Abstract
Neuroblastoma is a developmental tumor of young children arising from the embryonic sympathoadrenal lineage of the neural crest. Currently neuroblastoma is the primary cause of death from pediatric cancer for children between the age of 1 and 5 years and accounts for approximately 13% of all pediatric cancer mortality. Its clinical impact and its unique biology have made this aggressive malignancy the focus of a large concerted translational research effort. New insights into tumor biology are driving the development of new classification schemas; novel targeted therapeutic approaches include small molecule inhibitors, epigenetic, non-coding RNA, and cell-based immunologic therapies. Recent insights regarding the pathogenesis and biology of neuroblastoma will be placed in context with the current understanding of tumor biology and tumor/host interactions. Systematic classification of patients coupled with therapeutic advances point to a future of improved clinical outcomes for this biologically distinct and highly aggressive pediatric malignancy.
Keywords: Biology, epigenetics, epithelial-to-mesenchymal transition, neural crest development, oncogenes, review
Introduction
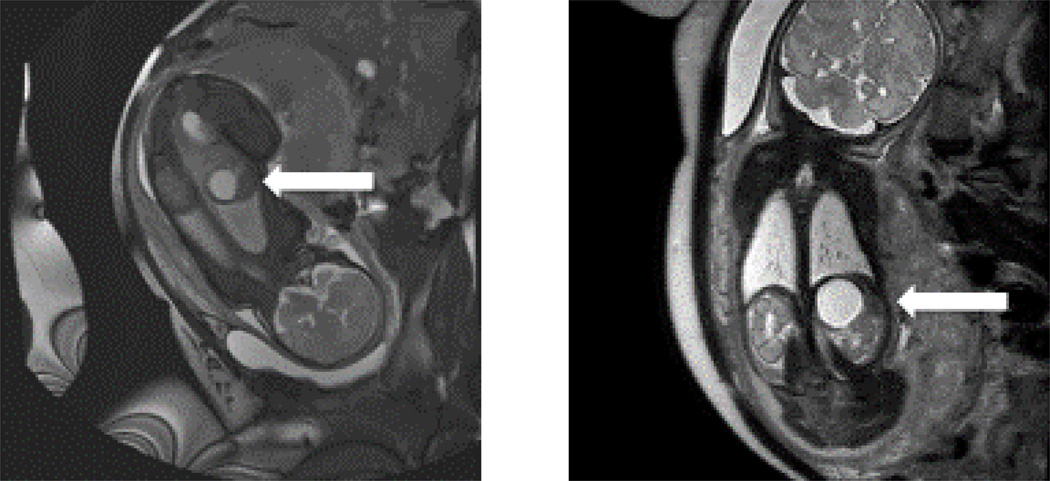
Neuroblastoma (NB) is the most common extra-cranial solid tumor in childhood. In 2010, the age-adjusted incidence in the United States was 10.7 cases per 1,000,000 persons aged 0–14 years (1). However, these numbers likely underestimate its true incidence as neuroblastoma regresses in some infants who therefore may never present to medical attention. The median age at the time of diagnosis is approximately 19 months and ranges from in-utero identification by fetal MRI (Figure 1) to the rare cases diagnosed each year in patients older than 19 years of age (2). Historically, neuroblastomas are slightly more common in boys and can be seen in all North American ethnic groups with 2010 incidence rates reported as 9.0 cases in Whites, 6.0 in Blacks, 6.3 in Asian/Pacific Islanders, 5 in American Indian/Alaska Native, and 6.5 in Hispanics per 1,000,000 children aged 0–19 (1).
Figure 1.
Cystic adrenal mass identified on a non-contrast fetal MRI obtained at 36 weeks and 2 days of gestation. The white arrows are pointing to a 3.1×3×2.8 cm lesion in the left adrenal gland.
Neuroblastoma remains distinct from other solid tumors due to its biological heterogeneity and range of clinical behavior spanning from spontaneous regression to cases of highly-aggressive metastatic disease unresponsive to standard and investigational anti-cancer treatment. Using historical overall and event-free survival rates combined with histological and biological criteria, patients with neuroblastoma can be assigned a pre-treatment risk classification (3). Although the histological and biologic characteristics used within classification schemas continue to evolve based on new scientific data, they are used to divide patients into low, intermediate and high-risk strata. In general, those with low risk disease have excellent event free and overall survival rates with observation only or minimal therapeutic interventions. The outcome of patients with intermediate risk disease, using primarily surgery and chemotherapy, have improved to the point where many groups are focused on using biological markers to help further decrease therapy in specific sub-populations of children (4). Patients with high-risk disease, comprising approximately half of all new neuroblastoma cases each year, require treatment with multi-modal therapy using induction chemotherapy, surgery, radiotherapy, high-dose chemotherapy with autologous stem cell rescue, biologic and immunotherapeutic maintenance therapy in order to improve their survival odds. Using this aggressive therapeutic strategy, the Children’s Oncology Group (COG) has reported a 4-year event-free survival (EFS) of 59+5% in patients treated on the most recent Phase III clinical trial using ch14.18 immunotherapy (5). Therefore, as a significant number of patients will still relapse and eventually die of disease, it remains important for investigators to both better understand the origins of this disease and develop novel treatment strategies for those who are diagnosed with it.
Molecular Pathogenesis of Neuroblastoma – A Tumor of the Neural Crest
Neuroblastoma is a developmental malignancy arising within the neuronal ganglia of the peripheral sympathetic nervous system. These neuronal structures derive from the venterolateral neural crest cells, which migrate away from the neural tube early during embryogenesis (6). Thirty percent of neuroblastoma tumors arise within the adrenal medulla, approximately 60% will arise from abdominal paraspinal ganglia, and the remaining is from the sympathetic ganglia in the chest, head/neck and pelvis. As such, the clinical presentation and subsequent outcomes of neuroblastoma are highly variable. Long-term survival is primarily dependent on the degree of differentiation, with patients exhibiting more primitive crest-like tumors doing worse than patients with more differentiated tumors who have a more favorable outcome (7). The extensive clinical and pathologic heterogeneity of this malignancy reflects the unique developmental biology of the neural crest (8). Placing the pathogenesis of neuroblastoma in the context of neural crest embryogenesis may help to explain the complex molecular heterogeneity of this disease and help identify molecules and pathways for specific biologically-targeted interventions.
Sometimes referred to as the fourth germ layer, the neural crest is a transient embryologic tissue derived from neuroectoderm (9). In vertebrates during neural tube formation, a remarkable maturation process occurs within the neural crest, which responds to a complex transcription factor/epigenetic regulatory schema (10, 11). Through this process, the earliest neural crest precursors gain multipotent differentiation potential and obtain a self-renewing phenotype reminiscent of embryonic stem cells. Subsequent cascading signaling gradients of BMP, Wnt, Notch and other ligands drive differentiation into epithelial, mesenchymal, and endothelial components of the face, trunk, and heart (12, 13) and include the peripheral sympathetic ganglia and neuroendocrine adrenal medulla (14). Inhibition of this maturation process may predispose early multipotent neural crest precursors to malignant transformation.
EMT and MET Transitions within the Neural Crest
A central component of neural crest maturation is a programmed epithelial-to-mesenchymal transition (EMT) (12, 15). During embryogenesis, a series of transcriptional factors including ZIC1, PAX3, TPAP2a, Notch and PRDM1A initiate the crest developmental pathway after the neural tube forms (16, 17). This distinguishes early neural crest cells from primitive neuroectoderm. Subsequent expression of the SOXE family (SOX 8, 9, 10) as well as ZEB2 and other factors, drive mesenchymal transformation (e.g. loss of E-Cadherins, loss of cell contacts, activation of metalloproteinases). Next, BMP, Wnt and FGF signaling within the microenvironment further drive differentiation of these mesenchymal migratory neural crest cells. The early neural crest is similar to other pluripotent cell populations -with regards to their self-renew capacity and ability to generate many different tissue types. Expression of pro-survival and pluripotency factors such as SOX10, FOXD3, C-Myc and MYCN allow these cells to become highly proliferative and resistant to apoptosis (18).
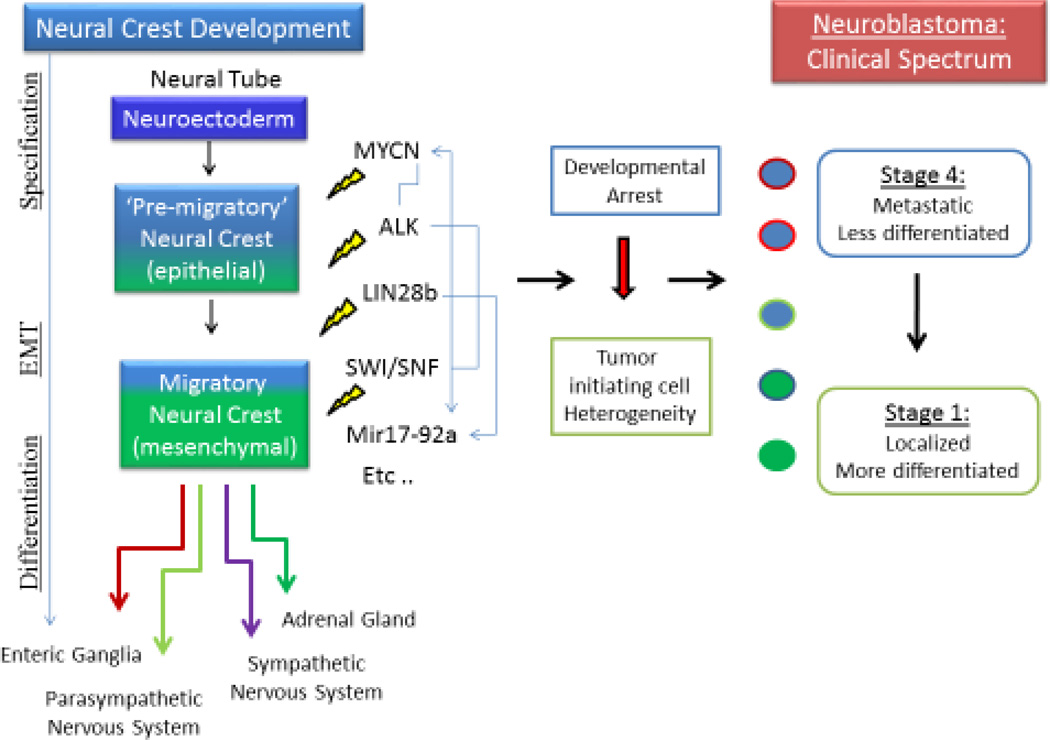
The observed clinical and pathological heterogeneity of neuroblastoma may well result from diverse molecular drivers disrupting this carefully orchestrated process at discrete stages of neural crest maturation (Figure 2). NB tumor initiating cells or cancer stem cells (CSCs) of various backgrounds may yield distinct tumor phenotypes according to the developmental stage of their crest precursors (19, 20). This concept is supported by the recent observation of tumorigenic stem cell-like subpopulations within neuroblastoma that differentially express elevated SOX10, E-Cadherin and other pre-migratory early crest markers (21). In addition, a distinct subset of highly undifferentiated neuroblastoma (Stage IVS or M4S) presents with metastatic disease in very young infants. Remarkably, some of these tumors spontaneously regress within months as the child matures, strongly suggesting that this subtype of NB requires non-cell autonomous growth factors for survival (22, 23). Alternatively, lesions arising from a more mesenchymal precursor may be highly metastatic and lack requirements for external growth factors. Controlled inhibition, but not mutation, of p53 is required for persistence of early crest precursors (24), which corresponds to the observation that NB is almost uniformly p53 wild-type at diagnosis yet resistant to apoptotic stresses (25, 26). Tumor initiating cells arising at later stages may yield more differentiated and therefore less malignant low stage tumors. Consideration of a uniquely dynamic and multipotent neural crest developmental program can guide the generation of novel and innovative therapeutics for crest derived malignancies such as neuroblastoma. Some of the well-defined oncogenic drivers of neuroblastoma are reviewed below.
Figure 2.
Neuroblastoma is a spectrum of diseases with a wide range of clinical behaviors. Disruption of the normal maturation progression with different genetic drivers at different times leads to heterogeneity of tumor initiating cells. Interaction between different epigenetic and genetic factors complicates the task of defining a primary oncogenic driver or pathway for this disease. This results in a wide range of pathologies with highly variable responses to treatment.
Neuroblastoma Oncogenic Drivers and Transcriptional Networks
While the origins of neuroblastoma tumorigenesis arise from the disrupted development of neural crest precursors, no single genetic or epigenetic mutation has been found, after the DNA and RNA sequencing of over one thousand cases, to account for all cases of NB (27). Likewise, structural genomic changes have not been linked to NB tumorigenesis. For example, 1p deletion, MYCN amplification, or gain of 17q may identify subtypes of neuroblastoma and impact survival (28, 29), yet there is no common neuroblastoma-specific genomic alteration, LOH or genetic translocation uniformly ascribed to all high-risk neuroblastoma tumors. Thus, this extensive molecular heterogeneity supports the concept that neuroblastoma represents a spectrum of disease. Clinically, this presents a challenge as tumors that are phenotypically and morphologically very similar can have highly disparate responses to treatment. Consequently, extensive efforts have focused on characterizing the transcriptomes and oncogenic pathways active in the most aggressive and fatal subtypes (30–32). In addition to elucidating the genetic and epigenetic origins of neuroblastoma, these efforts are motivated by the potential to yield actionable therapeutic targets for this highly fatal cancer.
MYCN
The MYCN oncogene plays a major role in neuroblastoma tumorigenesis and defines an aggressive subset of tumors. Amplification of MYCN (defined as more than 10 copies) is found in about 20% of cases overall and confers a particularly poor prognosis. Well-defined transgenic mouse models confirm that deregulated MYCN expression targeted to the neural crest is sufficient to drive tumorigenesis with high penetrance (33, 34). This transcription factor both activates and represses genetic targets (e.g. mRNA, miRNAs, lncRNAs) through direct DNA binding as well as indirect protein/protein interaction mechanisms (35–38). Both MYCN and MYCC (C-MYC) have well described anti-p53, pro-proliferative functions and pro-EMT functions (31, 39). During normal embryogenesis and neural crest development, MYCN is transiently expressed in the ventral-lateral migrating crest cells destined to become sympathetic ganglia (40). Thus, it is not surprising to find high levels of MYCN in a subset of poorly differentiated aggressive neuroblastomas (7). This has translated to clinical approaches targeting MYCN and other downstream pathways such as MDM2 (RG3788, Roche Pharmaceuticals) (41, 42), ODC1 (difluoromethylornithine -DFMO) (43) and mTOR (Temazolamide) (44, 45). However, many high-risk cases have minimal MYCN expression, suggesting additional mechanisms for tumorigenesis independent of MYCN deregulation (46).
ALK
Activating mutations of ALK (anaplastic lymphoma kinase) are also implicated as oncogenic drivers of neuroblastoma (47). Mutations are found in almost all cases of familial neuroblastoma (<1% of total NB cases) and between 6–10% of spontaneous cases (48). This receptor tyrosine kinase (RTK) is also implicated as an oncogene in lymphomas and lung cancers where it is typically found as a translocated fusion gene (ALK-NPM) (49, 50). Recent studies link ALK to sympathetic neuron development and survival of migratory neural crest cells (51), as well as being essential for neurogenesis in Zebrafish models. This gene is an important regulator of stem cell functions, including STAT3 dependent self-renewal, and as a transcriptional target of MYCN, high expression predicts poor outcome (52). Recent data from genetically engineered mouse models of neuroblastoma confirm that ALK and MYCN cooperate to promote tumorigenesis (53). Of note, this kinase is amenable to drug targeting, and potent ALK inhibitors are already in clinical trials for ALK mutant neuroblastoma.
PHOX2B
Germ line mutations of Paired-like Homeobox 2B (PHOX2B) are found in a subset of familial neuroblastoma and in about 4% of sporadic cases (54, 55). PHOX2B and PHOX2A drive differentiation of neural crest precursors toward sympathetic neurons (56). Mutations in this pathway are associated with neurocristopathies involving sympathetic and parasympathetic lineages such as Hirschsprung’s disease and central hypoventilation syndrome (57, 58). Recently, PHOX2B loss-of-function mutations have been shown to block neuroblastoma differentiation by disrupting calcium regulation (59). PHOX2B may also inhibit ALK expression in neuroblastoma (60); further suggesting that loss of PHOX2B function contributes to the pathogenesis of a subset of neuroblastoma tumors.
Epigenetics
Chromatin immunoprecipitation with high throughput sequencing (ChIPseq) and RNA sequencing studies have demonstrated specific epigenetic patterns which distinguish neuroectoderm, neural crest, and more mature neural states (61). For example, crest-specific patterns of histone modifications (H3k27ac, H3K4me1 and p300) mark enhancers of genes such as SNAIL, SOX10 and FOXD3 involved in EMT (62). A subset of these crest-specific promoters share the ‘bivalent’ H3k27me3/ H3K4me3 marks of poised promoters associated with pluripotent ESCs. In addition, the major chromatin modifying complex SWI/SNF and its subunits BRG1, ARID1 and BRN play critical roles in crest maturation (63), and mutations of these factors are linked to neuroblastoma tumorigenesis as detailed below. DNMT3B dependent DNA demethylation also participates in neural crest maturation by activation of differentiation specific genetic pathways (64), and alterations in this methyltransferase promote tumorigenesis (65). The development of effective and specific epigenetic drugs may soon permit a reverse of pathogenic epigenetic modifications and therefore promote neuroblastoma to differentiate along its programmed neural crest maturation pathway.
ATRX is another epigenetic factor mutated in a distinct subtype of NB tumors presenting in older children and adolescents. Interestingly, this gene contains an ATPase/helicase domain and is another member of the SWI/SNF family of chromatin modifiers. Functionally, it is involved in regulation of telomere length (66). Mutations were found in 44% of Stage IV4 neuroblastoma cases presenting in children ≥12 years of age, but only 9% of cases in children <12 years of age. Notably, no mutations were identified in children less than 1 year of age (67). Older children with this mutation typically have slow growing, yet inexorably progressive forms of neuroblastoma with a high overall mortality rate. As noted above, epigenetic modifiers critically regulate neural crest maturation, and this association with ATRX mutations provides further evidence of disrupted crest developmental pathways driving neuroblastoma.
Non-coding RNAs
Non-coding RNAs (microRNA, lncRNAs, piRNAs) are essential transcriptional regulators of stem cell biology, development and neural crest differentiation. Many of these microRNAs are deregulated in aggressive neuroblastomas and act to block p53 activity and promote EMT and metastasis (36, 68). Recently, neural crest directed expression of LIN28, an important regulator of microRNA processing, was shown to promote neuroblastoma tumorigenesis in part by inhibiting Let7a microRNA mediated tumor suppression (69). Additionally, miR-9, miR-17-92a, and the miR-25-106b cluster, as well as multiple other microRNAs are directly implicated in either tumorigenesis, metastasis or regulation of differentiation of neuroblastoma and other cancers (70, 71). Numerous studies of the biologic and prognostic impact of microRNAs in neuroblastoma have been reviewed elsewhere (36, 72). Due to the added complexity of non-coding RNAs, defining the entire transcriptomes of neuroblastomas becomes a monumental task; however, it is becoming increasingly possible to integrate coding and non-coding RNA transcriptomic and epigenetic data (e.g., ChIP-seq, and methylome analyses) with similar datasets obtained from models of neural crest development. This should help to further define novel therapeutic targets and pathways common to the heterogeneous subtypes of neuroblastoma.
Future Directions
Tumors are complex collections of varying subtypes of cells, all interacting with each other and the external environment to proliferate and spread. (8). The concepts of CSCs, cellular plasticity and tumor heterogeneity are providing new contexts for investigators to understand the clinical behavior of aggressive cancers such as neuroblastoma (19). Neuroblastoma CSC-like cells recently characterized from murine and human tumor models, as well as primary cancers, demonstrate selective responsiveness to inflammatory cytokines such as granulocyte colony-stimulating factor (G-CSF) and interleukin-6 (IL-6) (73). Signaling by soluble factors and cell-cell interactions between CSCs, non-stem tumor cells, tumor stromal and immune cells must all be considered in order to optimize therapeutic options (21). Disease progression, an all too common an occurrence for neuroblastoma, may be due to inadequately targeting resistant subpopulations. Inflammatory mediators likely promote tumor proliferation and facilitate tissue degradation leading to metastasis. In addition, it is now clear that tumors such as neuroblastoma co-opt the inflammatory immune response to tissue damage, activating and repressing subpopulations of T-cells and macrophages to generate an immune-privileged tumor microenvironment (74). This perspective is guiding the development of novel therapeutics based on adoptive immunotherapy and other approaches to sensitize and redirect the immune system to tumors.
Clinical Integration of Genomic and Biological Data
During the last decade, as neuroblastoma investigators have begun to better understand tumor biology and genetics, they have also begun to use this data to subdivide and risk classify newly diagnosed patients. Working as a collaborative body, the International Neuroblastoma Risk Group (INRG) task force generated a database containing the biological, histological, radiographic and clinical data from 8800 neuroblastoma patients treated across the globe (75). This database has been used to develop the current INRG staging and risk classification schemas that provide an international standard for patient classification. This standard will make it easier for investigators to compare clinical and research data between single institutions, national and international cooperative groups.
Staging and Pre-Treatment Classification
The International Neuroblastoma Staging System (INSS), first published in 1988, used the extent of the initial surgical procedure to define the stage of the patient (76). Small localized tumors that were completely resected were considered Stage I lesions. Stage II tumors were small, may or may not have had lymph node involvement, but could not be completely resected. Stage III lesions were large tumors that crossed the anatomical midline of the patient and could not be completely resected. Lastly, Stage IV and IVS metastatic tumors were differentiated by the fact that IVS patients were <1 year of age with metastatic disease located in liver, skin and less than 10% of the bone marrow (76).
Although used for over 20 years, staging per INSS was influenced by the location of the primary tumor (e.g., intrathoracic vs. abdominal primary), access to experienced pediatric surgery and pathology teams, and access to detailed radiographic imaging. To compensate for these variables, the new INRG Staging System (INRGSS) determines overall staging based on the extent of disease and presence of pre-operative, radiographic, image defined risk factors (IDRFs) that can be used to assess resectability (77, 78). For example, as seen in Table 1, L1 tumors are defined as localized lesions that do not involve vital structures, as defined within the IDRFs, and are confined to 1 body compartment; as compared to L2 tumors that are loco-regional lesions with 1 or more IDRFs.
Table 1.
International Neuroblastoma Risk Group (INRG) Staging System from Monclair et al. JCO 2009
| Stage | Definition |
|---|---|
| L1 | Localized tumor not involving vital structures as defined by the list of image-defined risk factors and confined to one body compartment. |
| L2 | Locoregional tumor with presence of one or more image-defined risk factors. |
| M | Distant metastatic disease (except stage MS). |
| MS | Metastatic disease in children younger than 18 months with metastases confined to skin, liver, and/or bone marrow. |
The current INRG pre-treatment classification schema combines different clinical and biological factors associated with prognosis, including INRG stage, age, histology, tumor differentiation, MYCN status, 11q LOH, and plody to determine a patient’s risk group (78). The risk groups have been formally expanded to include 4 categories: very low risk, low risk, intermediate risk, and high risk. The 4 risk groups depicted in Table 2 can then be used to assign treatment recommendations or assess a patient’s eligibility for participation on investigational studies (78).
Table 2.
International Neuroblastoma Risk Group (INRG) consensus pre-treatment classification schema from Cohn et al. JCO 2009
| INRG Stage |
Age (months) |
Histologic Category |
Grade of Tumor, Differentiation |
MYCN | 11q Aberration |
Plody | Pre-treatment Risk Group |
|---|---|---|---|---|---|---|---|
| L1/L2 | GN maturing GNB intermixed |
A Very Low | |||||
| L1 | Any, except GN maturing or GNB intermixed |
NA Amp |
B Very Low K High |
||||
| L2 | <18 | Any, except GN maturing or GNB intermixed |
NA NA |
No Yes |
D Low G Intermediate |
||
| L2 | >18 | GN nodular; Neuroblastoma |
Differentiating Differentiating Poorly differentiated or undifferentiated |
NA NA NA Amp |
No Yes |
E Low H Intermediate H Intermediate N High |
|
| M | <18 <12 12 to <18 <18 ≥18 |
NA NA NA Amp |
Hyperdiploid Diploid Diploid |
F Low J Intermediate J Intermediate O High P High |
|||
| MS | <18 | NA NA Amp |
No Yes |
C Very Low Q High R High |
GN: ganglioneuroma; GNB: Ganglioneuroblastoma; NA: Non-amplified; Amp: Amplified; blank field = “any”; diploid (DNA index ≤ 1.0); hyperdiploid (DNA index > 1.0 and includes near-triploid and near-tetraploid tumors); very low risk (5-year EFS > 85%); low risk (5-year EFS > 75% to ≤ 85%); intermediate risk (5-year EFS ≥ 50% to ≤ 75%); high risk (5-year EFS < 50%)
Treatment of Neuroblastoma
Neuroblastoma treatment recommendations range from observation only to intensive, multi-modal therapy and have been based on the event free and overall survival of patients enrolled on large, cooperative group clinical trials. The use of biologic correlates has further allowed the INRG to streamline therapy recommendations. For example, intermediate risk chemotherapy is recommended for patients with L2 tumors, who are <18 months of age, with any histology other than Ganglioneuroma (GN) maturing or Ganglioneuroblastoma (GNB) intermixed histology, and an 11q aberration as compared to a recommendation for observation only for that same patient population without an 11q aberration due to their low risk classification (78). With the impact that biological and genetic information has had on outcome analysis and associated future treatment recommendations, it becomes paramount for investigators to streamline testing on primary tumor and plasma samples while also saving as much material as possible to retrospectively analyze data from scientific discoveries yet to be made.
There have been a number of excellent reviews recently published describing the agents, both chemotherapeutic and immunotherapeutic, used in the treatment of neuroblastoma (79–81); therefore, the global recommendations for treatment by risk category are described below.
Non-High Risk
With event-free survival outcomes greater than 90% in these patients, there is a concerted effort to identify the subgroups where therapy can further reduced (4, 23, 82–84). In 2012, Nuchtern et al noted that in patients <6 months of age with small, localized adrenal lesions, observation alone was a safe treatment option (22). Of the 84 patients whose lesions were initially observed, 81% spontaneously regressed, and the overall 3-year disease free survival (DFS) was 97.7% (22). This data, coupled with the excellent outcome reported on patients <18 months of age with localized tumors and favorable genomics (hyperdiploid, no chromosome 1p or 11q LOH) treated on A3961 and P9641, is the rationale for the current proposal within the COG to investigate the use of expectant observation in children with a broader age range and slightly larger localized tumor size (4).
The current recommendations for other patients with localized tumors are associated with the presence or absence of IDRFs. Complete excision should be attempted for INRG L1 tumors. Historically larger lesions have been treated with neo-adjuvant chemotherapy in an effort to shrink the tumor prior to surgical resection (77). Low risk lesions with MYCN amplification have a significantly worse EFS (53% vs 72%, P<0.0001) and therefore additional therapy after resection is required (85). The outcomes for patients with local, but unresectable tumors (INRG L2; INSS Stage II or III) are dependent on the histology and biology of the lesion. Results from the COG P9641 and the SIOP European Neuroblastoma Group (SIOPEN) LNESG1 studies both found that patients with INSS Stage II lesions and unfavorable biological features had a worse disease free and overall survival (OS) as compared to those with favorable features (23, 86). In the COG trial, patients with INSS Stage IIB tumors that were either diploid or had an unfavorable histology had a poorer OS as compared to their hyperdiploid or favorable histology counterparts (82). The SIOPEN results noted an EFS and OS difference of 96.4% vs 75.9% and 85.5% vs 61.2%, respectively, for patients with Stage II tumors with favorable vs unfavorable features. Results were similar for those with Stage III disease. Matthay et al showed that older patients with Stage III disease with unfavorable features had a 15% difference in 4-year EFS and 25% in 4-year OS as compared to those with favorable features of any age (65% vs. 80% and 75% vs. 100%, respectively) (87). The COG is considering updating the criteria for high-risk patients to include those over the age of 18 months with INRG L2, MYCN non-amplified lesions, with unfavorable histology and genetics based on this data (4).
A subset of patients with metastatic neuroblastoma is considered non-high risk. Asymptomatic infants with INSS Stage IVS disease can be observed. However, those less than 2 months of age at diagnosis and those with hepatomegaly are at risk for rapid disease progression and strong consideration for the early initiation of chemotherapy should be made in these children (4, 88). The INRG classification schema defines patients <18 months with non-amplified, hyperdiploid stage IV disease as low risk and those of the same age with non-amplified, diploid disease as intermediate risk based on their biological features (78).
High Risk
In general, patients with MYCN amplification and/or those >18 months of age with INRG Stage M disease are considered high-risk. Treatment for patients with high-risk disease is multimodal and involves the use of chemotherapy, surgery, radiotherapy, biologics (cis-retinoic acid) and immunotherapy. Although there (89), the addition of immunotherapy has provided the largest impact on the EF and OS of children who have not progressed prior to that point. In the update by Yu et al to their 2010 report of the Phase III study randomizing patients to cis-retinoic acid alone vs cis-retinoic acid, Interleukin-2 (IL-2), granulocyte-macrophage colony-stimulating factor (GM-CSF) and a chimeric monoclonal antibody targeting GD2 (ch14.18), the use of immunotherapy is associated with an improvement in the 4-year EFS of 48% vs. 59% and OS of 59% vs. 74%, respectively (5, 90). To improve upon these results, other investigators are studying alternative infusion and immunotherapy administration schedules (91, 92).
COG has also considered adding other targeted agents to the next therapeutic protocol for patients with high-risk disease. As previously noted, ALK mutations and aberrations are found in a small percentage of neuroblastoma tumors. Therefore, one potential modification to the treatment schema would be the addition of an oral tyrosine kinase inhibitor targeting ALK for those patients with documented ALK mutations or aberrations.
Many groups are looking for correlative biomarkers to better and more accurately determine treatment responders and non-responders. The 2 most studied indicators include the use of a radiographic, semi-quantitative metaiodobenzylguanidine (MIBG) scoring system and the measurement of minimal residual disease markers in the blood and bone marrow of patients at various time points during treatment (4, 93–95). Once validated, these biomarkers could be important tools to help investigators determine if patients should continue with standard of care treatment plans or be offered alternative treatment options due to their risk of developing relapsed or refractory disease.
Relapsed/Refractory Disease
Treatment of patients with relapsed and refractory disease remains a challenge. Recent data reported by Modak et al shows that patients with a solitary lesion at the time of relapse can be salvaged and recommended using agents with known anti-neuroblastoma activity as compared to experimental therapy in this population (96). However, with the advances in targeted therapies and the understanding that the genetics and biology of tumors at the time of relapse may be different than the original diagnosis, obtaining tumor samples at the time of relapse will be required in order to generate rational treatment choices for children with this disease.
Acknowledgments
CUL is supported by a Sidney Kimmel Translational Science Award, NIH RO1 CA142636, and NIH P01 CA094237. JMS is supported by Alex’s Lemonade Stand, The Gillson-Longenbaugh Foundation, and NIH RO1 CA174808.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose related to the project. The Center for Cell and Gene Therapy has a collaborative research agreement with Celgene.
Literature Cited
- 1.CDC. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2010 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2013. [Google Scholar]
- 2.London WB, Castleberry RP, Matthay KK, et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children's Oncology Group. J Clin Oncol. 2005;23:6459–6465. doi: 10.1200/JCO.2005.05.571. [DOI] [PubMed] [Google Scholar]
- 3.Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol. 2009;27:298–303. doi: 10.1200/JCO.2008.16.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JR, Bagatell R, London WB, et al. Children's Oncology Group's 2013 blueprint for research: neuroblastoma. Pediatr Blood Cancer. 2013;60:985–993. doi: 10.1002/pbc.24433. [DOI] [PubMed] [Google Scholar]
- 5.Yu AL. Update on Outcome for High-Risk Neuroblastoma Treated on a Randomized Trial of Chimeric anti-GD2 Antibody (ch14.18) + GM-CSF/ IL2 in First Response: A Children's Oncology Group Study. Advances in Neuroblastom Research Conference ANR2014; Cologne, Germany. 2014. p. 108. 2014. [Google Scholar]
- 6.Betters E, Liu Y, Kjaeldgaard A, Sundstrom E, Garcia-Castro MI. Analysis of early human neural crest development. Dev Biol. 2010;344:578–592. doi: 10.1016/j.ydbio.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredlund E, Ringner M, Maris JM, Pahlman S. High Myc pathway activity and low stage of neuronal differentiation associate with poor outcome in neuroblastoma. Proc Natl Acad Sci U S A. 2008;105:14094–14099. doi: 10.1073/pnas.0804455105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi Y, Sipp D, Enomoto H. Tissue interactions in neural crest cell development and disease. Science. 2013;341:860–863. doi: 10.1126/science.1230717. [DOI] [PubMed] [Google Scholar]
- 9.Hall BK. The neural crest as a fourth germ layer and vertebrates as quadroblastic not triploblastic. Evol Dev. 2000;2:3–5. doi: 10.1046/j.1525-142x.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- 10.Prasad MS, Sauka-Spengler T, Labonne C. Induction of the neural crest state: Control of stem cell attributes by gene regulatory, post-transcriptional and epigenetic interactions. Dev Biol. 2012;366:10–21. doi: 10.1016/j.ydbio.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayanil CS. Transcriptional and Epigenetic Regulation of Neural Crest Induction during Neurulation. Dev Neurosci. 2013 doi: 10.1159/000354749. [DOI] [PubMed] [Google Scholar]
- 12.Strobl-Mazzulla PH, Bronner ME. Epithelial to mesenchymal transition: New and old insights from the classical neural crest model. Semin Cancer Biol. 2012 doi: 10.1016/j.semcancer.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pegoraro C, Monsoro-Burq AH. Signaling and transcriptional regulation in neural crest specification and migration: lessons from xenopus embryos. Wiley Interdiscip Rev Dev Biol. 2013;2:247–259. doi: 10.1002/wdev.76. [DOI] [PubMed] [Google Scholar]
- 14.Shtukmaster S, Schier MC, Huber K, Krispin S, Kalcheim C, Unsicker K. Sympathetic neurons and chromaffin cells share a common progenitor in the neural crest in vivo. Neural Dev. 2013;8:12. doi: 10.1186/1749-8104-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denecker G, Vandamme N, Akay O, et al. Identification of a ZEB2-MITF-ZEB1 transcriptional network that controls melanogenesis and melanoma progression. Cell Death Differ. 2014 doi: 10.1038/cdd.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell DR, Hernandez-Lagunas L, LaMonica K, Artinger KB. Prdm1a directly activates foxd3 and tfap2a during zebrafish neural crest specification. Development. 2013;140:3445–3455. doi: 10.1242/dev.096164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez-Lagunas L, Powell DR, Law J, Grant KA, Artinger KB. prdm1a and olig4 act downstream of Notch signaling to regulate cell fate at the neural plate border. Dev Biol. 2011;356:496–505. doi: 10.1016/j.ydbio.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers CD, Saxena A, Bronner ME. Sip1 mediates an E-cadherin-to-N-cadherin switch during cranial neural crest EMT. J Cell Biol. 2013;203:835–847. doi: 10.1083/jcb.201305050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer. 2013;13:727–738. doi: 10.1038/nrc3597. [DOI] [PubMed] [Google Scholar]
- 20.Howk CL, Voller Z, Beck BB, Dai D. Genetic Diversity in Normal Cell Populations is the Earliest Stage of Oncogenesis Leading to Intra-Tumor Heterogeneity. Front Oncol. 2013;3:61. doi: 10.3389/fonc.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu DM, Agarwal S, Benham A, et al. G-CSF Receptor Positive Neuroblastoma Subpopulations Are Enriched in Chemotherapy-Resistant or Relapsed Tumors and Are Highly Tumorigenic. Cancer Res. 2013;73:4134–4146. doi: 10.1158/0008-5472.CAN-12-4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuchtern JG, London WB, Barnewolt CE, et al. A prospective study of expectant observation as primary therapy for neuroblastoma in young infants: a Children's Oncology Group study. Ann Surg. 2012;256:573–580. doi: 10.1097/SLA.0b013e31826cbbbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strother DR, London WB, Schmidt ML, et al. Outcome after surgery alone or with restricted use of chemotherapy for patients with low-risk neuroblastoma: results of Children's Oncology Group study P9641. J Clin Oncol. 2012;30:1842–1848. doi: 10.1200/JCO.2011.37.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rinon A, Molchadsky A, Nathan E, et al. p53 coordinates cranial neural crest cell growth and epithelial-mesenchymal transition/delamination processes. Development. 2011;138:1827–1838. doi: 10.1242/dev.053645. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Lin Y, Barbieri E, et al. Mdm2 deficiency suppresses MYCN-Driven neuroblastoma tumorigenesis in vivo. Neoplasia. 2009;11:753–762. doi: 10.1593/neo.09466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim E, Shohet J. Targeted molecular therapy for neuroblastoma: the ARF/MDM2/p53 axis. J Natl Cancer Inst. 2009;101:1527–1529. doi: 10.1093/jnci/djp376. [DOI] [PubMed] [Google Scholar]
- 27.Pugh TJ, Morozova O, Attiyeh EF, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet. 2013;45:279–284. doi: 10.1038/ng.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita T, Igarashi J, Okawa ER, et al. CHD5, a tumor suppressor gene deleted from 1p36.31 in neuroblastomas. J Natl Cancer Inst. 2008;100:940–949. doi: 10.1093/jnci/djn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theissen J, Oberthuer A, Hombach A, et al. Chromosome 17/17q gain and unaltered profiles in high resolution array-CGH are prognostically informative in neuroblastoma. Genes Chromosomes Cancer. 2014 doi: 10.1002/gcc.22174. [DOI] [PubMed] [Google Scholar]
- 30.De Preter K, Vermeulen J, Brors B, et al. Accurate outcome prediction in neuroblastoma across independent data sets using a multigene signature. Clin Cancer Res. 2010;16:1532–1541. doi: 10.1158/1078-0432.CCR-09-2607. [DOI] [PubMed] [Google Scholar]
- 31.Westermann F, Muth D, Benner A, et al. Distinct transcriptional MYCN/c-MYC activities are associated with spontaneous regression or malignant progression in neuroblastomas. Genome Biol. 2008;9:R150. doi: 10.1186/gb-2008-9-10-r150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bilke S, Chen QR, Westerman F, Schwab M, Catchpoole D, Khan J. Inferring a tumor progression model for neuroblastoma from genomic data. J Clin Oncol. 2005;23:7322–7331. doi: 10.1200/JCO.2005.03.2821. [DOI] [PubMed] [Google Scholar]
- 33.Hansford LM, Thomas WD, Keating JM, et al. Mechanisms of embryonal tumor initiation: distinct roles for MycN expression and MYCN amplification. Proc Natl Acad Sci U S A. 2004;101:12664–12669. doi: 10.1073/pnas.0401083101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997;16:2985–2995. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schramm A, Koster J, Marschall T, et al. Next-generation RNA sequencing reveals differential expression of MYCN target genes and suggests the mTOR pathway as a promising therapy target in MYCN-amplified neuroblastoma. Int J Cancer. 2013;132:E106–E115. doi: 10.1002/ijc.27787. [DOI] [PubMed] [Google Scholar]
- 36.Stallings RL. MicroRNA involvement in the pathogenesis of neuroblastoma: potential for microRNA mediated therapeutics. Curr Pharm Des. 2009;15:456–462. doi: 10.2174/138161209787315837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valentijn LJ, Koster J, Haneveld F, et al. A functional MYCN signature predicts outcome of neuroblastoma irrespective of MYCN amplification. Proc Natl Acad Sci U S A. 2012;109 doi: 10.1073/pnas.1208215109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shohet JM, Ghosh R, Coarfa C, et al. A genome-wide search for promoters that respond to increased MYCN reveals both new oncogenic and tumor suppressor microRNAs associated with aggressive neuroblastoma. Cancer Res. 2011;71:3841–3851. doi: 10.1158/0008-5472.CAN-10-4391. [DOI] [PubMed] [Google Scholar]
- 39.Huang M, Weiss WA. Neuroblastoma and MYCN. Cold Spring Harb Perspect Med. 2013;3:a014415. doi: 10.1101/cshperspect.a014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakamatsu Y, Watanabe Y, Nakamura H, Kondoh H. Regulation of the neural crest cell fate by N-myc: promotion of ventral migration and neuronal differentiation. Development. 1997;124:1953–1962. doi: 10.1242/dev.124.10.1953. [DOI] [PubMed] [Google Scholar]
- 41.Van Maerken T, Ferdinande L, Taildeman J, et al. Antitumor activity of the selective MDM2 antagonist nutlin-3 against chemoresistant neuroblastoma with wild-type p53. J Natl Cancer Inst. 2009;101:1562–1574. doi: 10.1093/jnci/djp355. [DOI] [PubMed] [Google Scholar]
- 42.Barbieri E, Mehta P, Chen Z, et al. MDM2 inhibition sensitizes neuroblastoma to chemotherapy-induced apoptotic cell death. Mol Cancer Ther. 2006;5:2358–2365. doi: 10.1158/1535-7163.MCT-06-0305. [DOI] [PubMed] [Google Scholar]
- 43.Evageliou NF, Hogarty MD. Disrupting polyamine homeostasis as a therapeutic strategy for neuroblastoma. Clin Cancer Res. 2009;15:5956–5961. doi: 10.1158/1078-0432.CCR-08-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagatell R, Norris R, Ingle AM, et al. Phase 1 trial of temsirolimus in combination with irinotecan and temozolomide in children, adolescents and young adults with relapsed or refractory solid tumors: a Children's Oncology Group Study. Pediatr Blood Cancer. 2014;61:833–839. doi: 10.1002/pbc.24874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Giannatale A, Dias-Gastellier N, Devos A, et al. Phase II study of temozolomide in combination with topotecan (TOTEM) in relapsed or refractory neuroblastoma: a European Innovative Therapies for Children with Cancer-SIOP-European Neuroblastoma study. Eur J Cancer. 2014;50:170–177. doi: 10.1016/j.ejca.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ardini E, Magnaghi P, Orsini P, Galvani A, Menichincheri M. Anaplastic Lymphoma Kinase: role in specific tumours, and development of small molecule inhibitors for cancer therapy. Cancer Lett. 2010;299:81–94. doi: 10.1016/j.canlet.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Mosse YP, Laudenslager M, Longo L, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–935. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minuti G, D'Incecco A, Landi L, Cappuzzo F. Protein kinase inhibitors to treat non-small-cell lung cancer. Expert Opin Pharmacother. 2014 doi: 10.1517/14656566.2014.909412. [DOI] [PubMed] [Google Scholar]
- 50.Ulivi P, Zoli W, Capelli L, Chiadini E, Calistri D, Amadori D. Target therapy in NSCLC patients: Relevant clinical agents and tumour molecular characterisation. Mol Clin Oncol. 2013;1:575–581. doi: 10.3892/mco.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reiff T, Huber L, Kramer M, Delattre O, Janoueix-Lerosey I, Rohrer H. Midkine and Alk signaling in sympathetic neuron proliferation and neuroblastoma predisposition. Development. 2011;138:4699–4708. doi: 10.1242/dev.072157. [DOI] [PubMed] [Google Scholar]
- 52.Wang M, Zhou C, Sun Q, et al. ALK amplification and protein expression predict inferior prognosis in neuroblastomas. Exp Mol Pathol. 2013;95:124–130. doi: 10.1016/j.yexmp.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Schulte JH, Lindner S, Bohrer A, et al. MYCN and ALKF1174L are sufficient to drive neuroblastoma development from neural crest progenitor cells. Oncogene. 2013;32:1059–1065. doi: 10.1038/onc.2012.106. [DOI] [PubMed] [Google Scholar]
- 54.Mosse YP, Laudenslager M, Khazi D, et al. Germline PHOX2B mutation in hereditary neuroblastoma. Am J Hum Genet. 2004;75:727–730. doi: 10.1086/424530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trochet D, Bourdeaut F, Janoueix-Lerosey I, et al. Germline mutations of the paired-like homeobox 2B (PHOX2B) gene in neuroblastoma. Am J Hum Genet. 2004;74:761–764. doi: 10.1086/383253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pei D, Luther W, Wang W, Paw BH, Stewart RA, George RE. Distinct neuroblastoma-associated alterations of PHOX2B impair sympathetic neuronal differentiation in zebrafish models. PLoS Genet. 2013;9:e1003533. doi: 10.1371/journal.pgen.1003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Butler Tjaden NE, Trainor PA. The developmental etiology and pathogenesis of Hirschsprung disease. Transl Res. 2013;162:1–15. doi: 10.1016/j.trsl.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Lascio S, Bachetti T, Saba E, Ceccherini I, Benfante R, Fornasari D. Transcriptional dysregulation and impairment of PHOX2B auto-regulatory mechanism induced by polyalanine expansion mutations associated with congenital central hypoventilation syndrome. Neurobiol Dis. 2013;50:187–200. doi: 10.1016/j.nbd.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 59.Wang W, Zhong Q, Teng L, et al. Mutations that disrupt PHOXB interaction with the neuronal calcium sensor HPCAL1 impede cellular differentiation in neuroblastoma. Oncogene. 2013 doi: 10.1038/onc.2013.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bachetti T, Di Paolo D, Di Lascio S, et al. PHOX2B-mediated regulation of ALK expression: in vitro identification of a functional relationship between two genes involved in neuroblastoma. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burney MJ, Johnston C, Wong KY, et al. An epigenetic signature of developmental potential in neural stem cells and early neurons. Stem Cells. 2013;31:1868–1880. doi: 10.1002/stem.1431. [DOI] [PubMed] [Google Scholar]
- 62.Rada-Iglesias A, Bajpai R, Prescott S, Brugmann SA, Swigut T, Wysocka J. Epigenomic annotation of enhancers predicts transcriptional regulators of human neural crest. Cell Stem Cell. 2012;11:633–648. doi: 10.1016/j.stem.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eroglu B, Wang G, Tu N, Sun X, Mivechi NF. Critical role of Brg1 member of the SWI/SNF chromatin remodeling complex during neurogenesis and neural crest induction in zebrafish. Dev Dyn. 2006;235:2722–2735. doi: 10.1002/dvdy.20911. [DOI] [PubMed] [Google Scholar]
- 64.Martins-Taylor K, Schroeder DI, LaSalle JM, Lalande M, Xu RH. Role of DNMT3B in the regulation of early neural and neural crest specifiers. Epigenetics. 2012;7:71–82. doi: 10.4161/epi.7.1.18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ostler KR, Yang Q, Looney TJ, et al. Truncated DNMT3B isoform DNMT3B7 suppresses growth, induces differentiation, and alters DNA methylation in human neuroblastoma. Cancer Res. 2012;72:4714–4723. doi: 10.1158/0008-5472.CAN-12-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Newhart A, Rafalska-Metcalf IU, Yang T, Negorev DG, Janicki SM. Single-cell analysis of Daxx and ATRX-dependent transcriptional repression. J Cell Sci. 2012;125:5489–5501. doi: 10.1242/jcs.110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheung NK, Zhang J, Lu C, et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA. 2012;307:1062–1071. doi: 10.1001/jama.2012.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma L, Young J, Prabhala H, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Molenaar JJ, Domingo-Fernandez R, Ebus ME, et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat Genet. 2012 doi: 10.1038/ng.2436. [DOI] [PubMed] [Google Scholar]
- 70.Mestdagh P, Bostrom AK, Impens F, et al. The miR-17-92 microRNA cluster regulates multiple components of the TGF-beta pathway in neuroblastoma. Mol Cell. 2010;40:762–773. doi: 10.1016/j.molcel.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamilton MP, Rajapakshe K, Hartig SM, et al. Identification of a pan-cancer oncogenic microRNA superfamily anchored by a central core seed motif. Nat Commun. 2013;4:2730. doi: 10.1038/ncomms3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buechner J, Einvik C. N-myc and Noncoding RNAs in Neuroblastoma. Mol Cancer Res. 2012 doi: 10.1158/1541-7786.MCR-12-0244. [DOI] [PubMed] [Google Scholar]
- 73.Ara T, Nakata R, Sheard MA, et al. Critical role of STAT3 in IL-6-mediated drug resistance in human neuroblastoma. Cancer Res. 2013;73:3852–3864. doi: 10.1158/0008-5472.CAN-12-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu D, Song L, Wei J, et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J Clin Invest. 2012;122:2221–2233. doi: 10.1172/JCI59535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schleiermacher G, Mosseri V, London WB, et al. Segmental chromosomal alterations have prognostic impact in neuroblastoma: a report from the INRG project. Br J Cancer. 2012;107:1418–1422. doi: 10.1038/bjc.2012.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brodeur GM, Seeger RC, Barrett A, et al. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol. 1988;6:1874–1881. doi: 10.1200/JCO.1988.6.12.1874. [DOI] [PubMed] [Google Scholar]
- 77.Owens C, Irwin M. Neuroblastoma: the impact of biology and cooperation leading to personalized treatments. Crit Rev Clin Lab Sci. 2012;49:85–115. doi: 10.3109/10408363.2012.683483. [DOI] [PubMed] [Google Scholar]
- 78.Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morgenstern DA, Baruchel S, Irwin MS. Current and future strategies for relapsed neuroblastoma: challenges on the road to precision therapy. J Pediatr Hematol Oncol. 2013;35:337–347. doi: 10.1097/MPH.0b013e318299d637. [DOI] [PubMed] [Google Scholar]
- 80.Castel V, Segura V, Berlanga P. Emerging drugs for neuroblastoma. Expert Opin Emerg Drugs. 2013;18:155–171. doi: 10.1517/14728214.2013.796927. [DOI] [PubMed] [Google Scholar]
- 81.Heczey A, Louis CU. Advances in chimeric antigen receptor immunotherapy for neuroblastoma. Discov Med. 2013;16:287–294. [PMC free article] [PubMed] [Google Scholar]
- 82.Baker DL, Schmidt ML, Cohn SL, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med. 2010;363:1313–1323. doi: 10.1056/NEJMoa1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hero B, Simon T, Spitz R, et al. Localized infant neuroblastomas often show spontaneous regression: results of the prospective trials NB95-S and NB97. J Clin Oncol. 2008;26:1504–1510. doi: 10.1200/JCO.2007.12.3349. [DOI] [PubMed] [Google Scholar]
- 84.Rubie H, De Bernardi B, Gerrard M, et al. Excellent outcome with reduced treatment in infants with nonmetastatic and unresectable neuroblastoma without MYCN amplification: results of the prospective INES 99.1. J Clin Oncol. 2011;29:449–455. doi: 10.1200/JCO.2010.29.5196. [DOI] [PubMed] [Google Scholar]
- 85.Bagatell R, Beck-Popovic M, London WB, et al. Significance of MYCN amplification in international neuroblastoma staging system stage 1 and 2 neuroblastoma: a report from the International Neuroblastoma Risk Group database. J Clin Oncol. 2009;27:365–370. doi: 10.1200/JCO.2008.17.9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Bernardi B, Mosseri V, Rubie H, et al. Treatment of localised resectable neuroblastoma. Results of the LNESG1 study by the SIOP Europe Neuroblastoma Group. Br J Cancer. 2008;99:1027–1033. doi: 10.1038/sj.bjc.6604640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matthay KK, Perez C, Seeger RC, et al. Successful treatment of stage III neuroblastoma based on prospective biologic staging: a Children's Cancer Group study. J Clin Oncol. 1998;16:1256–1264. doi: 10.1200/JCO.1998.16.4.1256. [DOI] [PubMed] [Google Scholar]
- 88.Nickerson HJ, Matthay KK, Seeger RC, et al. Favorable biology and outcome of stage IV-S neuroblastoma with supportive care or minimal therapy: a Children's Cancer Group study. J Clin Oncol. 2000;18:477–486. doi: 10.1200/JCO.2000.18.3.477. [DOI] [PubMed] [Google Scholar]
- 89.Burton PR, Walls J. A selection adjusted comparison of hospitalization on continuous ambulatory peritoneal dialysis and haemodialysis. J Clin Epidemiol. 1989;42:531–539. doi: 10.1016/0895-4356(89)90149-2. [DOI] [PubMed] [Google Scholar]
- 90.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Endres S. Long-term In fusion of ch14-18/CHO combined with s.c. interleukin-2 Applied in a Single Center Treatment Program Effectively Srtimulates Anti-neuroblastoma Activity with Reduced Pain in High-risk Neuroblastoma Patients. Advances in Neuroblastoma Research Conference ANR2014; Cologne, Germany. 2014. p. 108. 2014. [Google Scholar]
- 92.Ladenstein R. Myeloablative Therapy (MAT) and Immunotherapy (IT) with ch14.18/CHO for High Risk Neuroblastoma: Update and News orf Randomised Results from the HR-NBL1/SIOPEN trial. Advances in Neuroblastoma Research ANR2014; Cologne, Germany. 2014. p. 137. 2014. [Google Scholar]
- 93.Schmidt M, Simon T, Hero B, Schicha H, Berthold F. The prognostic impact of functional imaging with (123)I-mIBG in patients with stage 4 neuroblastoma >1 year of age on a high-risk treatment protocol: results of the German Neuroblastoma Trial NB97. Eur J Cancer. 2008;44:1552–1558. doi: 10.1016/j.ejca.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 94.Matthay KK, Edeline V, Lumbroso J, et al. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol. 2003;21:2486–2491. doi: 10.1200/JCO.2003.09.122. [DOI] [PubMed] [Google Scholar]
- 95.Beiske K, Burchill SA, Cheung IY, et al. Consensus criteria for sensitive detection of minimal neuroblastoma cells in bone marrow, blood and stem cell preparations by immunocytology and QRT-PCR: recommendations by the International Neuroblastoma Risk Group Task Force. Br J Cancer. 2009;100:1627–1637. doi: 10.1038/sj.bjc.6605029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Modak S. generation and Administration of Autologous T cells Transduced with a 3rd Generation GD2 chimeric Antigen Receptor for Patients with Relapsed or Refractory Neuroblastoma. Andvances in Neuroblastoma Research ANR2014; Cologne, Germany. 2014. p. 137. 2014. [Google Scholar]