Abstract
Tumor hypoxia presents a unique therapeutic challenge in the treatment of solid malignancies. Its presence has been established to be a poor prognostic factor in multiple cancer types, and past hypoxia-directed approaches have yielded generally disappointing results. Previous approaches have centered on either increasing oxygen delivery or administering agents that preferentially radiosensitize or kill hypoxic cells. However, a novel and potentially more effective method may be to increase therapeutic benefit by decreasing tumor oxygen consumption via agents such as metformin or nelfinavir, in a patient population that is enriched for tumor hypoxia. This promising approach is currently being investigated in clinical trials and the subject of this article.
Background
Hypoxia/anoxia is a well-characterized component of the solid tumor microenvironment. In their comprehensive review of studies in the literature examining tumor hypoxia using polarographic needle electrode systems, Vaupel and colleagues found that the overall median pO2 levels in malignant brain tumors and cancers of the uterine cervix, head and neck and breast was 10 mm Hg with the hypoxic fraction (percentage of tumor with pO2 < 2.5 mm Hg) approximately 20–30% (1). In general there is not a correlation between tumor diameter and median pO2 or hypoxic fraction. For many tumors there is spatial heterogeneity of hypoxia, i.e. there is no characteristic topological distribution of pO2 within tumors (periphery versus center). For example, Evans and colleagues showed that there was substantial intra- and intertumoral hypoxic heterogeneity within human grade IV glial neoplasms (2). The majority of cells within these tumors had levels of hypoxia that were mild to moderate (defined as 10% to 0.5% pO2) rather than severe (approximately 0.1% pO2).
Even if only a minority of cells within a tumor contain are hypoxic, this can have a negative effect on outcome (3). Hypoxia is associated with chemoresistance, increased genomic instability, and the propensity for invasion and metastasis (4). Hypoxic cells are more resistant to radiotherapy due to the fact that O2 must be present for optimal fixation of DNA damage induced by ionizing radiation (5). Hypoxic cells are relatively resistant to radiotherapy, requiring 2.5–3 times the radiation dose as normoxic cells to result in the same level of cell killing (5). Gray and colleagues found this to be the case in a landmark study examining a wide range of cells and tissues (6). Furthermore, these authors showed that it was the presence of oxygen during the actual time of irradiation that resulted in sensitivity. A free radical is the primary product induced by ionizing radiation that leads to DNA damage/lethality. When oxygen, which is highly electron-affinic, is present, it reacts rapidly with the free radical, hence “fixing” the damage. Without oxygen, this free radical damage can be reversed by hydrogen donation from nonprotein sylfhydryls in the cell. For these multiple reasons, hypoxia correlates with poor prognosis in a variety of cancers, including carcinoma of the cervix (7), head and neck (8, 9), and sarcomas (10). In patients with head and neck squamous cell carcinomas (HNSCC) treated with definitive radiotherapy, hypoxia has been shown to adversely affect not only local-regional control but also survival (3).
For the reasons discussed above, there have been multiple approaches to hypoxia-directed therapy in patients receiving radiotherapy. One strategy to counter hypoxia is to reverse it by increasing oxygen delivery using hyperbaric oxygen (HBO). Although some trials demonstrated a modest benefit in cancers of the head and neck and cervix (11–13), other trials demonstrated no appreciable benefit (14, 15). An alternative approach is the use of hypoxic cell radiosensitizers, specifically nitroimidzaoles, such as misonidazole and nimorazole. These drugs are electron affinic, undergoing bioreductive activation under hypoxic conditions leading to the creation of reactive intermediates that can form adducts with target molecules within the cells. For example, misonidazole reacts with intracellular glutathinone (GSH) to form covalently bound conjugates. As GSH is an important free radical scavenger, conjugation/depletion of this antioxidant leads to increased DNA damage when cells are exposed to radiation. In this way nitroimidazoles preferentially radiosensitize hypoxic cells, since they do not undergo bioreduction under normoxic conditions. Hence, the use of nitroimidazoles should theoretically increase the therapeutic ratio of radiation. As discussed later, misonidazole can also be used to image hypoxia by positron emission tomography (PET) scanning when bound to radioactive fluoromisonidazole (18F-MISO). Multiple clinical studies using different nitroimidazoles in various cancer types have been performed, and again show mixed results, with modest benefit (16, 17), no benefit (18, 19), or even worse outcome (20).
Direct, comparative clinical evidence was provided by Overgaard who recently published a systematic review and meta-analysis of randomized trials with 4,805 patients with HNSCC treated with radiotherapy and some type of hypoxia-modifying therapy including HBO, nitroimidazole, or breathing carbogen or normobaric oxygen (21). The conclusion for this analysis was that hypoxic modification of radiotherapy in HNSCC did result in a significant improved therapeutic benefit, most clearaly seein in loco-regional control (odds ratio 0.71, 95% CI 0.63–0.80; p<0.001) and disease-specific survival. The improvement in loco-regional control translated into an improvement in overall survival for 60% of patients.
Another approach to combat hypoxia is with hypoxic cytotoxins, such as Mitomycin C and tirapazamine, which are reduced intracellularly to form an active cytotoxic species in the presence of hypoxia. Trials of Mitomycin C in head and neck cancer have shown mixed results, with some reporting improvement in local control (22, 23), while others demonstrate no benefit (24, 25). A phase III trial (TROG 02.02, HeadSTART) of tirapazamine for advanced stage head and neck cancers did not show an overall benefit (26), but a subset analysis demonstrated a trend towards improved locoregional control (92% vs 81% at 2 years) favoring the tirapazamine arm in p16-negative (with p16 a surrogate for human papilloma virus (HPV)) patients with oropharyngeal cancer (27). The investigators also found that the patients who derived the greatest benefit from the addition of tirapazmine were those with hypoxic tumors as demonstrated on 18F-MISO-PET imaging (28), suggesting that hypoxia-directed therapy can be beneficial, and that upfront patient selection via methods such as imaging, are of critical importance.
The clinical data reviewed above, particularly the Overgaard meta-analysis, suggest that there may be merit to hypoxia modification in patients treated with radiotherapy, although many individual trials have failed to show a clearcut improvement in loco-regional control or survival (21). Possible explanations for this may be the substantial dose-limiting toxicity associated with some of these agents (such as peripheral neuropathy with misonidazole) thus limiting their dosage in trials or the failure to enrich the study populations for hypoxic tumors, thus diluting the effect of hypoxia modification. The remainder of this review focuses on novel strategies for tackling the hypoxia problem in patients receiving radiotherapy, including drugs that may be better tolerated or imaging techniques that will better identify patients who might benefit from such therapy.
Clinical–Translational Advances
An alternative approach to attacking the problem of hypoxia is to address it on the demand side, i.e. to decrease O2 consumption. Based on mathematical modeling, Secomb et al. predicted that even a 30% decrease in O2 consumption would decrease the hypoxic fraction from 37% to 11% (29). This particular approach has not been as well explored as targeting the supply side or using agents that are preferentially toxic to hypoxic cells. There have been recent reports that describe clinically relevant agents that decrease O2 consumption and could lead to improved radiation response, which are described below.
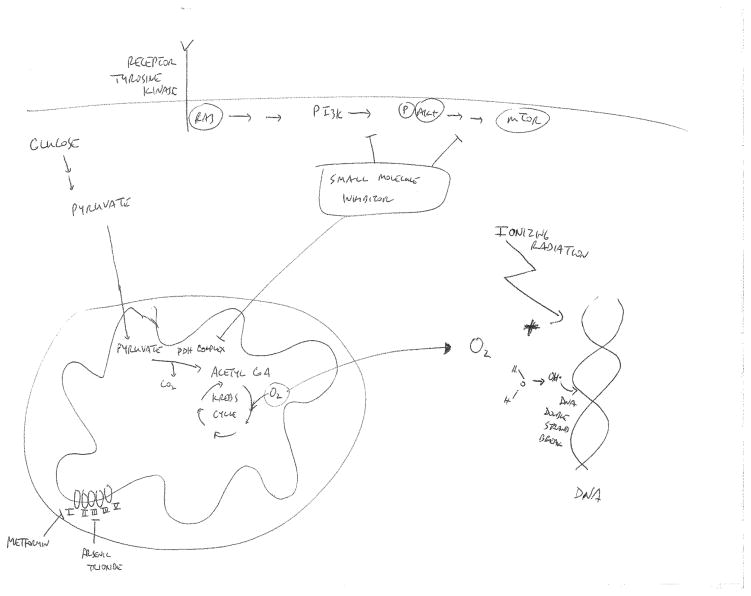
In pre-clinical models including spheroids, there are published data showing that inhibition of O2 consumption using respiratory inhibitors can lead to increased killing by radiation (30). Similar findings have been made using drugs such as meta-iodobenzylguanidine (31) and arsenic trioxide (32). Arsenic trioxide has been postulated to decrease oxygen consumption via inhibition of the electron transport chain (33) (see Fig. 1). However, the toxicity of these agents in vivo has prevented their use in patients. On the other hand, metformin and rosiglitazone, which are used to treat type 2 diabetes, have been also been shown to reduce O2 consumption in vitro by inhibiting complex I in the mitochondrial respiratory chain (34) (Fig. 1). As shown in this figure, complex 1 is the first complex in the electron transport chain that resides in the mitochondrial inner membrane. In Complex 1, two electrons are removed from NADH and transferred to a lipid-soluble carrier, ubiquinone. Complex 1 also translocates 4 protons across the membrane, thus producing a proton gradient. Zanella et al. recently showed that metformin increases oxygenation in vivo within tumor xenografts and improves radiotherapy response by delaying tumor regrowth of xenografts (35). This in vivo effect was not due to any change in the intrinsic (in vitro) radiation response of the tumor cells induced by metformin, so it was presumed to result from improved oxygenation. Furthermore, the authors went on to analyze clinical data to see whether metformin use during radiotherapy might be associated with better outcome. Indeed, they found that patients with localized prostate cancer who had been on metformin during their radiation had a reduction in biochemical relapse compared to those who had not been on the drug. While not proof that this was due to changes in oxygenation leading to better radiation effect, these data are consistent with this idea.
Figure 1.
The role of oxygen in radiation-induced DNA damage and agents that can reduce cellular oxygen consumption. Oxygen is required to elicit maximal DNA damage following ionizing radiation (primarily double strand breaks) through the generation of oxidative free radicals. Hence, hypoxic tumors are relatively resistant to cell killing in response to radiation. Drugs such as metformin and arsenic trioxide have been shown to reduce oxygen consumption, likely by inhibiting electron transport chain function. Inhibitors of the PI3K/AKT/mTOR signaling pathway have also been shown to decrease oxygen consumption, possibly by inhibiting the pyruvate dehydrogenase (PDH) complex. These drugs could be used to reduce tumor oxygen consumption and tumor hypoxia, thus potentially increasing radiosensitization.
Storozhuk et al. found that the addition of metformin to radiotherapy led to delayed tumor regrowth when given to mice bearing A549 and H1299 lung adenocarcinoma xenografts (36). Simone and colleagues made similar observations in a mouse model, and in a clinical correlate, went on show a dramatic decrease in local relapse in a subset of patients with stage III non-small cell lung cancer treated with chemoradiation who were taking metformin for diabetes compared to patients not taking the drug (37). Similar retrospective data analyses have suggested that metformin use is associated with improved treatment response following chemoradiation in patients treated for esophageal and rectal cancers (38, 39).
Drugs that target the PI3K Akt pathway also decrease tumor hypoxia (40, 41). In our own work, we have shown that the HIV protease inhibitor nelfinavir can decrease tumor hypoxia (42, 43). Nelfinavir has been shown to inhibit the PI3K/Akt pathway, although it probably does not do so directly (44). How does PI3K/Akt inhibition alter tumor oxygenation? There is some evidence (40, 41, 43) that these drugs may improve blood flow by “normalizing” vascular blood flow within tumors. Hence these drugs may address the problem on the supply side. However, there is also evidence that they may affect the demand side. Kelly et al. have shown that treatment of cells in vitro with inhibitors of the PI3K pathway including NVP-BEZ235 and NVP-BKM226, both inhibitors of PI3K/mTOR, results in decreased O2 consumption (45). We have made similar observations using multiple drugs such as the Akt inhibitor GDC-0068 and the dual PI3K/mTOR inhibitors NVP-BGT226 and GDC-0098 (A. Maity; unpublished data). Pharmacologic or genetic inhibition of this pathway decreased the oxygen consumption rate (OCR) in vitro in SQ20B head and neck squamous cell carcinoma cells and other cell lines by 30–40%. Inhibition of this pathway also increased phosphorylation of the E1α subunit of the pyruvate dehydrogenase (PDH complex of Ser293 in SQ20B cells) (Fig. 1). This phosphorylation inhibits activity of this critical gatekeeper of mitochondrial respiration, which catalyzes the conversion of pyruvate to acetyl coA which can then enter the Krebs cycle to start oxidative phosphorylation (OXPHOS). Hence, inhibition of the PDH complex would be predicted to decrease OXPHOS and reduce OCR and offers an explanation as to how the PI3K/Akt pathway affects O2 metabolism, although we have not yet determined the exact steps connecting Akt to E1α phosphorylation. As further evidence of a causal relationship, introduction of exogenous PDH-E1α that contains serine to alanine mutations, which can no longer be regulated by phosphorylation, blunted the decrease in OCR seen with PI3K/mTOR inhibition. We have also shown that nelfinavir also decreases in vitro OCR in a variety of cells. Studies are currently underway to determine mechanistically how this drug reduces OCR. Decreasing tumor hypoxia should increase in vivo radiation response. In fact, dual PI3K/mTOR inhibitors and nelfinavir have both been shown to delay tumor regrowth following radiation (41, 42, 44).
Other clinically useful agents have also been shown to decrease O2 consumption by cells. Using electron paramagnetic resonance (EPR) oximetry, a proven method to obtain direct absolute measurement of oxygen in tissue, Crokart et al. found that the in vivo administration of commonly used non-steroidal anti-inflammatory drugs (NSAIDs) including diclofenac, indomethacin, and piroxicam caused a rapid reduction in hypoxia within murine liver tumors and fibrosarcomas (46). The administration of NSAIDs led to a decrease in tumor perfusion, so the reduction in hypoxia was not due to an increase in oxygen supply, but more likely primarily mediated by a decrease in mitochondrial respiration. The administration of NSAID led to an augmentation in tumor regrowth delay following a single 18 Gy fraction of radiation. Using EPR oximetry, Danhier et al. showed that the chemotherapeutic agent paclitaxel in a micelle formulation (M-PTX) dramatically reduced hypoxia within tumors grown in mice (47). Additional experiments showed that this was due to both an increase in blood flow as well as an inhibition of O2 consumption. This dual effect led to synergistic, functional improvement when delivered with 10 Gy irradiation.
While these results are very interesting and suggestive that agents that improve tumor oxygenation can lead to improved radiation response, they are hardly definitive. First, these studies have been performed in mouse tumor models, which for obvious reasons may not reflect the situation in patients. Second, interpretation of these results is confounded by the fact that these drugs may also affect intrinsic radiosensitivity. Therefore, their in vivo effects when combined with radiation may not be exclusively due to altered tumor oxygenation. For example, metformin, which in the study cited above was not found to alter in vitro radiosensitzation (35), has been shown by others to impair the repair of DNA damage following radiation in vitro (48) and lead to radiosensitzation (36).
Current clinical trials
As discussed above, there is retrospective evidence that patients with prostate, lung, and gastrointestinal cancers taking metformin may have a better outcome following radiation therapy (35, 38, 39). There is currently a phase II trial open in the U.S. (NRG-LU001, NCT02186847; www.clinicaltrials.gov) that randomizes patients with stage III non-small cell lung cancer receiving chemoradiation to either receive metformin during radiation or not. A similar study (ALMERA, NCT02115464; www.clinicaltrials.gov) is currently recruiting patients in Canada. Metformin is also being investigated in the treatment of prostate cancer. A clinical trial (NCT01864096; www.clinicaltrials.gov) is currently being planned at Princess Margaret Cancer Center in which non-diabetic patients with early stage prostate cancer are given metformin as a lead-in prior to the initiation of definitive radiotherapy. Biopsies will be taken before metformin administration and then again prior to the start of radiotherapy, which will allow for assessment of biomarkers reporting on metformin activity and tumor hypoxia (R. Bristow and M. Koritzinsky; personal communication).
While these studies will give a clearer answer as to whether the use of metformin will improve outcomes when administered with chemoradiation, it is difficult to conclude that the effect is secondary to changes in oxygenation. One way of directly studying this would be to assess hypoxia non-invasively in patients receiving metformin or other drugs that can alter tumor oxygenation. Hypoxia imaging, using agents such as radiolabeled nitroimidazoles, has been available for some time and is being slowly introduced into the clinical setting (49). Therefore, the means currently exist to treat patients with a given agent and determine radiographically whether their tumors become less hypoxic. At our institution, we are employing such an approach in an open phase II trial (NCT02207439; www.clinicaltrials.gov) using nelfinavir in combination with cisplatin and radiation for locally-advanced, human papilloma virus-negative, larynx cancer. The patient population for this trial is enriched for hypoxia (tobacco-induced, HPV-negative), with evidence that hypoxia modification is of benefit (27, 28). Patients undergo baseline hypoxia imaging (18F-EF5 PET/CT), receive 2 weeks of a “lead-in” period of nelfinavir, undergo repeat 18F-EF PET/CT, and then are treated with standard platinum-based chemoradiation with nelfinavir. We hypothesize that the patients demonstrating the greatest decrease in tumor hypoxia secondary to nelfinavir, as assessed by EF5-PET/CT scanning, will derive the greatest benefit.
Conclusions
Tumor hypoxia remains a significant issue in multiple cancers, with its presence associated with poor clinical outcomes. We believe that decreasing oxygen consumption to be a promising method in reversing hypoxia. Identifying, selecting and enriching populations with hypoxic tumors will be paramount in order to conduct clinical trials of novel agents that we hope and anticipate will improve outcomes for our patients.
Acknowledgments
Grant Support
A. Lin and A. Maity were supported by the NIH under award number RO1CA174976.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Vaupel P, Hockel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal. 2007;9:1221–35. doi: 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- 2.Evans SM, Judy KD, Dunphy I, Jenkins WT, Nelson PT, Collins R, et al. Comparative measurements of hypoxia in human brain tumors using needle electrodes and EF5 binding. Cancer Res. 2004;64:1886–92. doi: 10.1158/0008-5472.can-03-2424. [DOI] [PubMed] [Google Scholar]
- 3.Brizel DM, Dodge RK, Clough RW, Dewhirst MW. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53:113–7. doi: 10.1016/s0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 4.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 5.Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 7. Philadelphia: Lippincott Williams & Wilkins; 2012. Oxygen effect and reoxygenation; pp. 86–103. [Google Scholar]
- 6.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638–48. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 7.Fyles A, Milosevic M, Hedley D, Pintilie M, Levin W, Manchul L, et al. Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. J Clin Oncol. 2002;20:680–7. doi: 10.1200/JCO.2002.20.3.680. [DOI] [PubMed] [Google Scholar]
- 8.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. J Radiat Oncol Biol Phys. 1997;38:285–9. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 9.Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 10.Nordsmark M, Alsner J, Keller J, Nielsen OS, Jensen OM, Horsman MR, et al. Hypoxia in human soft tissue sarcomas: adverse impact on survival and no association with p53 mutations. Br J Cancer. 2001;84:1070–5. doi: 10.1054/bjoc.2001.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henk JM, Kunkler PB, Smith CW. Radiotherapy and hyperbaric oxygen in head and neck cancer. Final report of first controlled clinical trial. Lancet. 1977;2:101–3. doi: 10.1016/s0140-6736(77)90116-7. [DOI] [PubMed] [Google Scholar]
- 12.Henk JM, Smith CW. Radiotherapy and hyperbaric oxygen in head and neck cancer. Interim report of second clinical trial. Lancet. 1977;2:104–5. doi: 10.1016/s0140-6736(77)90117-9. [DOI] [PubMed] [Google Scholar]
- 13.Watson ER, Halnan KE, Dische S, Saunders MI, Cade IS, McEwen JB, et al. Hyperbaric oxygen and radiotherapy: a Medical Research Council trial in carcinoma of the cervix. Br J Radiol. 1978;51:879–87. doi: 10.1259/0007-1285-51-611-879. [DOI] [PubMed] [Google Scholar]
- 14.Cade IS, McEwen JB, Dische S, Saunders MI, Watson ER, Halnan KE, et al. Hyperbaric oxygen and radiotherapy: a Medical Research Council trial in carcinoma of the bladder. Br J Radiol. 1978;51:876–8. doi: 10.1259/0007-1285-51-611-876. [DOI] [PubMed] [Google Scholar]
- 15.Overgaard J. Sensitization of hypoxic tumour cells--clinical experience. Int J Radiat Biol. 1989;56:801–11. doi: 10.1080/09553008914552081. [DOI] [PubMed] [Google Scholar]
- 16.Overgaard J, Hansen HS, Andersen AP, Hjelm-Hansen M, Jorgensen K, Sandberg E, et al. Misonidazole combined with split-course radiotherapy in the treatment of invasive carcinoma of larynx and pharynx: report from the DAHANCA 2 study. Int J Radiat Oncol Biol Physics. 1989;16:1065–8. doi: 10.1016/0360-3016(89)90917-6. [DOI] [PubMed] [Google Scholar]
- 17.Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiother Oncol. 1998;46:135–46. doi: 10.1016/s0167-8140(97)00220-x. [DOI] [PubMed] [Google Scholar]
- 18.Lee DJ, Cosmatos D, Marcial VA, Fu KK, Rotman M, Cooper JS, et al. Results of an RTOG phase III trial (RTOG 85-27) comparing radiotherapy plus etanidazole with radiotherapy alone for locally advanced head and neck carcinomas. Int J Radiat Oncol Biol Physics. 1995;32:567–76. doi: 10.1016/0360-3016(95)00150-W. [DOI] [PubMed] [Google Scholar]
- 19.Overgaard J. Clinical evaluation of nitroimidazoles as modifiers of hypoxia in solid tumors. Oncol Res. 1994;6:509–18. [PubMed] [Google Scholar]
- 20.A trial of Ro 03-8799 (pimonidazole) in carcinoma of the uterine cervix: an interim report from the Medical Research Council Working Party on advanced carcinoma of the cervix. Radiother Oncol. 1993;26:93–103. [PubMed] [Google Scholar]
- 21.Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck--a systematic review and meta-analysis. Radiother Oncol. 2011;100:22–32. doi: 10.1016/j.radonc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Haffty BG, Son YH, Sasaki CT, Papac R, Fischer D, Rockwell S, et al. Mitomycin C as an adjunct to postoperative radiation therapy in squamous cell carcinoma of the head and neck: results from two randomized clinical trials. Int J Radiat Oncol Biol Physics. 1993;27:241–50. doi: 10.1016/0360-3016(93)90234-m. [DOI] [PubMed] [Google Scholar]
- 23.Weissberg JB, Son YH, Papac RJ, Sasaki C, Fischer DB, Lawrence R, et al. Randomized clinical trial of mitomycin C as an adjunct to radiotherapy in head and neck cancer. Int J Radiat Oncol Biol Physics. 1989;17:3–9. doi: 10.1016/0360-3016(89)90362-3. [DOI] [PubMed] [Google Scholar]
- 24.Dobrowsky W, Naude J, Dobrowsky E, Millesi W, Pavelka R, Grasl M, et al. Mitomycin C (MMC) and unconventional fractionation (V-CHART) in advanced head and neck cancer. Acta Oncol. 1995;34:270–2. doi: 10.3109/02841869509093973. [DOI] [PubMed] [Google Scholar]
- 25.Grau C, Prakash Agarwal J, Jabeen K, Rab Khan A, Abeyakoon S, Hadjieva T, et al. Radiotherapy with or without mitomycin c in the treatment of locally advanced head and neck cancer: results of the IAEA multicentre randomised trial. Radiother Oncol. 2003;67:17–26. doi: 10.1016/s0167-8140(03)00020-3. [DOI] [PubMed] [Google Scholar]
- 26.Rischin D, Peters LJ, O’Sullivan B, Giralt J, Fisher R, Yuen K, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02. 02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol. 2010;28:2989–95. doi: 10.1200/JCO.2009.27.4449. [DOI] [PubMed] [Google Scholar]
- 27.Rischin D, Young RJ, Fisher R, Fox SB, Le QT, Peters LJ, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02. 02 phase III trial. J Clin Oncol. 2010;28:4142–8. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rischin D, Hicks RJ, Fisher R, Binns D, Corry J, Porceddu S, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol. 2006;24:2098–104. doi: 10.1200/JCO.2005.05.2878. [DOI] [PubMed] [Google Scholar]
- 29.Secomb TW, Hsu R, Dewhirst MW. Synergistic effects of hyperoxic gas breathing and reduced oxygen consumption on tumor oxygenation: a theoretical model. Int J Radiat Oncol Biol Physics. 2004;59:572–8. doi: 10.1016/j.ijrobp.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 30.Durand RE, Biaglow JE. Radiosensitization of hypoxic cells of an in vitro tumor model by respiratory inhibitors. Radiat Res. 1977;69:359–66. [PubMed] [Google Scholar]
- 31.Biaglow JE, Manevich Y, Leeper D, Chance B, Dewhirst MW, Jenkins WT, et al. MIBG inhibits respiration: potential for radio- and hyperthermic sensitization. Int J Radiat Oncol Biol Physics. 1998;42:871–6. doi: 10.1016/s0360-3016(98)00334-4. [DOI] [PubMed] [Google Scholar]
- 32.Diepart C, Karroum O, Magat J, Feron O, Verrax J, Calderon PB, et al. Arsenic trioxide treatment decreases the oxygen consumption rate of tumor cells and radiosensitizes solid tumors. Cancer Res. 2012;72:482–90. doi: 10.1158/0008-5472.CAN-11-1755. [DOI] [PubMed] [Google Scholar]
- 33.Pelicano H, Feng L, Zhou Y, Carew JS, Hileman EO, Plunkett W, et al. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J Biol Chem. 2003;278:37832–9. doi: 10.1074/jbc.M301546200. [DOI] [PubMed] [Google Scholar]
- 34.Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004;53:1052–9. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- 35.Zannella VE, Dal Pra A, Muaddi H, McKee TD, Stapleton S, Sykes J, et al. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin Cancer Res. 2013;19:6741–50. doi: 10.1158/1078-0432.CCR-13-1787. [DOI] [PubMed] [Google Scholar]
- 36.Storozhuk Y, Hopmans SN, Sanli T, Barron C, Tsiani E, Cutz JC, et al. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer. 2013;108:2021–32. doi: 10.1038/bjc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simone CB, II, Csiki I, Heskel M, Gabriel P, Kim H, Corradetti MN, et al. Metformin as a radiosensitizer for lung cancer [abstract]. Proceedings of the 15th World Conference on Lung Cancer; 2013 Oct 27–31; Sydney, Australia. Vancouver (BC): IASLC; 2013. p. Abstract nr MO05.10. [Google Scholar]
- 38.Skinner HD, McCurdy MR, Echeverria AE, Lin SH, Welsh JW, O’Reilly MS, et al. Metformin use and improved response to therapy in esophageal adenocarcinoma. Acta Oncol. 2013;52:1002–9. doi: 10.3109/0284186X.2012.718096. [DOI] [PubMed] [Google Scholar]
- 39.Skinner HD, Crane CH, Garrett CR, Eng C, Chang GJ, Skibber JM, et al. Metformin use and improved response to therapy in rectal cancer. Cancer Med. 2013;2:99–107. doi: 10.1002/cam4.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qayum N, Muschel RJ, Im JH, Balathasan L, Koch CJ, Patel S, et al. Tumor vascular changes mediated by inhibition of oncogenic signaling. Cancer Res. 2009;69:6347–54. doi: 10.1158/0008-5472.CAN-09-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fokas E, Im JH, Hill S, Yameen S, Stratford M, Beech J, et al. Dual inhibition of the PI3K/mTOR pathway increases tumor radiosensitivity by normalizing tumor vasculature. Cancer Res. 2012;72:239–48. doi: 10.1158/0008-5472.CAN-11-2263. [DOI] [PubMed] [Google Scholar]
- 42.Pore N, Gupta AK, Cerniglia GJ, Jiang Z, Bernhard EJ, Evans SM, et al. Nelfinavir down-regulates hypoxia-inducible factor 1alpha and VEGF expression and increases tumor oxygenation: implications for radiotherapy. Cancer Res. 2006;66:9252–9. doi: 10.1158/0008-5472.CAN-06-1239. [DOI] [PubMed] [Google Scholar]
- 43.Cerniglia GJ, Pore N, Tsai JH, Schultz S, Mick R, Choe R, et al. Epidermal growth factor receptor inhibition modulates the microenvironment by vascular normalization to improve chemotherapy and radiotherapy efficacy. PloS One. 2009;4:e6539. doi: 10.1371/journal.pone.0006539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta AK, Cerniglia GJ, Mick R, McKenna WG, Muschel RJ. HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both in vitro and in vivo. Cancer Res. 2005;65:8256–65. doi: 10.1158/0008-5472.CAN-05-1220. [DOI] [PubMed] [Google Scholar]
- 45.Kelly CJ, Hussien K, Fokas E, Kannan P, Shipley RJ, Ashton TM, et al. Regulation of O2 consumption by the PI3K and mTOR pathways contributes to tumor hypoxia. Radiother Oncol. 2014;111:72–80. doi: 10.1016/j.radonc.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crokart N, Radermacher K, Jordan BF, Baudelet C, Cron GO, Gregoire V, et al. Tumor radiosensitization by antiinflammatory drugs: evidence for a new mechanism involving the oxygen effect. Cancer Res. 005;65:7911–6. doi: 10.1158/0008-5472.CAN-05-1288. [DOI] [PubMed] [Google Scholar]
- 47.Danhier F, Danhier P, Magotteaux N, De Preter G, Ucakar B, Karroum O, et al. Electron paramagnetic resonance highlights that the oxygen effect contributes to the radiosensitizing effect of paclitaxel. PloS One. 2012;7:e40772. doi: 10.1371/journal.pone.0040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang T, Zhang L, Zhang T, Fan J, Wu K, Guan Z, et al. Metformin sensitizes prostate cancer cells to radiation through EGFR/p-DNA-PKCS in vitro and in vivo. Radiation research. 2014;181:641–9. doi: 10.1667/RR13561.1. [DOI] [PubMed] [Google Scholar]
- 49.Lin A, Hahn SM. Hypoxia imaging markers and applications for radiation treatment planning. Semin Nucl Med. 2012;42:343–52. doi: 10.1053/j.semnuclmed.2012.04.002. [DOI] [PubMed] [Google Scholar]