Abstract
Cognitive performance is an important component of healthy aging. Type 2 diabetes (T2D) is associated with negative outcomes for the brain and cognition, although causal mechanisms have not been definitely determined. Genetic risk factors warrant further consideration in this context. This study examined the heritability of cognitive function as assessed by (1) the Digit Symbol Substitution Task; (2) the Modified Mini-Mental State Examination; (3) the Stroop Task; (4) the Rey Auditory-Verbal Learning Task; and (5) the Controlled Oral Word Association Task for Phonemic and Semantic Fluency, in the family-based, T2D-enriched, Diabetes Heart Study sample (n = 550 participants from 257 families). The genetic basis of these cognitive measures was further evaluated by association analysis with candidate single-nucleotide polymorphisms (SNPs) and genome-wide SNP data. Measures of cognitive function were significantly heritable (ĥ2 = 0.28–0.62) following adjustment for age, gender, and education. A total of 31 SNPs (from 26 genes/regions) selected to form an a priori set of candidate SNPs showed limited evidence of association with cognitive function when applying conservative metrics of significance. Genome-wide assessment of both noncoding and coding variants revealed suggestive evidence of association for several coding variants including rs139509083 in CNST (p = 4.9 × 10−9), rs199968569 in PLAA (p = 4.9 × 10−9) and rs138487371 in PCDH8 (p = 3.7 × 10−8). The identification of a heritable component to cognitive performance in T2D suggests a role for genetic contributors to cognitive performance even in the presence of metabolic disease and other associated comorbidities and is supported by the identification of genetic association signals in functionally plausible candidates.
Keywords: Cognitive function, Heritability, Genetics, Type 2 diabetes
1. Introduction
Over the past 10 years, research has revealed that type 2 diabetes (T2D) is associated with negative outcomes for the brain and cognition. This ranges from relatively mild decline in a variety of cognitive domains (Arvanitakis et al., 2006; Awad et al., 2004; Brands et al., 2007), akin to acceleration of typical aging-related cognitive declines, to an increased risk for dementia (Ott et al., 1999; Peila et al., 2002), a pathologic process. In the brain T2D is linked with decreased brain volume and increased white matter lesion burden (Manschot et al., 2006; Tiehuis et al., 2008). A definitive causal link between T2D and poorer cognitive function is currently lacking, but brain insulin resistance (Awad et al., 2004; Baker et al., 2011) and cardiovascular disease (CVD) (Hugenschmidt et al., 2013; Warsch and Wright, 2010) have both been implicated. However, one risk factor that has been little explored is the potential contribution of genetic risk to cognitive decline in people with T2D.
The genetic influence on general cognition has been evaluated on a global basis by estimating the heritability of measures of cognitive function and at the level of individual genetic variation by genetic association studies. While the heritability of cognitive performance has been estimated previously (Cirulli et al., 2010; Giubilei et al., 2008; Sleegers et al., 2007), few, if any, studies have involved populations affected by extensive metabolic disease and the associated comorbidities of T2D. Equally, genetic association studies have revealed evidence for association of single-nucleotide polymorphisms (SNPs) in a variety of genes with diverse measures of cognitive function in largely healthy population groups (Davies et al., 2014; Houlihan et al., 2009; Luciano et al., 2011; Need et al., 2009; Papassotiropoulos et al., 2006). However, these associations have proven difficult to replicate in subsequent studies. Moreover, there is a dearth of information as to whether these genetic variants may also underpin a heritable risk for cognitive decline in populations at increased risk, such as in individuals with T2D.
The Diabetes Heart Study (DHS) is a single-site family-based study that provides a useful starting point for exploring genetic contributions to cognition in a population enriched for T2D. The DHS collected abundant data on cardiovascular risk factors from 1998 to 2006, and a follow-up study from 2008 to 2013 collected cognitive testing and neuroimaging data on 550 of the original cohort. Here, we first examined the heritability of cognitive function in this T2D-enriched sample. This analysis was then extended by analysis of a number of candidate SNPs, reported in prior publications, for association with available measures of cognitive function. Subsequently, we extended the genetic analysis with an unbiased genome-wide association study (GWAS) using both genome-wide and exome-wide array data in the DHS cohort.
2. Methods
2.1. Study design and sample
The DHS is a family-based study examining risk for macro-vascular and other complications in T2D. Briefly, the DHS includes siblings concordant for T2D but without advanced renal insufficiency. When possible, unaffected siblings were also recruited. T2D was clinically defined as diabetes developing after the age of 35 years and initially treated with oral agents and/or diet and exercise, in the absence of historical evidence of ketoacidosis. Diagnoses were confirmed by measurement of fasting blood glucose and glycosylated hemoglobin (HbA1c). Extensive measurements of CVD risk factors were obtained during baseline exams, which occurred from 1998 to 2006. Ascertainment and recruitment have been previously described in detail (Bowden et al., 2008, 2010).
The DHS-Mind study is an ancillary study to the DHS initiated in 2008 that included a cognitive testing component to investigate the relationships between cognitive function and vascular disease in T2D. Participants returning from the original DHS investigation were re-examined on average 6.7 ± 1.6 years after their initial visit. Participant examinations were conducted in the General Clinical Research Center of the Wake Forest Baptist Medical Center. The current analyses are based on a subset of 550 participants returning from the baseline DHS exam with measured phenotypes from the DHS-Mind study visit and available genotype data. For these analyses level of educational attainment was classified as less than high school, high school, or greater than high-school based on self-report by participants. Study protocols were approved by the Institutional Review Board at Wake Forest School of Medicine and all study procedures were carried out in accordance with the Declaration of Helsinki. All participants provided written informed consent before participation.
2.2. Cognitive testing
Participants were administered a battery of cognitive tests as described previously (Hugenschmidt et al., 2013). This included: (1) the Digit Symbol Substitution Task (DSST), a test of processing speed and to a lesser extent, working memory (Wechsler, 1981); (2) the Modified Mini-Mental State Examination (3MSE), a test of global cognitive function often used clinically in assessment of dementia (Teng and Chui, 1987); (3) the Stroop Task (Stroop), a test of executive function (Houx et al., 1993) (reported here as the difference in response times between subtest 2 and subtest 3); (4) the Rey Auditory-Verbal Learning Task (RAVLT), a word-list recall task (Lezak et al., 2004) (reported here as the total number of words recalled across the first 5 trials); and (5) the Controlled Oral Word Association Task (COWA) for phonemic fluency (reported here as the sum of words generated for 3 different letters [F, A, S]) and semantic fluency (reported here as the sum of words generated for 2 different categories [kitchen, animals]), accepted as testing another aspect of executive function (Benton et al., 1994; Strauss et al., 2006). As the aim of the study was to examine cognition in a T2D-affected population, subjects were not excluded for 3MSE scores or other indices of cognitive function indicative of mild cognitive impairment or dementia, however individuals with color blindness were excluded from the Stroop task.
2.3. Heritability analysis
Heritability estimates for measures of cognitive function were assessed in 526 related individuals from 188 families; unrelated individuals were excluded from this analysis. The measurements of cognition were transformed to approximate the normality assumptions of the analysis if necessary. To determine the contribution of genetic factors to cognition, the data in family members were analyzed using Sequential Oligogenic Linkage Analysis Routines (SOLAR) version 6.3.4 (Texas Biomedical Research Institute, San Antonio, TX, USA). SOLAR performs a variance components analysis of family data where the total phenotypic variation is partitioned into genetic and nongenetic sources of variation. This approach has been used previously in the DHS (Hsu et al., 2005; Lange et al., 2006). To minimize the bias associated with shared environmental factors, the estimates of heritability (ĥ2) were based on all available family data and were controlled for covariates related to cognition. Three models were developed that incorporated an increasing number of covariates to determine the extent that genetic factors contribute to variation in cognition independent of other confounding variables. The first model was an unadjusted model. The second model was adjusted for age and gender. The third model was adjusted for age, gender, and education. The significance of the heritability estimates was obtained by likelihood ratio tests.
2.4. Genetic data
Genetic association analysis was performed to investigate the relationships between previously reported cognition-associated genetic variants and the measures of cognitive function available in the DHS. To perform these analyses genotype data for SNPs of interest was obtained from genetic data sets available in the DHS derived from (1) the Affymetrix Genome-wide Human SNP Array 5.0 (Affymetrix, CA, USA) (the GWAS set; predominately common variants); (2) the Illumina Infinium Human Exome Beadchip v1.0 (Illumina, CA, USA) (the Exome set; predominately low-frequency and rare coding variants); (3) GWAS Imputed data (the Imputed set) imputed from the 1000 Genomes Project SNPs using IMPUTE2 (http://mathgen.stats.ox.ac.uk/impute/impute_v2.html) and the Phase I v2, cosmopolitan (integrated) reference panel, build 37 (Howie et al., 2009). Genotype data for one SNP (rs429358, in APOE) was not available from the array-based data sets and was directly genotyped using the MassARRAY SNP Genotyping System as described previously (Buetow et al., 2001; Cox et al., 2013).
The genetic data sets were processed as follows. For the GWAS set, genotype calling was completed using the BRLLM-P algorithm in Genotyping Console v4.0 (Affymetrix). Samples failing to meet an intensity quality control threshold were not included for genotype calling and those failing to meet a minimum acceptable call rate of 95% were excluded from further analyses. An additional 39 samples were included as blind duplicates within the genotyping set to serve as quality controls; the concordance rate for these blind duplicates was 99.0 ± 0.72% (mean ± standard deviation). For the GWAS set, exclusion criteria for SNP performance included call rate <95% (n = 11,085), Hardy–Weinberg equilibrium p < 1 × 10−6 (n = 332), and minor allele frequency <0.01 (n = 57,382); 371,951 SNPs were retained for analysis. For imputed data only SNPs with a confidence score >0.90 and information score >0.50 were used.
For the Exome set, genotype calling was completed using Genome Studio Software v1.9.4 (Illumina). Samples failing to meet a minimum acceptable call rate of 98% were excluded from further analyses. An additional 58 samples were included as blind duplicates within the genotyping set to serve as quality controls; the concordance rate for blind duplicates was 99.9 ± 0.0001% (mean ± standard deviation). For the Exome set, exclusion criteria for SNP performance included call rate <99% (n = 972), monomorphic SNPs (n = 157,754), and Hardy–Weinberg equilibrium p < 1 × 10−6 (n = 26); 88,483 SNPs were retained for analysis.
Following genotype calling, exploratory analyses of genotype data were performed using PLINK v1.07 (http://pngu.mgh.harvard.edu/purcell/plink/) and identified samples with poor quality genotype calls, gender errors, or unclear and/or unexpected sibling relationships; all of these were excluded from further analysis.
Targeted genetic association analyses were performed using a set of 31 SNPs selected a priori based on an extensive search of the existing literature (Cirulli et al., 2010; Davies et al., 2014; De Jager et al., 2012; Houlihan et al., 2009; Luciano et al., 2011; Marioni et al., 2011; Need et al., 2009; Papassotiropoulos et al., 2006; Sedille-Mostafaie et al., 2012; Seshadri et al., 2007; Sigmund et al., 2008). SNPs were selected from previous studies examining genetic associations with specific indices of cognitive function in studies of largely healthy population groups, encompassing both young and elderly adults, mixed ethnicities, and ranging in size from several hundred to several thousand participants. Studies reporting genetic associations with measures of cognitive function in Alzheimer’s disease and/or dementia or neuropsychiatric disease were not included. In addition, genome-wide discovery analyses were performed using the entire GWAS and Exome data sets. All analyses were performed using variance components methods as implemented in SOLAR version 6.4.1 (Texas Biomedical Research Institute, San Antonio, TX, USA) to account for family relationships. Association analyses were performed assuming an additive model of inheritance with adjustment for age, sex, T2D affected status, and education. For the candidate SNPs statistical significance was accepted at p < 0.002 based on a Bonferroni correction. For the discovery analyses, genome-wide significance was accepted at p < 5 × 10−8 and exome-wide significance was accepted at p < 2 × 10−7.
Gene-based tests of polymorphic exonic variants from the Exome set were also performed using the sequence kernel association test (SKAT) program with default weights using minor allele frequency. SKAT is a variance components based test that aggregates weighted test statistics for all variants in a gene which is applicable to family data for continuous traits, incorporating a kinship matrix into the models (Chen et al., 2013; Lee et al., 2013). All analysis were adjusted for age, sex, T2D affected status, and education.
3. Results
The demographic and clinical characteristics of the DHS-Mind cohort are summarized in Table 1. As anticipated in a T2D-enriched sample, a predominance of traditional CVD risk factors were evident including high body mass index, prevalent hypertension, and self-reported history of prior CVD events. The cognitive tests demonstrate substantial heterogeneity in cognitive function with scores ranging from levels indicative of mild dementia (3MSE < 77), to scores above average. Overall, mean, and median scores on cognitive tests are slightly lower than would be expected in the general population.
Table 1.
Demographic characteristics for the DHS-Mind participants
| Mean ± SD or % | Median (range) | |
|---|---|---|
| Demographic information | ||
| Age (y) | 67.3 ± 8.8 | 64.4 (41.3–89.2) |
| Gender (% female) | 55.3% | |
| BMI (kg/m2) | 31.5 ± 6.5 | 30.4 (17.6–58.4) |
| % Smoking (current or past) | 55.1% | |
| Hypertension (%) | 78.8% | |
| Self-reported history of prior CVD | 30.9% | |
| Type 2 diabetes | ||
| Type 2 diabetes affected (%) | 76.9% | |
| Diabetes duration (y) | 16.6 ± 6.6 | 14.6 (4.9–44.3) |
| Glucose (mg/dL) | 134 ± 50 | 121 (40–349) |
| Hemoglobin A1C (%) | 7.1 ± 1.3 | 6.8 (4.9–14.8) |
| Medication use | ||
| Anti-diabetic medicationa | 74.0% | |
| Cholesterol-lowering medication | 67.4% | |
| Anti-hypertensive medication | 81.8% | |
| Education | ||
| Less than high school | 18% | |
| High school | 54% | |
| Greater than high school | 28% | |
| Cognitive function test scores | ||
| Modified mini mental state exam (3MSE) | 90.4 ± 7.2 | 92 (43–100) |
| Digit symbol substitution (DSST) | 47.5 ± 15.3 | 47 (10–98) |
| Stroop | 36.2 ± 20.6 | 30 (−8–161) |
| Phonemic fluency | 29.8 ± 7.9 | 28 (2–67) |
| Semantic fluency | 28.7 ± 11.7 | 29 (11–60) |
| Rey auditory-verbal learning task (RAVLT) | 41.6 ± 10.3 | 42 (11–66) |
Key: BMI, body mass index; CVD, cardiovascular disease; DHS, diabetes heart study; SD, standard deviation.
Either oral hypoglycemic medications or insulin.
Results from the heritability analysis support a statistically significant heritable component of all measures of cognitive function in this T2D-enriched cohort (Table 2). Heritability estimates decreased after adjustment for covariates, including education but remained statistically significant. The various cognitive measures differed dramatically in the magnitude of estimated heritability (Table 2); the DSST had the highest calculated heritability (ĥ2 = 0.62 in the fully adjusted model), whereas the Stroop appeared the least heritable (ĥ2 = 0.28).
Table 2.
Heritability estimates for cognitive variables in related individuals from the Diabetes Heart Study Cohort
| Covariates | Stroop | DSST | 3MSE | RAVLT | Semantic fluency | Phonemic fluency |
|---|---|---|---|---|---|---|
| ĥ2 (SE) | ĥ2 (SE) | ĥ2 (SE) | ĥ2 (SE) | ĥ2 (SE) | ĥ2 (SE) | |
| None | 0.43 (0.11) | 0.89 (0.10) | 0.54 (0.10) | 0.60 (0.11) | 0.68 (0.11) | 0.84 (0.11) |
| Age, gender | 0.33 (0.11) | 0.80 (0.11) | 0.49 (0.10) | 0.45 (0.11) | 0.59 (0.11) | 0.80 (0.11) |
| Age, gender, education | 0.28 (0.10) | 0.62 (0.12) | 0.31 (0.10) | 0.31 (0.11) | 0.40 (0.11) | 0.58 (0.12) |
All p-values were <0.05.
Key: DSST, digit symbol substitution task; 3MSE, modified mini-mental state examination; RAVLT, rey auditory-verbal learning task; SE, standard error.
A total of 31 SNPs (from26 genes/regions; Table 3) were selected from prior reports to form an a priori set of candidate SNPs to examine association with measures of cognitive function. Broadly speaking, the previously reported cognition-associated SNPs were not significantly associated with the measures of cognitive function available in the DHS using the Bonferonni corrected significance threshold (Table 3). That said, the strongest association was observed between rs4420638, which is downstream of the apolipoprotein C-1 (APOC1) gene, and phonemic fluency (p = 0.002). Trends for association (0.002 < p < 0.05) were noted between rs7547519 (calmodulin binding transcription activator 1 [CAMTA1], p = 0.005) and rs1130214 (v-akt murine thyoma viral oncogen homolog 1 [AKT1], p = 0.03) and the Stroop task, and between rs429358 (apolipoprotein E [APOE], p = 0.01), rs6265 (brain derived neurotrophic factor [BDNF], p = 0.03), and rs10769565 (olfactory receptor family 56 subfamily A member4/member 1 [OR56A4/OR56A1], p = 0.03) and the RAVLT. Statistically significant associations were not observed between the APOE risk haplotype (Davies et al., 2014) and measures of cognitive function (p = 0.05–0.87). Further, results for the candidate SNP associations were essentially unchanged following additional adjustment for the APOE haplotype (Supplementary Table 1).
Table 3.
Genetic association (assuming an additive model of inheritance) between candidate SNPs and measure of cognitive function
| Chromosome | Position | SNP | Gene | Location | DHS analysis | Association p-values (covariates: age, sex, affected, education) |
Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | Alleles (major/minor) |
MAF | Stroop | DSST | 3MSE | RAVLT | Semantic fluency |
Phonemic fluency |
||||||
| 1 | 7316136 | rs7547519 | CAMTA1 | Intronic | GWAS | G/A | 0.243 | 0.005 | 0.49 | 0.34 | 0.81 | 0.06 | 0.71 | (Cirulli et al., 2010) |
| 1 | 88802012 | rs2179965 | PKN2 | Upstream | Exome | A/G | 0.160 | 0.77 | 0.55 | 0.42 | 0.60 | 0.27 | 0.42 | (Seshadri et al., 2007) |
| 1 | 160321065 | rs12239747 | NCSTN | Exonic | Imputed | A/G | 0.048 | 0.34 | 0.67 | 0.38 | 0.81 | 0.87 | 0.51 | (Houlihan et al., 2009) |
| 1 | 160321180 | rs2274185 | NCSTN | Intronic | Imputed | C/G | 0.058 | 0.07 | 0.80 | 0.47 | 0.23 | 0.66 | 0.61 | (Houlihan et al., 2009) |
| 1 | 160323903 | rs7528638 | NCSTN | Intronic | Imputed | C/G | 0.045 | 0.66 | 0.96 | 0.55 | 0.43 | 0.62 | 0.73 | (Houlihan et al., 2009) |
| 1 | 160327820 | rs17370539 | NCSTN | Intronic | Imputed | G/C | 0.058 | 0.29 | 0.64 | 0.36 | 0.76 | 0.95 | 0.74 | (Houlihan et al., 2009) |
| 3 | 139682495 | rs6439886 | CLSTN2 | Intronic | Imputed | A/G | 0.137 | 0.32 | 0.76 | 0.85 | 0.56 | 0.10 | 0.50 | (Need et al., 2009; Papassotiropoulos et al., 2006; Sedille-Mostafaie et al., 2012) |
| 4 | 67733857 | rs1155865 | Intergenic | — | GWAS | A/G | 0.150 | 0.60 | 0.40 | 0.43 | 0.76 | 0.05 | 0.48 | (Seshadri et al., 2007) |
| 4 | 155491759 | rs4220 | FGB | Exonic | Exome | G/A | 0.153 | 0.08 | 0.19 | 0.71 | 0.77 | 0.33 | 0.49 | (Marioni et al., 2011) |
| 5 | 167845791 | rs17070145 | KIBRA (WWC1) | Intronic | GWAS | C/T | 0.310 | 0.70 | 0.97 | 0.51 | 0.69 | 0.70 | 0.61 | (Cirulli et al., 2010; Need et al., 2009; Papassotiropoulos et al., 2006; Sedille-Mostafaie et al., 2012) |
| 7 | 136592969 | rs2350780 | CHRM2 | Intronic | Imputed | A/G | 0.332 | 0.66 | 0.69 | 0.58 | 0.07 | 0.89 | 0.93 | (Cirulli et al., 2010) |
| 8 | 31474141 | rs35753505 | NRG1 | Upstream | Imputed | T/C | 0.345 | 0.93 | 0.55 | 0.94 | 0.96 | 0.58 | 0.98 | (Need et al., 2009) |
| 8 | 66644713 | rs10808746 | PDE7A | Intronic | GWAS | G/A | 0.459 | 0.53 | 0.22 | 0.14 | 0.11 | 0.43 | 0.71 | (De Jager et al., 2012) |
| 10 | 16689683 | rs7087965 | RSU1 | Intronic | Imputed | G/A | 0.301 | 0.62 | 0.60 | 0.90 | 0.16 | 0.44 | 0.16 | (Luciano et al., 2011) |
| 10 | 117512588 | rs10490919 | ATRNL1 | Intronic | GWAS | T/G | 0.500 | 0.87 | 0.14 | 0.92 | 0.61 | 0.82 | 0.26 | (Luciano et al., 2011) |
| 11 | 1782594 | rs17571 | CTSD | Exonic | Exome | G/A | 0.097 | 0.14 | 0.34 | 0.92 | 0.76 | 0.43 | 0.76 | (Cirulli et al., 2010; Need et al., 2009) |
| 11 | 6034259 | rs10769565 | OR56A4/ | Intergenic | Imputed | T/C | 0.326 | 0.78 | 0.18 | 0.89 | 0.03 | 0.06 | 0.55 | (De Jager et al., 2012) |
| 11 | 27679916 | rs6265 | BDNF/BDNF-AS | Exonic | Exome | G/A | 0.201 | 0.54 | 1.00 | 0.17 | 0.03 | 0.39 | 0.17 | (Cirulli et al., 2010; Houlihan et al., 2009; Need et al., 2009) |
| 12 | 30451040 | rs10506065 | Intergenic | — | Imputed | C/A | 0.358 | 0.63 | 0.25 | 0.18 | 0.75 | 0.57 | 0.64 | (Seshadri et al., 2007) |
| 13 | 33628138 | rs9536314 | KL | Exonic | Exome | A/C | 0.169 | 0.27 | 0.28 | 0.75 | 0.65 | 0.81 | 0.50 | (Houlihan et al., 2009) |
| 13 | 47409034 | rs6314 | HTR2A | Exonic | Imputed | G/A | 0.081 | 0.07 | 0.25 | 0.49 | 0.48 | 0.58 | 0.97 | (Cirulli et al., 2010; Need et al., 2009; Sigmund et al., 2008) |
| 13 | 106185749 | rs3918342 | DAOA | Downstream | Imputed | C/T | 0.494 | 0.45 | 0.24 | 0.65 | 0.99 | 0.87 | 0.45 | (Need et al., 2009) |
| 13 | 106198235 | rs1421292 | DAOA | Downstream | Imputed | T/A | 0.472 | 0.41 | 0.22 | 0.61 | 0.62 | 0.59 | 0.41 | (Need et al., 2009) |
| 14 | 105259734 | rs1130214 | AKT1 | 3′ UTR | GWAS | C/A | 0.302 | 0.03 | 0.29 | 0.90 | 0.20 | 0.71 | 0.36 | (Need et al., 2009) |
| 19 | 45395619 | rs2075650 | TOMM40 | Intronic | Exome | A/G | 0.136 | 0.10 | 0.64 | 0.92 | 0.05 | 0.47 | 0.06 | (Davies et al., 2014) |
| 19 | 45411941 | rs429358 | APOE | Exonic | Genotyped | T/C | 0.047 | 0.26 | 0.56 | 0.22 | 0.01 | 0.40 | 0.12 | (Davies et al., 2014) |
| 19 | 45412079 | rs7412 | APOE | Exonic | Exome | G/A | 0.080 | 0.86 | 0.13 | 0.15 | 0.57 | 0.52 | 0.07 | (Davies et al., 2014) |
| — | — | — | APOE_hapa | — | — | — | — | 0.21 | 0.79 | 0.88 | 0.06 | 0.75 | 0.01 | (Davies et al., 2014) |
| 19 | 45422946 | rs4420638 | APOC1 | Downstream | Exome | A/G | 0.190 | 0.17 | 0.45 | 0.89 | 0.14 | 0.10 | 0.002 | (De Jager et al., 2012) |
| 21 | 30141021 | rs2832077 | Intergenic | — | Exome | G/A | 0.184 | 0.16 | 0.13 | 0.51 | 0.43 | 0.08 | 0.14 | (Luciano et al., 2011) |
| 21 | 32216253 | rs7283316 | KRTAP7-1 | Upstream | Exome | G/A | 0.413 | 0.29 | 0.13 | 0.65 | 0.72 | 0.33 | 0.96 | (Luciano et al., 2011) |
| 22 | 19951271 | rs4680 | COMT | Exonic | Exome | G/A | 0.473 | 0.91 | 0.62 | 0.06 | 0.36 | 0.94 | 0.35 | (Cirulli et al., 2010; Houlihan et al., 2009; Need et al., 2009) |
Key: DSH, diabetes heart study; DSST, digit symbol substitution task; GWAS, genome-wide association study; MAF, minor allele frequencies; 3MSE, modified mini-mental state examination; RAVLT, rey auditory-verbal learning task; SNPs, single-nucleotide polymorphisms.
Association reported for the E4 haplotype.
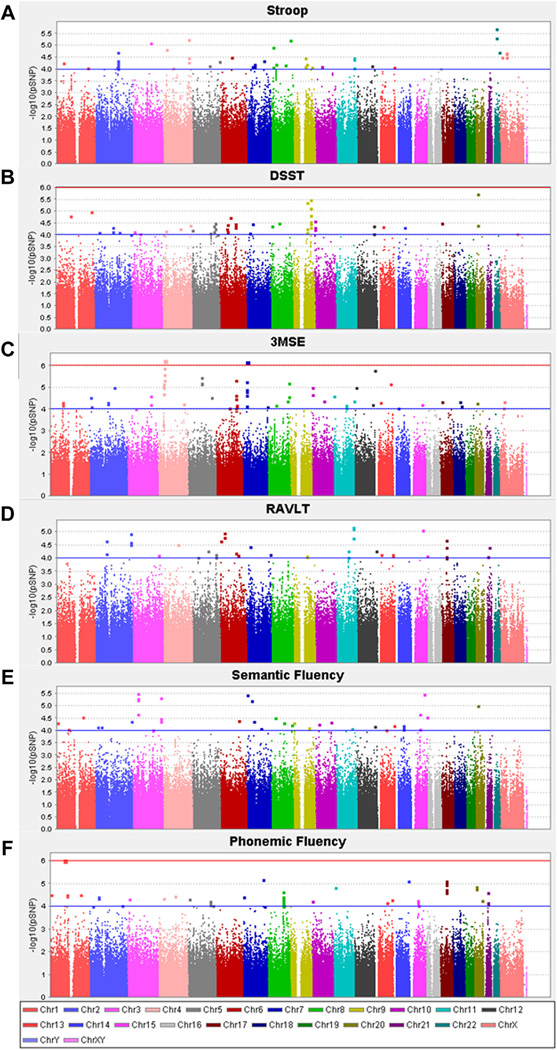
Genome-wide analyses were performed using array-based SNP genotype data (i.e., largely common noncoding variants). This analysis revealed no SNP associations with any of the cognitive traits at a level of conventional genome-wide significance (p > 5 × 10−8). However, there were multiple loci with evidence of nominal association (Fig. 1A–F). The top 50 SNPs associated with each of the measures of cognitive function are included in Supplementary Table 2A–F. Among the most strongly associated loci, a number of SNPs were intergenic and as such, possible functional relationships underpinning genetic risk for cognitive function are more difficult to discern. However, other genes had multiple associated variants and are therefore of potential interest for subsequent consideration including: WD repeat domain 19 (WDR19; 8 SNPs associated with 3MSE 5.62 × 10−7 < p < 4.91 × 10−6); paralemmin (PALM2; 12 SNPs associated with the DSST 3.32 × 10−6 < p < 1.50 × 10−5); membrane protein palmitoylated 2 (MPP2; 7 SNPs associated with phonemic fluency 7.99 × 10−6 < p < 1.10 × 10−5); activin A receptor type IIA (ACVR2A; 6 SNPs associated with the Stroop 2.0 × 10−5 < p < 9.8 × 10−5); acid sensing proton gated ion channel 2 (ACCN1; 4 SNPs associated with RAVLT 2.1 × 10−5 < p < 9.8 × 10−5) and radixin/feredoxin 1 (RDX/FDX1; rs7945071, rs7931910 associated with RAVLT p = 6.73 × 10−6 and p = 7.86 × 10−6, respectively).
Fig. 1.
Manhattan plots for GWAS associations with (A) Stroop (B) DSST, (C) 3MSE, (D) RAVLT (E) Semantic Fluency (F) Phonemic Fluency. Association analyses were performed assuming an additive model of inheritance with adjustment for age, sex, T2D affected status, and education. Abbreviations: DSST, digit symbol substitution task; 3MSE, modified mini-mental state examination; GWAS, genome-wide association study; RAVLT, rey auditory-verbal learning task; T2D, type 2 diabetes.
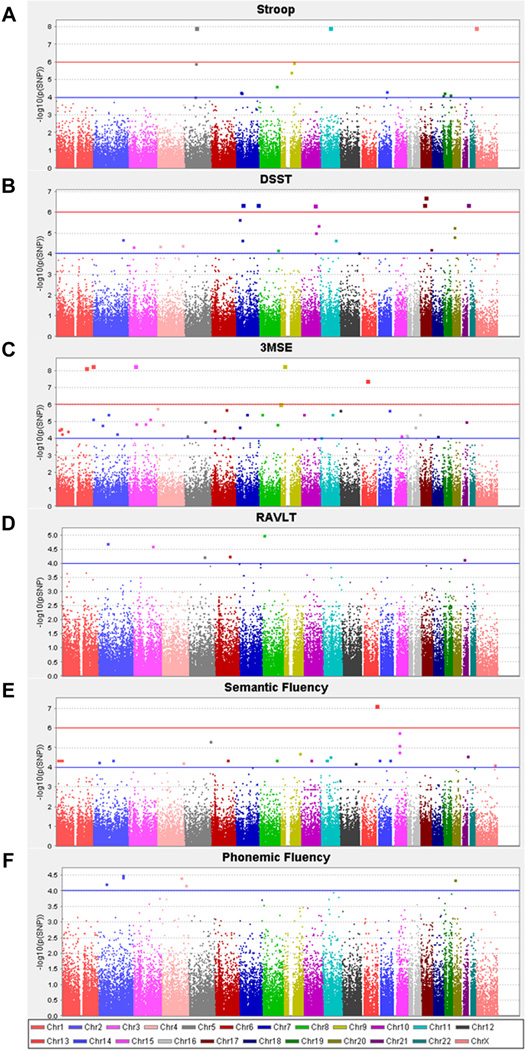
Additional analysis was also completed using an array-derived Exome set of approximately 88,000 less common and rare, predominantly coding variants. For the purposes of the present study, associated variants with only a single observation of the rare allele were excluded from further consideration. The top 50 SNPs associated with each of the measures of cognitive function are included in Supplementary Table 3A–F. Manhattan plots for each phenotype (Fig. 2A–F) suggest there were a number of coding variants associated with given cognitive traits at a level consistent with the corrected exome-wide significance threshold (p < 2 × 10−7). The most strongly associated signals included variants in: consortin connexin sorting protein (CNST; rs139509083), phospholipase A2-activiating protein (PLAA; rs199968569), pleckstrin homology domain containing family A member 6 (PLEKHA6; rs139222464), and protocadherin 8 (PCDH8; rs138487371) for 3MSE (p < 3.8 × 10−8); cardiomyopathy associated 5 (CMYA5; rs201459496) and N-acetylated alpha-linked acidic dipeptidase-like 1 (NAALADL1; rs201741811) for Stroop (p = 1.1 × 10−8); keratin 34 (KRT34; rs149344143) for DSST (p = 1.8 × 10−7); and MCL.2 cell line derived transforming sequence like (MCF2L; rs74949017) for semantic fluency (p = 6.8 × 10−8). Full association statistics for these significantly associated variants are listed in Table 4. The minor allele frequencies for all these significantly associated variants was <1%.
Fig. 2.
Manhattan plots for exome associations with (A) Stroop (B) DSST, (C) 3MSE, (D) RAVLT (E) Semantic Fluency (F) Phonemic Fluency. Association analyses were performed assuming an additive model of inheritance with adjustment for age, sex, T2D affected status and education. Abbreviations: DSST, digit symbol substitution task; 3MSE, modified mini-mental state examination; RAVLT, rey auditory-verbal learning task; T2D, type 2 diabetes.
Table 4.
Statistically significant associations with measures of cognitive function for exome variants
| Phenotype | Chromosome | Position | Gene | Gene name | Variant impact | Alleles (major/minor) |
MAF | B (SE) | p |
|---|---|---|---|---|---|---|---|---|---|
| Stroop | 5 | 79024833 | CMYA5 | Cardiomyopathy associated 5 | Missense -G82E | G/A | 0.0018 | −1.81 (0.31) | 1.08 × 10−8 |
| 11 | 64813784 | NAALADL1 | N-acetylated alpha-linked acidic dipeptidase-like 1 | Missense -R578W | G/A | 0.0027 | −1.81 (0.31) | 1.08 × 10−8 | |
| DSST | 17 | 39534363 | KRT34 | Keratin 34 | Missense -S420T | G/C | 0.0018 | 41.99 (7.92) | 1.78 × 10−7 |
| 3MSE | 1 | 246810819 | CNST | Consortin connexin sorting protein | Missense -C493F | C/A | 0.0018 | −26.15 (4.41) | 4.85 × 10−9 |
| 3 | 46658737 | LOC100132146 | — | Missense -H33R | G/A | 0.0018 | −26.15 (4.41) | 4.85 × 10−9 | |
| 9 | 26935046 | PLAA | Phospholipase A2-activating protein | Missense -M103T | A/G | 0.0018 | −26.15 (4.41) | 4.85 × 10−9 | |
| 1 | 204217977 | PLEKHA6 | Plexkstrin homology domain containing, family A member 6 | Missense -I599S | A/C | 0.0037 | −18.98 (3.25) | 6.84 × 10−9 | |
| 13 | 53419568 | PCDH8 | Protocadherin 8 | Missense -M944T | A/G | 0.0073 | −13.28 (2.39) | 3.73 × 10−8 | |
| Semantic fluency | 13 | 113718663 | MCF2L | MCL.2 cell line derived transforming sequence like | Missense -K179E | A/G | 0.0018 | 25.02 (4.55) | 6.78 × 10−8 |
Exome-wide significance was accepted at p < 2 × 10−7. Effect size (β) and standard error (SE) are shown along with association p-value.
Key: DSST, digit symbol substitution task; MAF, minor allele frequency; 3MSE, modified mini-mental state examination; RAVLT, rey auditory-verbal learning task.
Gene-based association test for all genes with 2 or more polymorphic exonic variants (n = 10,636 genes) revealed additional regions with significant evidence of association when using a Bonferroni corrected p-value threshold of p < 4.7 × 10−6. The top 25 associated genes from SKAT analyses are displayed in Supplementary Table 4A–F.
4. Discussion
Trajectories of age-related cognitive decline have been shown to vary considerably between individuals (De Jager et al., 2012) and, as with other diabetes-associated complications, risk for cognitive decline is likely to vary, perhaps to an even greater extent, between individuals with T2D. As such, identifying individuals at elevated risk is crucial for targeting future treatment and management strategies aimed at reducing the burden of cognitive impairment and disability in individuals with T2D, as well as helping patients and families plan for the impact of cognitive frailty and dysfunction on daily life. Genetic data may be an important component in this context and quantifying the heritability of measures of cognitive function and identifying genetic associations are an important first step in this process. To this end, the present study evaluated the heritability of measures of cognitive function in the T2D-enriched Diabetes Heart Study sample and also examined both specific and more global genetic associations with these indices of cognitive function. Specific measures of cognitive function were found to be highly and significantly heritable in this cohort. This represents an important finding when considering risk for cognitive performance in individuals with extensive metabolic disease. While associations with candidate SNPs were not strongly replicated in the DHS, using genome-wide genetic data as a discovery tool, several previously implicated SNPs showed nominal association and several exonic SNPs showed evidence of chip-wide significant association. Thus, genetic risk loci were identified that may represent potential regions underpinning heritable risk for cognitive performance individuals with T2D.
The heritability of cognitive performance, assessed using an array of cognitive tests, has been examined previously. Heritability estimates for specific cognitive abilities are highly variable, ĥ2 = 0.2–0.7 (Cirulli et al., 2010; Giubilei et al., 2008; Sleegers et al., 2007), likely the result of varying study designs and ascertainment criteria. Whether metabolic disease and associated comorbidities in T2D may confound the full impact of the heritable component of cognitive function has not been extensively studied. However, findings from this study support the presence of a heritable component of cognitive function in the presence of metabolic disease. The DSST, a test of processing speed, was found to have the highest estimated heritability (ĥ2 = 0.62 in adjusted models) in the DHS cohort followed by measures of phonemic and semantic fluency, global cognitive function (3MSE), verbal memory (RAVLT), and executive function (Stroop; ĥ2 = 0.28 in adjusted models). These heritability estimates are in line with previous estimates in relatively healthy aging populations (Haworth et al., 2010) and suggest that genetic factors are also an important contributor to cognitive performance in people with T2D.
Recognizing the heritability of cognitive function in a T2D-enriched sample, we also examined the genetic association of 31 SNPs selected from previous studies examining relationships with specific indices of cognitive function in studies of predominately healthy population groups (Cirulli et al., 2010; Houlihan et al., 2009; Need et al., 2009; Papassotiropoulos et al., 2006). In the DHS, this set of SNPs was largely not associated with the available measures of cognitive function at the conservative level of statistical significance, which we applied to these results. However, several trends for association were noted. Of most interest, the SNP rs4420638 downstream from APOC1, was the most strongly associated variant (p = 0.002 for its association with phonemic fluency) and has recently been reported as associated with rate of cognitive decline (De Jager et al., 2012) and longevity (Beekman et al., 2013). While the SNPs selected for candidate gene analysis represent the most promising variants from the literature examining genetic associations with cognitive function, the challenge of replicating genetic associations with cognition has been acknowledged by others previously and is likely a result of the small sample sizes of the early genetic association studies (Need et al., 2009), complex genetic regulation of cognition (Cirulli et al., 2010) and residual confounding by environmental factors. Some subtle differences in methods of reporting cognitive test performance coupled with the history of extensive disease in the DHS cohort may be additional factors here.
To further understand the genetic underpinning of cognition in T2D we used genome-wide data from both a conventional GWAS array (i.e., largely common noncoding SNPs) and Exome array data (i.e., primarily rare and low frequency coding variants) to identify additional genetic association signals that may warrant further follow-up. While variants from the GWAS array were not associated with measures of cognitive function at a level of traditional genome-wide significance, a number of coding variants from the Exome array were associated with various measures of cognitive function at a level meeting the set exome-wide significance threshold (Table 4). Among these, the missense variant (rs138487371) in PCHD8 is of particular interest given the reported expression of protocadherin family members in the central nervous system and functions as synaptic components (Yagi and Takeichi, 2000). Given the rare nature of these variants (minor allele frequencies <1%), further replication of these findings is required. The use of gene-based analysis methods provides a set of additional targets that could also be considered in subsequent studies.
Although not statistically significant, association results for the common variants do allow for further insight into potential mechanisms underpinning the influence of T2D on cognition. Of the genes containing multiple associated variants, several have biological plausibility with regard to potential roles in cognitive function. For example PALM2 (with >10 intronic SNPs from a region of linkage disequilibrium; associated here with DSST) is part of the paralemmin gene family, which is highly expressed in the nervous system (Hultqvist et al., 2012); variants in this gene have previously been associated with general cognitive ability in school-age children (Davis et al., 2010). Similarly, ACCN1 (with the intronic SNPs from a region of linkage disequilibrium; associated here with RAVLT) is expressed in central and peripheral neurons and has suggested roles in neurotransmission (Chai et al., 2007). WDR19 (with multiple variants from a region of linkage disequilibrium in the last intron and downstream of the gene; associated here with 3MSE) is also of interest in this population given the expression of this transmembrane protein in the pancreas and its suggested roles in vesicular trafficking (Lin et al., 2003). ACVR2A (with multiple variants from a region of linkage disequilibrium upstream of the gene; associated with the Stroop) with demonstrated expression patterns in the hypothalamus and basal forebrain (Miller et al., 2012) represents another functionally plausible candidate. Last, variants lying upstream of RDX, associated here with RAVLT, are also of interest; RDX encodes the cytoskeletal protein Radixin, which has recently been suggested as playing a role in signal transduction pathways (Neisch and Fehon, 2011).
Other SNP associations among the 50 most strongly associated loci (both GWAS and Exome) for each of the different cognitive measures revealed additional promising functional candidate genes including: astrotactin 2 (ASTN2; rs9695439 and rs1415377 associated with the Stroop), which has reported associations with hippocampal volume (Bis et al., 2012); chromodomain helicase DNA binding protein 5 (CHD5; rs731975 associated with semantic fluency), which has suggested roles in nervous system development (Thompson et al., 2003); and protocadherin gamma subfamily A 1 (PCDHGA1; rs115370042 and rs202113404 associated with 3MSE), a member of protocadherin gene family expressed in synaptic junctions within the brain (Wu and Maniatis, 1999). Confirmation of these associations in additional cohorts is required. Further, some of the observations in the DHS provide additional support for the existing literature examining cognitive function in a range of different contexts and include: low density lipoprotein receptor-related protein 1B (LRP1B), associated previously with maintained cognitive function in an elderly population (Poduslo et al., 2010) and associated here with the Stroop (rs493102); and phosphotyrosine interaction domain containing 1 (PID1), previously associated with processing speed in a schizophrenic cohort (McClay et al., 2011) and associated with here with the RAVLT (rs6739369, rs31276, rs16825626). Despite the different settings, these observations further support the likelihood of genetic mechanisms underpinning cognitive performance and its change in individuals with T2D.
In conclusion, the present study, focused on a cognitively heterogenous T2D-enriched cohort with overall poorer cognitive function than anticipated in a similar population without T2D, demonstrated a heritable component to cognitive performance in T2D. Genetic analysis revealed a number of functionally plausible loci that warrant further consideration. This suggests a role for including genetic contributors in approaches to identify a subgroup of individuals with T2D at the highest risk for cognitive decline and cognition-related disability. Such identification is critical to understanding new pathways to prevent and treat this insidious complication of this increasingly prevalent disease.
Supplementary Material
Acknowledgements
This study was supported in part by the National Institutes of Health through R01 HL67348, R01 HL092301, R01 NS058700 (to Donald W. Bowden), 1F32DK083214-01 (to Christina E. Hugenschmidt), NIA F31AG044879 (to Laura M. Raffield), and the General Clinical Research Centre of the Wake Forest School of Medicine (M01 RR07122, F32 HL085989). The authors thank the other investigators, the staff, and the participants of the Diabetes Heart Study for their valuable contributions.
Appendix A
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neurobiolaging.2014.03.005.
Footnotes
Disclosure statement
The authors declare no conflicts of interest in relation to this work.
References
- Arvanitakis Z, Wilson RS, Li Y, Aggarwal NT, Bennett DA. Diabetes and function in different cognitive systems in older individuals without dementia. Diabetes Care. 2006;29:560–565. doi: 10.2337/diacare.29.03.06.dc05-1901. [DOI] [PubMed] [Google Scholar]
- Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J. Clin. Exp. Neuropsychol. 2004;26:1044–1080. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch. Neurol. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman M, Blanche H, Perola M, Hervonen A, Bezrukov V, Sikora E, Flachsbart F, Christiansen L, De Craen AJ, Kirkwood TB, Rea IM, Poulain M, Robine JM, Valensin S, Stazi MA, Passarino G, Deiana L, Gonos ES, Paternoster L, Sorensen TI, Tan Q, Helmer Q, van den Akker EB, Deelen J, Martella F, Cordell HJ, Ayers KL, Vaupel JW, Tornwall O, Johnson TE, Schreiber S, Lathrop M, Skytthe A, Westendorp RG, Christensen K, Gampe J, Nebel A, Houwing-Duistermaat JJ, Slagboom PE, Franceschi C. Genome-wide linkage analysis for human longevity: genetics of Healthy Aging Study. Aging Cell. 2013;12:184–193. doi: 10.1111/acel.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton A, Hamsher K, Sivan A. Multilingual Aphasia Examination. Iowa City: AJA Associates; 1994. [Google Scholar]
- Bis JC, DeCarli C, Smith AV, van der Lijn F, Crivello F, Fornage M, Debette S, Shulman JM, Schmidt H, Srikanth V, Schuur M, Yu L, Choi SH, Sigurdsson S, Verhaaren BF, DeStefano AL, Lambert JC, Jack CR, Jr, Struchalin M, Stankovich J, Ibrahim-Verbaas CA, Fleischman D, Zijdenbos A, den Heijer T, Mazoyer B, Coker LH, Enzinger C, Danoy P, Amin N, Arfanakis K, van Buchem MA, de Bruijn RF, Beiser A, Dufouil C, Huang J, Cavalieri M, Thomson R, Niessen WJ, Chibnik LB, Gislason GK, Hofman A, Pikula A, Amouyel P, Freeman KB, Phan TG, Oostra BA, Stein JL, Medland SE, Vasquez AA, Hibar DP, Wright MJ, Franke B, Martin NG, Thompson PM, Nalls MA, Uitterlinden AG, Au R, Elbaz A, Beare RJ, van Swieten JC, Lopez OL, Harris TB, Chouraki V, Breteler MM, De Jager PL, Becker JT, Vernooij MW, Knopman D, Fazekas F, Wolf PA, van der Lugt A, Gudnason V, Longstreth WT, Jr, Brown MA, Bennett DA, van Duijn CM, Mosley TH, Schmidt R, Tzourio C, Launer LJ, Ikram MA, Seshadri S. Common variants at 12q14 and 12q24 are associated with hippocampal volume. Nat. Genet. 2012;44:545–551. doi: 10.1038/ng.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden DW, Cox AJ, Freedman BI, Hugenschimdt CE, Wagenknecht LE, Herrington D, Agarwal S, Register TC, Maldjian JA, Ng MC, Hsu FC, Langefeld CD, Williamson JD, Carr JJ. Review of the Diabetes Heart Study (DHS) family of studies: a comprehensively examined sample for genetic and epidemiological studies of type 2 diabetes and its complications. Rev. Diabet. Stud. 2010;7:188–201. doi: 10.1900/RDS.2010.7.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden DW, Lehtinen AB, Ziegler JT, Rudock ME, Xu J, Wagenknecht LE, Herrington DM, Rich SS, Freedman BI, Carr JJ, Langefeld CD. Genetic epidemiology of subclinical cardiovascular disease in the Diabetes Heart Study. Ann. Hum. Genet. 2008;72:598–610. doi: 10.1111/j.1469-1809.2008.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands AM, Van den Berg E, Manschot SM, Biessels GJ, Kappelle LJ, De Haan EH, Kessels RP. A detailed profile of cognitive dysfunction and its relation to psychological distress in patients with type 2 diabetes mellitus. J. Int. Neuropsychol. Soc. 2007;13:288–297. doi: 10.1017/S1355617707070312. [DOI] [PubMed] [Google Scholar]
- Buetow KH, Edmonson M, MacDonald R, Clifford R, Yip P, Kelley J, Little DP, Strausberg R, Koester H, Cantor CR, Braun A. High-throughput development and characterization of a genomewide collection of gene-based single nucleotide polymorphism markers by chip-based matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 2001;98:581–584. doi: 10.1073/pnas.021506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai S, Li M, Lan J, Xiong ZG, Saugstad JA, Simon RP. A kinase-anchoring protein 150 and calcineurin are involved in regulation of acid-sensing ion channels ASIC1a and ASIC2a. J. Biol. Chem. 2007;282:22668–22677. doi: 10.1074/jbc.M703624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Meigs JB, Dupuis J. Sequence kernel association test for quantitative traits in family samples. Genet. Epidemiol. 2013;37:196–204. doi: 10.1002/gepi.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli ET, Kasperaviciute D, Attix DK, Need AC, Ge D, Gibson G, Goldstein DB. Common genetic variation and performance on standardized cognitive tests. Eur. J. Hum. Genet. 2010;18:815–820. doi: 10.1038/ejhg.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A, Lehtinen A, Xu J, Langefeld C, Freedman B, Carr J, Bowden D. Polymorphisms in the Selenoprotein S gene and subclinical cardiovascular disease in the Diabetes Heart Study. Acta Diabetol. 2013;50:391–399. doi: 10.1007/s00592-012-0440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G, Harris SE, Reynolds CA, Payton A, Knight HM, Liewald DC, Lopez LM, Luciano M, Gow AJ, Corley J, Henderson R, Murray C, Pattie A, Fox HC, Redmond P, Lutz MW, Chiba-Falek O, Linnertz C, Saith S, Haggarty P, McNeill G, Ke X, Ollier W, Horan M, Roses AD, Ponting CP, Porteous DJ, Tenesa A, Pickles A, Starr JM, Whalley LJ, Pedersen NL, Pendleton N, Visscher PM, Deary IJ. A genome-wide association study implicates the APOE locus in nonpathological cognitive ageing. Mol. Psychiatry. 2014;19:76–87. doi: 10.1038/mp.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis OS, Butcher LM, Docherty SJ, Meaburn EL, Curtis CJ, Simpson MA, Schalkwyk LC, Plomin R. A three-stage genome-wide association study of general cognitive ability: hunting the small effects. Behav. Genet. 2010;40:759–767. doi: 10.1007/s10519-010-9350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager PL, Shulman JM, Chibnik LB, Keenan BT, Raj T, Wilson RS, Yu L, Leurgans SE, Tran D, Aubin C, Anderson CD, Biffi A, Corneveaux JJ, Huentelman MJ, Rosand J, Daly MJ, Myers AJ, Reiman EM, Bennett DA, Evans DA. A genome-wide scan for common variants affecting the rate of age-related cognitive decline. Neurobiol. Aging. 2012;33:1017.e1–1017.e15. doi: 10.1016/j.neurobiolaging.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giubilei F, Medda E, Fagnani C, Bianchi V, De Carolis A, Salvetti M, Sepe-Monti M, Stazi MA. Heritability of neurocognitive functioning in the elderly: evidence from an Italian twin study. Age Ageing. 2008;37:640–646. doi: 10.1093/ageing/afn132. [DOI] [PubMed] [Google Scholar]
- Haworth CM, Wright MJ, Luciano M, Martin NG, de Geus EJ, van Beijsterveldt CE, Bartels M, Posthuma D, Boomsma DI, Davis OS, Kovas Y, Corley RP, Defries JC, Hewitt JK, Olson RK, Rhea SA, Wadsworth SJ, Iacono WG, McGue M, Thompson LA, Hart SA, Petrill SA, Lubinski D, Plomin R. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Mol. Psychiatry. 2010;15:1112–1120. doi: 10.1038/mp.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlihan LM, Harris SE, Luciano M, Gow AJ, Starr JM, Visscher PM, Deary IJ. Replication study of candidate genes for cognitive abilities: the Lothian Birth Cohort 1936. Genes Brain Behav. 2009;8:238–247. doi: 10.1111/j.1601-183X.2008.00470.x. [DOI] [PubMed] [Google Scholar]
- Houx PJ, Jolles J, Vreeling FW. Stroop interference: aging effects assessed with the Stroop Color-Word Test. Exp. Aging Res. 1993;19:209–224. doi: 10.1080/03610739308253934. [DOI] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FC, Lenchik L, Nicklas BJ, Lohman K, Register TC, Mychaleckyj J, Langefeld CD, Freedman BI, Bowden DW, Carr JJ. Heritability of body composition measured by DXA in the Diabetes Heart Study. Obes. Res. 2005;13:312–319. doi: 10.1038/oby.2005.42. [DOI] [PubMed] [Google Scholar]
- Hugenschmidt CE, Hsu FC, Hayasaka S, Carr JJ, Freedman BI, Nyenhuis DL, Williamson JD, Bowden DW. The influence of subclinical cardiovascular disease and related risk factors on cognition in type 2 diabetes mellitus: the DHS-Mind study. J. Diabetes Complications. 2013;27:422–428. doi: 10.1016/j.jdiacomp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultqvist G, Ocampo Daza D, Larhammar D, Kilimann MW. Evolution of the vertebrate paralemmin gene family: ancient origin of gene duplicates suggests distinct functions. PLoS One. 2012;7:e41850. doi: 10.1371/journal.pone.0041850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange LA, Burdon K, Langefeld CD, Liu Y, Beck SR, Rich SS, Freedman BI, Brosnihan KB, Herrington DM, Wagenknecht LE, Bowden DW. Heritability and expression of C-reactive protein in type 2 diabetes in the Diabetes Heart Study. Ann. Hum. Genet. 2006;70:717–725. doi: 10.1111/j.1469-1809.2006.00280.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Teslovich TM, Boehnke M, Lin X. General framework for meta-analysis of rare variants in sequencing association studies. Am. J. Hum. Genet. 2013;93:42–53. doi: 10.1016/j.ajhg.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M, Howieson D, Loring D. Neuropsychological Assessment. New York: Oxford University Press; 2004. [Google Scholar]
- Lin B, White JT, Utleg AG, Wang S, Ferguson C, True LD, Vessella R, Hood L, Nelson PS. Isolation and characterization of human and mouse WDR19, a novel WD-repeat protein exhibiting androgen-regulated expression in prostate epithelium. Genomics. 2003;82:331–342. doi: 10.1016/s0888-7543(03)00151-4. [DOI] [PubMed] [Google Scholar]
- Luciano M, Hansell NK, Lahti J, Davies G, Medland SE, Raikkonen K, Tenesa A, Widen E, McGhee KA, Palotie A, Liewald D, Porteous DJ, Starr JM, Montgomery GW, Martin NG, Eriksson JG, Wright MJ, Deary IJ. Whole genome association scan for genetic polymorphisms influencing information processing speed. Biol. Psychol. 2011;86:193–202. doi: 10.1016/j.biopsycho.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschot SM, Brands AM, van der Grond J, Kessels RP, Algra A, Kappelle LJ, Biessels GJ. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55:1106–1113. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- Marioni RE, Deary IJ, Murray GD, Lowe GD, Strachan MW, Luciano M, Houlihan LM, Gow AJ, Harris SE, Rumley A, Stewart MC, Fowkes FG, Price JF. Genetic associations between fibrinogen and cognitive performance in three Scottish cohorts. Behav. Genet. 2011;41:691–699. doi: 10.1007/s10519-011-9449-2. [DOI] [PubMed] [Google Scholar]
- McClay JL, Adkins DE, Aberg K, Bukszar J, Khachane AN, Keefe RS, Perkins DO, McEvoy JP, Stroup TS, Vann RE, Beardsley PM, Lieberman JA, Sullivan PF, van den Oord EJ. Genome-wide pharmacogenomic study of neurocognition as an indicator of antipsychotic treatment response in schizophrenia. Neuropsychopharmacology. 2011;36:616–626. doi: 10.1038/npp.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MC, Lambert-Messerlian GM, Eklund EE, Heath NL, Donahue JE, Stopa EG. Expression of inhibin/activin proteins and receptors in the human hypothalamus and basal forebrain. J. Neuroendocrinol. 2012;24:962–972. doi: 10.1111/j.1365-2826.2012.02289.x. [DOI] [PubMed] [Google Scholar]
- Need AC, Attix DK, McEvoy JM, Cirulli ET, Linney KL, Hunt P, Ge D, Heinzen EL, Maia JM, Shianna KV, Weale ME, Cherkas LF, Clement G, Spector TD, Gibson G, Goldstein DB. A genome-wide study of common SNPs and CNVs in cognitive performance in the CANTAB. Hum. Mol. Genet. 2009;18:4650–4661. doi: 10.1093/hmg/ddp413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisch AL, Fehon RG. Ezrin, radixin and moesin: key regulators of membrane-cortex interactions and signaling. Curr. Opin. Cell Biol. 2011;23:377–382. doi: 10.1016/j.ceb.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Stephan DA, Huentelman MJ, Hoerndli FJ, Craig DW, Pearson JV, Huynh KD, Brunner F, Corneveaux J, Osborne D, Wollmer MA, Aerni A, Coluccia D, Hanggi J, Mondadori CR, Buchmann A, Reiman EM, Caselli RJ, Henke K, de Quervain DJ. Common kibra alleles are associated with human memory performance. Science. 2006;314:475–478. doi: 10.1126/science.1129837. [DOI] [PubMed] [Google Scholar]
- Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- Poduslo SE, Huang R, Spiro A. A genome screen of successful aging without cognitive decline identifies LRP1B by haplotype analysis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010;153B:114–119. doi: 10.1002/ajmg.b.30963. [DOI] [PubMed] [Google Scholar]
- Sedille-Mostafaie N, Sebesta C, Huber KR, Zehetmayer S, Jungwirth S, Tragl KH, Fischer P, Krugluger W. The role of memory-related gene polymorphisms, KIBRA and CLSTN2, on replicate memory assessment in the elderly. J. Neural. Transm. 2012;119:77–80. doi: 10.1007/s00702-011-0667-9. [DOI] [PubMed] [Google Scholar]
- Seshadri S, DeStefano AL, Au R, Massaro JM, Beiser AS, Kelly-Hayes M, Kase CS, D’Agostino RB, Sr, Decarli C, Atwood LD, Wolf PA. Genetic correlates of brain aging on MRI and cognitive test measures: a genome-wide association and linkage analysis in the Framingham Study. BMC Med. Genet. 2007;8(Suppl. 1):S15. doi: 10.1186/1471-2350-8-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund JC, Vogler C, Huynh KD, de Quervain DJ, Papassotiropoulos A. Fine-mapping at the HTR2A locus reveals multiple episodic memory-related variants. Biol. Psychol. 2008;79:239–242. doi: 10.1016/j.biopsycho.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Sleegers K, de Koning I, Aulchenko YS, van Rijn MJ, Houben MP, Croes EA, van Swieten JC, Oostra BA, van Duijn CM. Cerebrovascular risk factors do not contribute to genetic variance of cognitive function: the ERF study. Neurobiol. Aging. 2007;28:735–741. doi: 10.1016/j.neurobiolaging.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests: Asministration, Norms and Commentary. New York: Oxford Univeristy Press; 2006. [Google Scholar]
- Teng EL, Chui HC. The modified Mini-Mental State (3MS) examination. J. Clin. Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- Thompson PM, Gotoh T, Kok M, White PS, Brodeur GM. CHD5, a new member of the chromodomain gene family, is preferentially expressed in the nervous system. Oncogene. 2003;22:1002–1011. doi: 10.1038/sj.onc.1206211. [DOI] [PubMed] [Google Scholar]
- Tiehuis AM, van der Graaf Y, Visseren FL, Vincken KL, Biessels GJ, Appelman AP, Kappelle LJ, Mali WP. Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke. 2008;39:1600–1603. doi: 10.1161/STROKEAHA.107.506089. [DOI] [PubMed] [Google Scholar]
- Warsch JR, Wright CB. The aging mind: vascular health in normal cognitive aging. J. Am. Geriatr. Soc. 2010;58(Suppl. 2):S319–S324. doi: 10.1111/j.1532-5415.2010.02983.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechlser Adult Intelligence Scale-Revised. New York: Pssychological Corporation; 1981. [Google Scholar]
- Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell. 1999;97:779–790. doi: 10.1016/s0092-8674(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Yagi T, Takeichi M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 2000;14:1169–1180. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.