [PubMed] [Google Scholar]
- 13.Di Cesare E, Costanzi A, Fedele F, et al. MRI postoperative monitoring in patients surgically treated for aortic dissection. Magn Reson Imaging. 1996;14:1149–56. doi: 10.1016/s0730-725x(96)00221-4. [DOI] [PubMed] [Google Scholar]
- 14.Luan Y, Kong L, Howell DR, et al. Inhibition of ADAMTS-7 and ADAMTS-12 degradation of cartilage oligomeric matrix protein by alpha-2-macroglobulin. Osteoarthritis Cartilage. 2008;16:1413–20. doi: 10.1016/j.joca.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu H, Zhu Y. Expression of ADAMTS-7 and ADAMTS-12 in the nucleus pulposus during degeneration of rat caudal intervetebral disc. J Vet Med Sci. 2012;74:9–15. doi: 10.1292/jvms.10-0556. [DOI] [PubMed] [Google Scholar]
- 16.Reilly MP, Li M, He J, et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet. 2011;377:383–92. doi: 10.1016/S0140-6736(10)61996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schunkert H, Konig IR, Kathiresan S, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–38. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peden JF, Hopewell JC, Saleheen D, et al. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43:339–44. doi: 10.1038/ng.782. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Zheng J, Bai X, et al. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ Res. 2009;104:688–98. doi: 10.1161/CIRCRESAHA.108.188425. [DOI] [PubMed] [Google Scholar]
- 20.Pu X, Xiao Q, Kiechl S, et al. ADAMTS7 cleavage and vascular smooth muscle cell migration is affected by a coronary-artery-disease-associated variant. Am J Hum Genet. 2013;92:366–7421. doi: 10.1016/j.ajhg.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai Y, Yu XP, Zhang Y, et al. Enhanced COMP catabolism detected in serum of patients with arthritis and animal disease models through a novel capture ELISA. Osteoarthritis Cartilage. 2012;20:854–62. doi: 10.1016/j.joca.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu CJ, Kong W, Xu K, et al. ADAMTS-12 associates with and degrades cartilage oligomeric matrix protein. J Biol Chem. 2006;281:15800–08. doi: 10.1074/jbc.M513433200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appleton CT, McErlain DD, Pitelka V, et al. Forced mobilization accelerates pathogenesis: characterization of a preclinical surgical model of osteoarthritis. Arthritis Res Ther. 2007;9:R13. doi: 10.1186/ar2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsiao G, Huang HY, Fong TH, et al. Inhibitory mechanisms of YC-1 and PMC in the induction of iNOS expression by lipoteichoic acid in RAW 264. 7 macrophages. Biochem Pharmacol. 2004;67:1411–19. doi: 10.1016/j.bcp.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Zufferey R, Donello JE, Trono D, et al. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol. 1999;73:2886–92. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu CJ. The role of ADAMTS-7 and ADAMTS-12 in the pathogenesis of arthritis. Nat Clin Pract Rheumatol. 2009;5:38–45. doi: 10.1038/ncprheum0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuhold LA, Killar L, Zhao W, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone. 2012;51:241–48. doi: 10.1016/j.bone.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma HL, Blanchet TJ, Peluso D, et al. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthritis Cartilage. 2007;15:695–700. doi: 10.1016/j.joca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Glasson SS. In vivo osteoarthritis target validation utilizing genetically-modified mice. Curr Drug Targets. 2007;8:367–76. doi: 10.2174/138945007779940061. [DOI] [PubMed] [Google Scholar]
- 31.Malfait AM, Ritchie J, Gil AS, et al. ADAMTS-5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthritis Cartilage. 2010;18:572–80. doi: 10.1016/j.joca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Chen FH, Thomas AO, Hecht JT, et al. Cartilage oligomeric matrix protein/ thrombospondin 5 supports chondrocyte attachment through interaction with integrins. J Biol Chem. 2005;280:32655–61. doi: 10.1074/jbc.M504778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen FH, Herndon ME, Patel N, et al. Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan. J Biol Chem. 2007;282:24591–98. doi: 10.1074/jbc.M611390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du Y, Gao C, Liu Z, et al. Upregulation of a disintegrin and metalloproteinase with thrombospondin motifs-7 by miR-29 repression mediates vascular smooth muscle calcification. Arterioscler Thromb Vasc Biol. 2012;32:2580–88. doi: 10.1161/ATVBAHA.112.300206. [DOI] [PubMed] [Google Scholar]
- 35.Furst DE. Development of TNF inhibitor therapies for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2010;28:S5–12. [PubMed] [Google Scholar]
- 36.Rasheed Z, Haqqi TM. Update on targets of biologic therapies for rheumatoid arthritis. Curr Rheumatol Rev. 2008;4:246. doi: 10.2174/157339708786263915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang W, Lu Y, Tian QY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–84. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.