Abstract
Objective
To examine the effect of an individually-tailored, motivationally-matched prenatal exercise intervention on gestational diabetes mellitus (GDM) and other measures of glucose intolerance among ethnically diverse prenatal care patients at increased risk for GDM.
Methods
The Behaviors Affecting Baby and You Study randomized eligible women at a mean (SD) of 18.2 (4.1) weeks gestation to a 12-week individually tailored, motivationally matched exercise intervention or a comparison health and wellness intervention. The goal of the exercise intervention was to achieve the American College of Obstetricians and Gynecologists guidelines for physical activity during pregnancy. Diagnosis of GDM, impaired glucose tolerance (IGT), abnormal glucose screen, and screening glucose values (mg/dL) were abstracted from medical records. A sample size of 352 women (176 per group) was planned to have 80% power to detect reductions in risk of 35% or larger.
Results
From July, 2007 to December, 2012, a total of 251 (86.5%) women completed the intervention; n=124 and 127 in the exercise and comparison interventions, respectively. Based on an intention-to-treat analysis, no statistically significant differences between the intervention groups were observed; the relative odds of GDM in the exercise group was 0.61 (95% Confidence Interval [CI] 0.28–1.32) as compared to the health and wellness comparison group. Odds ratios for IGT and abnormal glucose screen were 0.68 (95% CI 0.35–1.34) and 0.86 (95% CI 0.51–1.47), respectively. The intervention had no effect on birth outcomes.
Conclusion
In this randomized trial among ethnically diverse pregnant women at increased risk for GDM, we found that a prenatal exercise intervention implemented in the second trimester did not result in a statistically significant reduction in relative odds for GDM, IGT, or abnormal glucose screen.
Introduction
Gestational diabetes mellitus (GDM) is one of the most common complications of pregnancy with a prevalence rate varying from 1–20% depending on the population studied and diagnostic criteria applied (1, 2). Women with histories of GDM have elevated cardiovascular disease risk factors including higher blood pressure, triglyceride levels, and lower HDL (3) as well as a 7-fold risk for type 2 diabetes (4).
A meta-analysis of observational studies among healthy pregnant women found a 24% reduction in odds of GDM (95% CI 0.70–0.83) among women reporting higher levels of exercise in early pregnancy (5). However, a meta-analysis of randomized trials found no significant difference in GDM risk between exercise intervention and control groups (RR=0.91, 95% CI: 0.57–1.44) (6). The majority of these trials were conducted among non-Hispanic white women. This is critical as Hispanics are the largest minority group in the U.S. and have the highest birth rates (7). Hispanics are half as likely as non-Hispanic whites to meet the American College of Obstetricians and Gynecologists (ACOG) guidelines for pregnancy physical activity (8, 9) and are more likely to develop GDM (10) and type 2 diabetes (11).
Therefore, we conducted a randomized trial of an individually- tailored, motivationally-matched exercise intervention among an ethnically diverse group of pregnant women at increased risk for GDM. We hypothesized that participants in the exercise intervention would have a lower risk of GDM, impaired glucose tolerance (IGT), abnormal glucose screen, and lower screening glucose values as compared to a health and wellness control intervention.
Materials and Methods
The Behaviors Affecting Baby and You (B.A.B.Y.) study was a randomized controlled trial of an exercise intervention to prevent the development of GDM in pregnant women at increased risk. Details of the study design have been published elsewhere (12). The B.A.B.Y. study was based in the ambulatory obstetrical practices of Baystate Medical Center, a large tertiary care facility in Western Massachusetts which serves an ethnically and socio-economically diverse population. Health educators pre-screened eligible patients from 2007 to 2012 using demographic and medical characteristics provided on a daily roster of scheduled patients to generate a list of potential participants. Interviews were conducted in Spanish or English (based on patient preference).
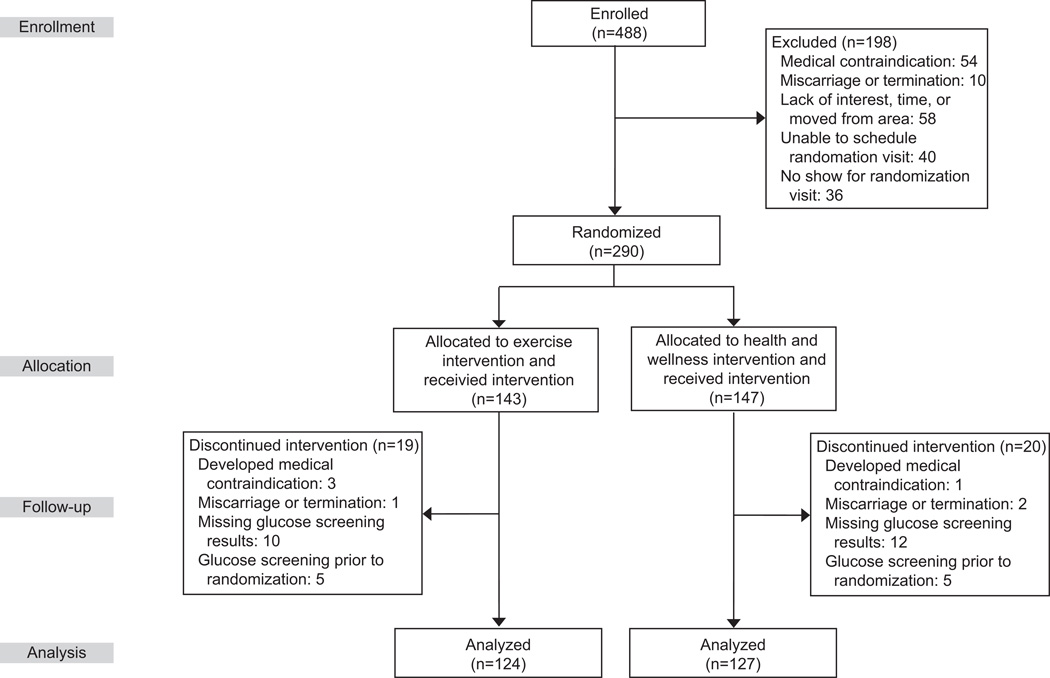
Women were considered eligible for the study if they were in their first trimester of pregnancy, between the ages of 16 and 40, and at increased risk for GDM defined as either: 1) overweight/obese (pre-pregnancy BMI≥25 kg/m2) with a family history of diabetes or 2) a diagnosis of GDM in a prior pregnancy defined according to the American Diabetes Association criteria (13). Exclusion criteria were the following: 1) contraindications to participating in moderate physical activity, 2) inability to read English at a 6th grade level, 3) self-reported current participation in >30 minutes of moderate or vigorous intensity exercise on more than 3 days/week, 4) diagnosis of diabetes outside of pregnancy, hypertension, heart disease, or chronic renal disease, 5) use of current medications that adversely influence glucose tolerance, or 6) nonsingleton pregnancy. All women signed a written informed consent and the study was approved by the Institutional Review Boards of the University of Massachusetts Amherst and Baystate Medical Center. A total of 251 participants met these criteria (n=124 in the exercise and n=127 in the health and wellness group) and were enrolled at a mean (SD) of 11.8 (3.3) weeks gestation (interquartile range [IQR] 9.3 to 13.1) (Figure 1).
Figure 1.
Flow diagram for the Behaviors Affecting Baby and You Study, 2007–2012.
Eligible women were block randomized based on age group (<30 or >30 years), pre-pregnancy BMI (≥25 kg/m2 or <25 kg/m2), and ethnicity (Hispanic or non-Hispanic) into either an exercise intervention group or a comparison health and wellness intervention. Block randomization factors were selected based on the strength of their association with GDM as well as their anticipated prevalence in the study population. The intervention was designed to encompass a 12-week time period, as that was the calculated average time between randomization and GDM screen. Women were not blinded to their assigned intervention group.
The intervention commenced with one in-person session with a health educator who administered a tailoring questionnaire and set behavioral goals. Neither the exercise nor the health and wellness intervention groups received a dietary intervention. However, counseling was routinely provided to all prenatal care patients at Baystate regarding the Institute of Medicine Guidelines for appropriate nutrition and weight gain in pregnancy (14).
The intervention drew from the trans-theoretical model (15) and social cognitive theory (16) constructs for physical activity which account for the individual’s stage of motivational readiness for change as well as the processes that help facilitate that change. The intervention took into account findings by our research group on the specific social, cultural, economic, and environmental challenges faced by women of diverse backgrounds (17).
The overall goal of the exercise intervention was to encourage pregnant women to achieve ACOG guidelines for physical activity during pregnancy; that is, 30 minutes or more of moderate-intensity physical activity on most days of the week (8). Specific activities were self-selected and included dancing, walking, and yard work. Weekly goals were to increase time spent in moderate intensity physical activity by 10% to safely progress towards the overall activity goal. Participants were provided a digital pedometer as a motivational tool and an activity diary to encourage self-monitoring.
At the baseline visit, health educators administered a 65-item tailoring questionnaire which assessed current stage of motivational readiness for physical activity adoption, self-efficacy, decisional balance, use of cognitive and behavioral processes of change, and time spent in physical activity. In light of responses to this questionnaire, health educators discussed barriers and facilitators to adopting physical activity. A stage-matched manual targeting the specific stage of motivational readiness to adopt physical activity was then given to the participants. These manuals described the benefits of physical activity, tips for stretching, building social support, goal setting, and strategies for overcoming barriers to physical activity.
Participants’ progress toward their behavioral goals was assessed via follow-up tailoring questionnaires which were mailed monthly (at 4, 8, and 12 weeks post-randomization) with a postage paid return envelope. Based upon responses to these questionnaires, individually tailored reports were generated and also mailed monthly to the participant along with the corresponding stage-matched manual. Each tailored report described the individual’s current stage of motivational readiness for becoming active, mediators for physical activity (i.e. self-efficacy, benefits and barriers for physical activity, cognitive and behavioral processes), normative feedback, and feedback regarding progress towards physical activity goals since prior assessment. Monthly booster telephone calls also provided individualized feedback as well as reviewed participants’ progress toward their behavioral goals. Additionally, tip sheets were mailed weekly for the first 4 weeks of the intervention and then every other week thereafter.
The overall goal of the health and wellness intervention was general health and wellness during pregnancy instead of issues related to physical activity. This intervention group received tips sheets and telephone booster calls on the same contact schedule as the exercise group which controlled for contact time while keeping the content of the two interventions distinct. Specifically, after completion of the initial tailoring questionnaire, the health educator focused on such topics as alcohol and drug use during pregnancy, easing back pain, and travel during pregnancy. A series of ACOG informational booklets on these topics was mailed to the participants weekly during the first four weeks and then biweekly thereafter. These booklets were selected to represent high-quality, low-cost self-help material currently available to the public. A follow-up tailoring questionnaire was mailed at week twelve. Monthly booster telephone calls provided an opportunity for participants to ask questions about the materials they received.
Baystate obstetric practices routinely screen all prenatal care patients for GDM in mid-pregnancy at the recommended range of 24 and 28 weeks of gestation using a 50 g, 1-hour glucose tolerance test (OGTT). Those with 1-hour plasma glucose values >135mg/dL were defined as having abnormal glucose screen and underwent the diagnostic 3-hour OGTT. Diagnosis of GDM was defined as 2 or more elevated values at fasting, and 1, 2, and 3 hours, based on the American Diabetes Association criteria of 95, 180, 155, and 140 mg/dL, respectively (18). Diagnosis of impaired glucose tolerance (IGT) was defined as exceeding one or more elevated values at fasting, and 1, 2, and 3 hours, respectively, and was inclusive of GDM. Recent studies designed to identify the diagnostic threshold between maternal hyperglycemia and adverse perinatal outcomes have observed a consistent, continuous increase in risk of adverse pregnancy outcomes over the range of maternal blood glucose levels, even at degrees not diagnostic of GDM (19). Therefore, we also evaluated the 50g OGTT screening glucose value as a continuous outcome. Diagnoses were confirmed by the study obstetrician who reviewed the medical records of each suspected case.
Information on adverse birth outcomes were abstracted from medical records. Low birth weight was defined as <2500 grams and preterm birth as <37 weeks gestation. Small-for-gestational-age (SGA) was defined as <10th percentile for gestational age at birth and large-for-gestational-age (LGA) was defined as >90th percentile for gestational age at birth based upon standard reference values (20).
Sociodemographic factors, including age, ethnicity, education, annual household income, marital status, living situation (e.g., with a spouse or partner), and the number of adults and children in the household were collected at the time of enrollment via standardized questionnaires. Health behaviors included early-pregnancy alcohol consumption and cigarette smoking. Medical factors included parity, personal history of GDM, and family history of diabetes. Pre-pregnancy weight and height and gestational weight gain were abstracted from the medical record. If pre-pregnancy weight was missing from the medical record (n=3, 1%), it was based upon self-reported weight collected at the time of enrollment.
The first 20% of participants completed a satisfaction survey at the end of follow-up that assessed the acceptability and feasibility of the intervention. The satisfaction survey was an adapted version of the consumer satisfaction questionnaire that has been used across multiple trials (21, 22) streamlined with an emphasis on assessing barriers to participation and comfort with study activities.
All analyses were carried out using an intent-to-treat approach. Chi square tests or Fisher’s Exact tests were used to compare the distribution of socio-demographic, medical history characteristics, and health behaviors between the intervention groups at baseline. Logistic regression was used to assess the effect of the intervention on GDM, IGT, and abnormal glucose screen. Unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CIs) were computed. Linear regression was used to assess the effect of the intervention on screening glucose values. Beta coefficients, standard errors, and p values were computed.
We a priori chose to evaluate age (<30 years, ≥30 years), ethnicity (Hispanic, non-Hispanic), pre-pregnancy BMI (<30 kg/m2≥30 kg/m2) and parity as potential effect modifiers by including multiplicative interaction terms in the multivariable models and assessing their statistical significance at p<0.1 using likelihood ratio chi-square tests. Finally, because baseline education level and parity differed significantly between intervention groups, these factors were included in subsequent multivariable models. As gestational weight gain may be on the causal pathway between physical activity and GDM, we also compared gestational weight gain between the intervention groups.
Finally, we conducted several sensitivity analyses. First, we conducted a sensitivity analysis according to the dose of intervention, defined as the length of time the intervention was administered. We then conducted sensitivity analyses to take into account adherence to the study protocol. Adherence was defined in two ways. First, we compared participants who were adherent to the exercise intervention, defined as meeting ACOG guidelines for physical activity at the time of the GDM screen, to all participants in the health and wellness group. Secondly, we defined adherence as the number of returned completed tailoring questionnaires in each intervention arm.
We a priori projected that 30% of women at the study site would meet the inclusion criteria of a history of GDM in a prior pregnancy and, based on prior literature, have an expected GDM recurrence rate of 50% (23, 24, 25). We anticipated that 50% of women would meet the inclusion criteria of BMI ≥30kg/m2 and a family history of diabetes and would have an expected GDM incidence rate of 15.5% (26). Lastly, we projected that 20% of women would meet the inclusion criteria of BMI ≥25–<30kg/m2 and a family history of diabetes and would have an expected GDM incidence rate of 11%. These figures result in a weighted incidence rate of GDM of 25%. Based on this incidence rate, a sample size of 352 would have the ability to detect reductions in risk of 35% or larger (i.e., an odds ratio of 0.65) with statistical significance based on a 0.05 two-sided significance level. For screening glucose, we had 80% power to detect a clinically significant mean difference as small as 5.99 ng/mL based on a standard deviation of 20 mg/dL (27).
Based upon prior literature available when the B.A.B.Y. Study was designed (28, 29, 30), achieving ACOG guidelines in the exercise arm by the time of GDM screen would require that the intervention lead to an increase in 3.8 MET-hours/week of recreational activity which represented 0.19 of a standard deviation or a “small effect” according to Cohen’s effect size (31). In practical terms, this translated to an additional hour per week or 10 minutes per day of brisk walking.
Results
A total of 290 women were randomized to the exercise intervention (n=143, 49%) or to the health and wellness comparison group (n=147, 51%) at a mean (SD) of 18.2 (4.1) weeks gestation (IQR 15.0 to 21.9) (Figure 1). Overall, the majority of participants were young (49.0% <25 yrs. of age), Hispanic (60.2%), and obese (62.2%) (Table 1). While 75.4% were unmarried, 62.8% reported living with a partner. There were no statistically significant differences between the intervention groups in any sociodemographic characteristic, medical history characteristic (including personal history of GDM, p=0.22), or health behavior with the exception of education and parity (Table 1). Specifically, the exercise group was more likely to have post-high school levels of education (p=0.03) and be nulliparous (p=0.01) as compared to the health and wellness group.
Table 1.
Baseline Characteristics of Participants According to Intervention Group; Behaviors Affecting Baby and You Study, 2007–2012.
| Total (n=251) |
Exercise Group (n=124) |
Health & Wellness Group (n=127) |
|||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | p- value* |
|
| Sociodemographic characteristics | |||||||
| Age (years) | |||||||
| 18–19 | 27 | 10.8 | 16 | 12.9 | 11 | 8.7 | 0.34 |
| 20–24 | 96 | 38.2 | 51 | 41.1 | 45 | 35.4 | |
| 25–29 | 61 | 24.3 | 25 | 20.2 | 36 | 28.3 | |
| 30–40 | 67 | 26.7 | 32 | 25.8 | 35 | 27.6 | |
| Ethnicity | |||||||
| Hispanic | 151 | 60.2 | 69 | 55.6 | 82 | 64.6 | 0.15 |
| Non-Hispanic | 100 | 39.8 | 55 | 44.4 | 45 | 35.4 | |
| Education | |||||||
| Less than high school | 57 | 24.8 | 26 | 22.2 | 31 | 27.4 | 0.03 |
| High school graduate or GED | 74 | 32.2 | 31 | 26.5 | 43 | 38.1 | |
| Post high school | 99 | 43.0 | 60 | 51.3 | 39 | 34.5 | |
| Marital status | |||||||
| Single/separated/divorced/widowed | 187 | 75.4 | 91 | 73.4 | 96 | 77.4 | 0.46 |
| Married | 61 | 24.6 | 33 | 26.6 | 28 | 22.6 | |
| Live with Partner | |||||||
| Yes | 145 | 62.8 | 75 | 64.1 | 70 | 61.4 | 0.67 |
| No | 86 | 37.2 | 42 | 35.9 | 44 | 38.6 | |
| Adults in household | |||||||
| 1 | 58 | 24.4 | 25 | 20.7 | 33 | 28.2 | 0.40 |
| 2 | 121 | 50.8 | 65 | 53.7 | 56 | 47.9 | |
| ≥3 | 59 | 24.8 | 31 | 25.6 | 28 | 23.9 | |
| Children in household | |||||||
| 0 | 58 | 24.0 | 34 | 27.6 | 24 | 20.2 | 0.27 |
| 1 | 93 | 38.4 | 49 | 39.8 | 44 | 37.0 | |
| 2 | 61 | 25.2 | 25 | 20.3 | 36 | 30.3 | |
| ≥3 | 30 | 12.4 | 15 | 12.2 | 15 | 12.6 | |
| Medical History Characteristics | |||||||
| Parity | |||||||
| Nulliparous | 65 | 27.0 | 41 | 34.5 | 24 | 19.7 | 0.01 |
| Parous | 176 | 73.0 | 78 | 65.5 | 98 | 80.3 | |
| Pre-pregnancy BMI | |||||||
| <25 kg/m2 | 8 | 3.2 | 3 | 2.4 | 5 | 3.9 | 0.28 |
| 25-<30 kg/m2 | 87 | 34.7 | 38 | 30.6 | 49 | 38.6 | |
| 30-<40 kg/m2 | 156 | 62.2 | 83 | 66.9 | 73 | 57.5 | |
| Personal history of GDM | |||||||
| Yes | 24 | 9.6 | 9 | 7.3 | 15 | 11.8 | 0.22 |
| No | 227 | 90.4 | 115 | 92.7 | 112 | 88.2 | |
| Family history of diabetes mellitus | |||||||
| Yes | 235 | 98.7 | 115 | 98.3 | 120 | 99.2 | 0.62 |
| No | 3 | 1.3 | 2 | 1.7 | 1 | 0.8 | |
| Health Behaviors | |||||||
| Smoking during pregnancy | |||||||
| Yes | 33 | 14.7 | 17 | 15.3 | 16 | 14.2 | 0.81 |
| No | 191 | 85.3 | 94 | 84.7 | 97 | 85.8 | |
| Alcohol use during pregnancy | |||||||
| Yes | 5 | 2.2 | 5 | 4.4 | 0 | 0.0 | 0.06 |
| No | 222 | 97.8 | 109 | 95.6 | 113 | 100.0 | |
Chi-square test or Fisher's exact test if cell sizes less than 5
Numbers may not total to 251 due to missing data on covariates.
Retention to GDM screening did not differ significantly between the 2 groups (86.7% in the exercise group vs. 86.3% in the health and wellness group). The GDM screen took place at a mean (SD) of 28.1 (2.9) weeks gestation (IQR 26.9, 29.4) and also did not differ between intervention groups (p=0.78). A total of 12.4% (n=31) of participants developed GDM; 9.7% of women (n=12) in the exercise group as compared to 15.0% (n=19) in the health and wellness group (Table 2). In an intent-to-treat analysis, the relative odds of GDM in the exercise group was 0.61 (95% Confidence Interval [CI] 0.28–1.32) as compared to the health and wellness group. After adjusting for education and parity, the findings were essentially unchanged (OR=0.60, 95% CI 0.27–2.32). Given the high risk of GDM among women with a personal history of GDM, we also repeated this analysis adjusting for this variable; findings were slightly attenuated (OR 0.74; 95% CI 0.33, 1.65). Restricting to participants with no personal history of GDM resulted in an odds ratio of 0.65 (95% CI 0.27, 1.58).
Table 2.
GDM and Markers of Glucose Intolerance According to Intervention Arm; Behaviors Affecting Baby and You Study, 2007–2012.
| Exercise Group |
Health & Wellness group |
Unadjusted | Adjusted for Education and Parity |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | OR1 | 95% CI | p-value | OR1 | 95% CI | p- value |
|
| Gestational diabetes mellitus | 12 | 9.7 | 19 | 15.0 | 0.61 | (0.28, 1.32) | 0.21 | 0.60 | (0.27, 1.32) | 0.20 |
| Impaired glucose tolerance | 17 | 13.7 | 24 | 18.9 | 0.68 | (0.35, 1.34) | 0.27 | 0.64 | (0.31, 1.29) | 0.21 |
| Abnormal glucose screen | 37 | 29.8 | 42 | 33.1 | 0.86 | (0.51, 1.47) | 0.58 | 0.86 | (0.49, 1.48) | 0.58 |
| mean | SD | mean | SD | β | SE | p-value | β | SE | p- value |
|
| Screening glucose value (mg/dL) | 117.8 | 26.5 | 119.7 | 33.7 | −1.95 | 3.86 | 0.61 | −1.76 | 3.98 | 0.66 |
Logistic regression was used to model the odds of GDM and other measure of glucose intolerance in the exercise group versus the health and wellness group.
Findings for IGT were of similar direction and magnitude with the exercise group having a lower odds of IGT as compared to the health and wellness group, but not statistically so (OR= 0.68, 95% CI 0.35–1.34) (Table 2). In terms of abnormal glucose screen, findings were of similar direction but further attenuated; the exercise arm had a relative odds of 0.86 of developing abnormal glucose screen as compared to the health and wellness group (95% CI 0.51–1.47). Adjustment for education and parity did not substantively alter findings for either IGT or abnormal glucose screen. In terms of the effect of the intervention on continuous 50g OGTT screening glucose values, the exercise group had, on average, a 1.95 mg/dL lower screening glucose value as compared to the health and wellness comparison group although this was not statistically significant (p=0.61) (Table 2). We found no statistically significant differences in total gestational weight gain (β=−1.08±2.71 pounds, p=0.69) or gestational weight gain up to the time of GDM screen (β=−0.98±SE 2.18 pounds, p=0.65) in the exercise group compared to the health and wellness group.
We evaluated age, ethnicity, pre-pregnancy BMI, and parity as effect modifiers of the effect of the exercise intervention on glucose outcomes. Only the likelihood ratio test for the Hispanic ethnicity interaction term was less than p=0.1. Among non-Hispanic white women, the effect of the intervention on GDM (OR=0.38, 95% CI 0.13–1.12) and IGT (OR=0.39, 95% CI 0.15–1.01) remained non-significant (Table 3). In contrast, findings among Hispanic women were attenuated and close to the null value (i.e., for GDM: OR=0.88, 95% CI 0.29–2.68). A significant difference between groups was noted on the continuous 50g OGTT screening glucose values for non-Hispanic white women in the exercise intervention group as compared to the health and wellness group (mean [SD]: −6.65 [6.40] vs. −0.33 [4.80] mg/dL, p=0.029).
Table 3.
Effects of the Exercise Intervention on GDM and Markers of Glucose Intolerance According to Hispanic Ethnicity; Behaviors Affecting Baby and You Study, 2007–2012.
| Hispanic (n=151) |
Non-Hispanic White (n=100) |
|||||
|---|---|---|---|---|---|---|
| OR1 | 95% CI | p-value | OR1 | 95% CI | p- value |
|
| Gestational diabetes mellitus | 0.88 | (0.29, 2.68) | 0.82 | 0.38 | (0.13, 1.12) | 0.08 |
| Impaired glucose tolerance | 1.06 | (0.39, 2.92) | 0.91 | 0.39 | (0.15, 1.01) | 0.05 |
| Abnormal glucose screen | 1.05 | (0.52, 2.13) | 0.90 | 0.61 | (0.27, 1.39) | 0.24 |
| β | SE | p-value | β | SE | p- value |
|
| Screening glucose value (mg/dL) | −0.33 | 4.80 | 0.95 | −6.65 | 6.40 | 0.30 |
Logistic regression was used to model the odds of GDM and other measure of glucose intolerance in the exercise group versus the health and wellness group.
We then conducted a sensitivity analysis according to the dose of intervention. The mean (SD) intervention dose was 9.9 (4.7) weeks (IQR 6.4, 13.1). The odds of GDM did not differ according to dose of intervention; women who received 10 or more weeks of the intervention (72%) had an odds of GDM of 0.62 (95% CI 0.23–1.68) as compared to women who received less than 10 weeks of intervention (28%) (OR=0.60; 95% CI 0.18–2.07). Findings also did not differ for IGT or screening glucose value.
We then conducted a sensitivity analysis according to adherence to the study protocol. First, we compared the 62.1% of participants in the exercise arm who met the ACOG guidelines for physical activity during pregnancy at the time of GDM screen to the entire health and wellness comparison group; the relative odds of GDM was 0.76 (95% CI 0.33–1.79). Secondly, we defined adherence as returning completed tailoring questionnaires in each intervention arm. There were no significant differences between the exercise arm (41% of the women complied with this protocol) and the health and wellness group (49%, p=0.21). Finally, based upon findings from the satisfaction survey, participants (95%) reported being satisfied with the amount of information received and 86% reported finding the study materials interesting and useful.
The incidence of adverse birth outcomes including low birth weight, preterm birth, small and large for gestational age, and cesarean section did not differ significantly between the exercise group and the health and wellness group (Table 4). Similarly there were no differences in continuous birth weight or gestational age values between the intervention groups.
Table 4.
Birth Outcomes According to Intervention Arm; Behaviors Affecting Baby and You Study, 2007–2012.
| Exercise Group | Health & Wellness Group (n=123) |
p- value |
|||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Low birth weight | 9 | 7.2 | 6 | 4.8 | 0.43 |
| Preterm birth | 11 | 8.7 | 11 | 8.7 | 0.99 |
| Small-for-gestational-age | 13 | 10.4 | 10 | 8.2 | 0.55 |
| Large-for-gestational-age | 15 | 12.0 | 21 | 17.2 | 0.25 |
| Cesarean section | 48 | 37.8 | 44 | 34.7 | 0.60 |
| Mean | SD | Mean | SD | ||
| Birth weight (g) | 3363.17 | 573.49 | 3402.52 | 613.56 | 0.60 |
| Gestational age (wks) | 39.22 | 1.65 | 39.21 | 2.00 | 0.96 |
Discussion
In this randomized trial among ethnically diverse pregnant women at increased risk for GDM, we found that an individually-tailored motivationally-matched exercise intervention implemented in the second trimester did not result in a statistically significant reduction in GDM, IGT, or abnormal glucose screen. The intervention had no effect on birth outcomes.
Our findings for the impact of the exercise intervention on GDM are consistent with a recent meta-analysis by Yin et al. which concluded that evidence was insufficient to suggest that physical activity during pregnancy might be effective in lowering the risk of GDM (6). Of the trials which also evaluated the effect of exercise interventions on other measures of glucose intolerance, two found a beneficial effect on screening or fasting glucose values (32, 33) while the remainder found no effect (34, 35, 36, 37, 38). Consistent with the majority of these studies, we did not find a statistically significant effect of the exercise intervention on other measures of glucose intolerance.
There are several explanations for the observed findings. It is possible that a beneficial effect of exercise on GDM prevention may be limited to women who participated in vigorous intensity activity prior to pregnancy and continued some activity into early pregnancy (5). For example, a meta-analysis of observational studies demonstrated that greater total physical activity before or during early pregnancy was significantly associated with a lower risk of GDM, with the magnitude of the association being stronger for pre-pregnancy physical activity (5). In contrast, only one trial began the intervention in the first trimester (32) and, similarly in our study, only 16.8% of participants began the intervention prior to 14 weeks gestational age.
In the current study, a likely reason for the lack of statistically significant results is insufficient statistical power. While our study was powered to detect odds ratios of 0.65 or smaller this was based on an anticipated enrollment of 352 eligible participants. In contrast, 290 eligible participants were recruited and 251 participants completed follow-up. In addition, the projected percentage of participants with a personal history of GDM, and therefore at highest risk of GDM recurrence, was lower than expected resulting in fewer cases. However, our observed effect size of 0.6 was similar to a recent meta-analysis of observational studies regarding physical activity and GDM (5), suggesting that an appropriately powered study might find a significant effect.
Another reason for the lack of statistical significance could be that the exercise goals were too modest to have an effect on GDM prevention, or lack of adherence with the exercise goals may have diminished any potential effect on GDM development (6). Prior studies have found compliance rates ranging from 16.3% (36) to greater than 95% (33, 39). However, studies with higher compliance rates did not consistently show a greater reduction in GDM incidence. In the current study, the dose of intervention was, on average, 10 weeks gestation which may not have sufficiently impacted behavior change. However, in our recently published paper evaluating the effect of the exercise intervention on change in activity in the B.A.B.Y. Study, the exercise intervention group increased their sports/exercise activity from a mean (SD) of 7.9 (11.2) to 13.1 (11.4) MET hours/week while the health and wellness group increased their sports/exercise from 6.7 (7.8) to 7.0 (9.1) MET hours/week. The difference in change between groups (5.3 vs. 0.3) was statistically significant, p=0.002). In addition, the exercise group was more likely to achieve ACOG guidelines for physical activity as compared to the health and wellness group (odds ratio = 2.12; 95% confidence interval = 1.45, 3.10) (40). However, it is important to note that ACOG guidelines were not developed to specifically address GDM prevention. A recent review has estimated that increasing energy expenditure to a minimum of 16 MET-hours/week (equivalent to approximately one hour of moderate activity on 5 days of the week) may be required to reduce the risk of GDM (41). This is challenging, as pregnancy is a time during which levels of exercise generally decline even among women who were active before pregnancy.
In summary, we found that a prenatal exercise intervention did not have a statistically significant effect on GDM and other measures of glucose intolerance in high-risk ethnically diverse women. However, the high prevalence of pregnant women at risk for GDM and the growing rates of obesity in this population underscore the need for further research on possible interventions that can prevent GDM.
Acknowledgments
Supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grant 1R01DK074876.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Clinical Trial Registration: ClinicalTrials.gov, www.clinicaltrials.gov, NCT00728377.
References
- 1.Metzger B, Gabbe S, Persson B, Buchanan T, Catalano P, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabetic Med. 2014;31:292–301. doi: 10.1111/dme.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellamy L, Casas J, Hingorani A, Williams D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. The Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 5.Tobias D, Zhang C, van Dam R, Bowers K, Hu F. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: A meta-analysis. Diabetes Care. 2011;34:223–229. doi: 10.2337/dc10-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin Y, Li X, Tao T, Luo B, Liao S. Physical activity during pregnancy and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2014;48:290–295. doi: 10.1136/bjsports-2013-092596. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Commerce Economics and Statistics Administration. US Census Bureau. The American Community—Hispanics: 2004. American Community Survey Reports. 2007:1–22. [Google Scholar]
- 8.ACOG Committee Obstetric Practice. ACOG committee opinion. number 267, january 2002: Exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99:171–173. doi: 10.1016/s0029-7844(01)01749-5. [DOI] [PubMed] [Google Scholar]
- 9.Evenson KR, Savitz DA, Huston SL. Leisure-time physical activity among pregnant women in the US. Paediatr Perinat Epidemiol. 2004;18:400–407. doi: 10.1111/j.1365-3016.2004.00595.x. [DOI] [PubMed] [Google Scholar]
- 10.Caughey A, Cheng Y, Stotland N, Washington AE, Escobar G. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Obstet Gynecol. 2010;202 doi: 10.1016/j.ajog.2010.01.082. 616-5. [DOI] [PubMed] [Google Scholar]
- 11.Prevalence of diabetes among hispanics--selected areas, 1998–2002. MMWR Morb Mortal Wkly Rep. 2004;53:941–944. [PubMed] [Google Scholar]
- 12.Chasan-Taber L, Marcus B, Stanek E, Ciccolo J, Marquez D, Solomon C, et al. A randomized controlled trial of prenatal physical activity to prevent gestational diabetes: Design and methods. Journal of Women's Health. 2009;18:851–859. doi: 10.1089/jwh.2008.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Standards of medical care in diabetes--2009. Diabetes Care. 2009;32(Suppl 1):S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute of Medicine, Subcommittee on Nutritional Status and Weight Gain during Pregnancy. Nutrition during pregnancy. 1990 [Google Scholar]
- 15.Prochaska JO, DiClemente CC, Velicer WF, Rossi JS. Standardized, individualized, interactive, and personalized self-help programs for smoking cessation. Health Psychol. 1993;12:399–405. doi: 10.1037//0278-6133.12.5.399. [DOI] [PubMed] [Google Scholar]
- 16.Bandura A. Self-Efficacy: The Exercise of Control. New York: Freeman; 1997. [Google Scholar]
- 17.Marquez D, Bustamante E, Bock B, Markenson G, Tovar A, Chasan-Taber L. Perspectives of latina and non-latina white women on barriers and facilitators to exercise in pregnancy. Women Health. 2009;49:505–521. doi: 10.1080/03630240903427114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kriketos AD, Gan SK, Poynten AM, Furler SM, Chisholm DJ, Campbell LV. Exercise increases adiponectin levels and insulin sensitivity in humans. Diabetes Care. 2004;27:629–630. doi: 10.2337/diacare.27.2.629. [DOI] [PubMed] [Google Scholar]
- 19.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. International Classification of Diseases and Related Health Problems. Geneva: World Health Organization; 1992. [Google Scholar]
- 21.Marcus BH, Napolitano MA, King AC, Lewis BA, Whiteley JA, Albrecht A, et al. Telephone versus print delivery of an individualized motivationally tailored physical activity intervention: Project STRIDE. Health Psychol. 2007;26:401–409. doi: 10.1037/0278-6133.26.4.401. [DOI] [PubMed] [Google Scholar]
- 22.Marcus BH, Lewis BA, Williams DM, Dunsiger S, Jakicic JM, Whiteley JA, et al. A comparison of internet and print-based physical activity interventions. Arch Intern Med. 2007;167:944–949. doi: 10.1001/archinte.167.9.944. [DOI] [PubMed] [Google Scholar]
- 23.Spong CY, Guillermo L, Kuboshige J, Cabalum T. Recurrence of gestational diabetes mellitus: Identification of risk factors. Am J Perinatol. 1998;15:29–33. doi: 10.1055/s-2007-993894. [DOI] [PubMed] [Google Scholar]
- 24.Gaudier FL, Hauth JC, Poist M, Corbett D, Cliver SP. Recurrence of gestational diabetes mellitus. Obstet Gynecol. 1992;80:755–758. [PubMed] [Google Scholar]
- 25.MacNeill S, Dodds L, Hamilton DC, Armson BA, VandenHof M. Rates and risk factors for recurrence of gestational diabetes. Diabetes Care. 2001;24:659–662. doi: 10.2337/diacare.24.4.659. [DOI] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics. National health interview survey. Vital Health Stat. 1991;10:185. [Google Scholar]
- 27.Pols MA, Peeters PH, Bueno-De-Mesquita HB, Ocke MC, Wentink CA, Kemper HC, et al. Validity and repeatability of a modified baecke questionnaire on physical activity. Int J Epidemiol. 1995;24:381–388. doi: 10.1093/ije/24.2.381. [DOI] [PubMed] [Google Scholar]
- 28.Dempsey JC, Butler CL, Sorensen TK, Lee IM, Thompson ML, Miller RS, et al. A case-control study of maternal recreational physical activity and risk of gestational diabetes mellitus. Diabetes Res Clin Pract. 2004;66:203–215. doi: 10.1016/j.diabres.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Dempsey JC, Sorensen TK, Williams MA, Lee IM, Miller RS, Dashow EE, et al. Prospective study of gestational diabetes mellitus risk in relation to maternal recreational physical activity before and during pregnancy. Am J Epidemiol. 2004;159:663–670. doi: 10.1093/aje/kwh091. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt MD, Pekow P, Freedson PS, Markenson G, Chasan-Taber L. Physical activity patterns during pregnancy in a diverse population of women. J Womens Health (Larchmt) 2006;15:909–918. doi: 10.1089/jwh.2006.15.909. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 32.Barakat R, Cordero Y, Coteron J, Luaces M, Montejo R. Exercise during pregnancy improves maternal glucose screen at 24–28 weeks: A randomised controlled trial. Br J Sports Med. 2011 doi: 10.1136/bjsports-2011-090009. [DOI] [PubMed] [Google Scholar]
- 33.Callaway L, Colditz P, Byrne N, Lingwood B, Rowlands I, Foxcroft K, et al. Prevention of gestational diabetes: Feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care. 2010;33:1457. doi: 10.2337/dc09-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stafne S, Salvesen KÅ, Romundstad PR, Eggebø TM, Carlsen S, Mørkved S. Regular exercise during pregnancy to prevent gestational diabetes: A randomized controlled trial. Obstet Gynecol. 2012;119:29–36. doi: 10.1097/AOG.0b013e3182393f86. [DOI] [PubMed] [Google Scholar]
- 35.Luoto R, Kinnunen T, Aittasalo M, Kolu P, Raitanen J, Ojala K, et al. Primary prevention of gestational diabetes mellitus and large-for-gestational-age newborns by lifestyle counseling: A cluster-randomized controlled trial. PLoS Medicine. 2011;8:e1001036. doi: 10.1371/journal.pmed.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oostdam N, van Poppel MNM, Wouters MGAJ, Eekhoff EMW, Bekedam DJ, Kuchenbecker WKH, Quartero HWP, Heres MHB, van Mechelen W. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: Results of a randomised controlled trial. BJOG: An International Journal of Obstetrics and Gynaecology. 2012;119:1098–1107. doi: 10.1111/j.1471-0528.2012.03366.x. [DOI] [PubMed] [Google Scholar]
- 37.Korpi-Hyövälti EAL, Laaksonen D, Schwab U, Vanhapiha T, Vihla K, Heinonen S, et al. Feasibility of a lifestyle intervention in early pregnancy to prevent deterioration of glucose tolerance. BMC Public Health. 2011;11 doi: 10.1186/1471-2458-11-179. 179-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP. Supervised home-based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes Metabolism. 2009;35:418–421. doi: 10.1016/j.diabet.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Barakat R, Perales M, Bacchi M, Coteron J, Refoyo I. A program of exercise throughout pregnancy. is it safe to mother and newborn? American Journal of Health Promotion. 2014;29:2–8. doi: 10.4278/ajhp.130131-QUAN-56. [DOI] [PubMed] [Google Scholar]
- 40.Hawkins M, Chasan Taber L, Marcus B, Stanek E, Braun B, Ciccolo J, et al. Impact of an exercise intervention on physical activity during pregnancy: The behaviors affecting baby and you study. Am J Public Health. 2014;104:e74–e81. doi: 10.2105/AJPH.2014.302072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zavorsky G, Longo L. Exercise guidelines in pregnancy: New perspectives. Sports Medicine. 2011;41:345–360. doi: 10.2165/11583930-000000000-00000. [DOI] [PubMed] [Google Scholar]