Abstract
Background
Among infants born prematurely, competence at oral feeding is necessary for growth and hospital discharge. Extremely preterm infants (EP, ≤ 28 weeks gestational age [GA]) are at risk for a variety of medical complications, which can limit the infant’s capacity to develop oral feeding competence.
Objective
This study examined feeding progression by assessing timing of acquisition of five early feeding milestones among EP infants, and the impact of immaturity and medical complications.
Design
A chart review was conducted for 94 EP infants who participated in a larger longitudinal randomized study. Feeding progression was defined as infants’ postmenstrual age (PMA) at five milestones: first enteral feeding, full enteral feeding, first oral feeding, half oral feeding, and full oral feeding. GA at birth and five medical complications (neurological risk, bronchopulmonary dysplasia [BPD], necrotizing entercolitis [NEC], patent ductus arteriosus [PDA], and gastroesophageal reflux disease [GERD]) were used as potential factors influencing the feeding progression. Linear mixed models were used to examine feeding progression across the milestones and contributions of GA at birth, and five medical complications on the progression, after controlling for milk type as a covariate.
Result
EP infants gradually achieved feeding milestones; however, the attainment of the feeding milestones slowed significantly for infants with younger GA at birth and the presence of medical complications, including neurological risk, BPD, NEC, and PDA, but not GERD. Milk type was a significant covariate for all analyses, suggesting infants fed with breast milk achieved each of five milestones earlier than formula-fed infants.
Discussion
Improved understanding of the timing of essential feeding milestones among EP infants, and the contribution of specific medical conditions to the acquisition of these milestones may allow for more targeted care to support feeding skill development.
Keywords: breast milk, extremely preterm infant, feeding milestones, health trajectory, human milk, medical complications
Among infants born prematurely, competence in oral feeding is necessary for growth and a criterion for hospital discharge (American Academy of Pediatrics, 2008). Failure to develop oral feeding competence often leads to poor nutritional status, growth failure, longer hospital stays, and increased costs of care (Bakewell-Sachs, Medoff-Cooper, Escobar, Silber, & Lorch, 2009; Jadcherla, Wang, Vijayapal, & Leuthner, 2010; Russell et al., 2007), and influences longer-term growth and neurodevelopmental outcomes (Samara, Johnson, Lamberts, Marlow, & Wolke, 2010). Preterm infants have difficulty establishing oral feeding skills because their neurologic, cardio-respiratory, gastrointestinal, and oral-motor systems are functionally immature. As a result, they require some degree of tube feeding in the weeks following birth, until they develop the necessary skills to feed by mouth and complete a successful transition from tube to independent oral feeding. Despite the required progression from tube to oral feeding, there is no consensus in scientific evidence to support clinical decisions regarding how to advance feedings for preterm infants, and feeding practices are highly variable across hospitals and typically rely on local customs and provider experience (Breton & Steinwender, 2008; Klingenberg, Embleton, Jacobs, O'Connell, & Kuschel, 2011).
Healthy preterm infants typically achieve independent oral feeding skills by 36 to 38 weeks of postmenstrual age (PMA); however, preterm infants who are younger at birth and have medical complications often require longer time to achieve oral feeding competency (Dodrill, Donovan, Cleghorn, McMahon, & Davies, 2008; Hwang, Ma, Tseng, & Tsai, 2013; Jadcherla et al., 2010). Extremely preterm (EP) infants who are born ≤ 28 weeks of gestational age (GA) are at risk for a variety of medical complications, including necrotizing entercolitis (NEC), bronchopulmonary dysplasia (BPD), patent ductus arteriosus (PDA), and intraventricular hemorrhage (IVH) (Costeloe et al., 2012), any of which further limits the infant’s capacity to progress to oral feeding competence. Depending on types and degrees of medical complications as well as degrees of immaturity at birth, EP infants may exhibit different paths to competent oral feeding. Much research has documented EP infants’ oral feeding skills development are constrained by immaturity and medical complications, mainly BPD (Amaizu, Shulman, Schanler, & Lau, 2008; Gewolb & Vice, 2006; Mizuno et al., 2007; Pickler, Best, & Crosson, 2009); however we could only find three studies that investigated how EP infants make the transition from tube to full oral feeding by assessing the timing of specific feeding milestones and impact of various medical complications on the milestones (Dodrill et al., 2008; Hwang et al., 2013; Jadcherla et al., 2010).
In an assessment of infants’ age at first and full oral feeding milestones and the impact of neonatal morbidity on the progression with 472 preterm infants, including 65 EP infants, infants with younger GA at birth and higher morbidity scores took longer time to initiate and attain full oral feeding, and the transition from first to full oral feeding was lengthened (Dodrill et al., 2008). In another study of 117 preterm infants born before 32 weeks GA, infants born at less than 28 weeks GA (n = 29) took significantly longer time to achieve full oral feeding than those born between 28 and 32 weeks GA; infants with medical complications also took significantly longer time to reach full oral feeding than those without (Hwang et al., 2013).
Feeding progression was examined more extensively by including both enteral and oral feeding milestones, such as first and full enteral feedings and full oral feedings, among 175 preterm infants (Jadcherla et al., 2010). Each feeding milestone was examined in three groups stratified by GA at birth (< 28 weeks [n = 35], 28–32 weeks [n = 59], 32–35 weeks [n = 81]), and specific types and degrees of medical complications. Infants < 28 weeks GA at birth demonstrated significant delays in initiation and progression to full enteral and oral feedings—compared to other preterm groups—and gastroesophageal reflux disease (GERD) and respiratory disease were the most significant factors impeding attainment of both full enteral and oral feeding milestones. The infant’s age at first and full enteral feeding was significantly and positively related to age at full oral feeding, which suggests that the timing of attaining earlier milestones may facilitate or delay attainment of later milestones.
In addition, breast milk is the recommended form of enteral nutrition for EP infants (Eidelman et al., 2012), and may be a significant factor facilitating feeding progression because it contains immune-protective and growth factors that may promote intestinal adaptation and maturation, improve feeding tolerance, and protect against infective and inflammatory disorders, such as NEC (Arslanoglu, Ziegler, Moro, & World Association of Perinatal Medicine Working Group on Nutrition, 2010; Quigley & McGuire, 2014; Underwood, 2013). However, the effects of breast milk on feeding progression were not assessed in previous studies.
Purpose
The current study builds on this previous research to improve understanding of EP infant feeding progression by:
Evaluating the process of achieving oral feeding competence during hospitalization using the full range of feeding milestones;
Examining feeding milestones as a continuous process—from commencement of enteral feeding to attainment of full oral feeding—to allow estimation of the influence of earlier milestones on later milestones;
Evaluating a larger sample size of EP infants to examine feeding progression in the most vulnerable group of preterm infants, including the contribution of medical complications to that progression; and
Evaluating the confounding effects of milk type—either breast milk or formula—on feeding progression.
The purpose of this study was, therefore, to examine the feeding progression of EP infants during hospitalization and the impact of medical complications on the progression—controlling for milk type. Specific aims were to examine the infant’s PMA at acquisition of five early feeding milestones necessary for successful feeding skill development during hospitalization, and the influence of GA at birth and medical complications (neurologic risk score, BPD, NEC, PDA, and GERD) on timing of the acquisition of each feeding milestone.
Methods
Design
This descriptive exploratory study is a secondary analysis of data from a longitudinal two-group randomized controlled trial of the effects of early and late cycled light on the health and developmental outcomes of EP infants (Brandon et al., under review). In the parent study, infants were randomly assigned to one of two intervention groups: (a) early cycled light (28 weeks PMA; 0–5 weeks of near darkness, followed by 12–16 weeks of cycled light); or (b) late cycled light (36 weeks PMA; 8–13 weeks of near darkness, 4–8 weeks of cycled light). Infants receiving cycled light at 28 and 36 weeks PMA had similar health and developmental outcomes. This study was approved by the Duke Medicine Institutional Review Board.
Sample and Setting
The original sample included118 infants who were born at ≤ 28 weeks of GA. Infants were excluded from the parent study if they had a history of anomalies associated with neurological or visual problems, such as congenital glaucoma or Down syndrome. In the current analysis, 24 infants were further excluded because they had structural anomalies that interfered with oral feeding (tracheoesophageal fistula, n = 1; cleft palate, n = 1), died before achieving all feeding milestones (n = 5), or were transferred to a nonstudy nursery before discharge to home (n = 17). A total of 94 infants were included in the current analysis. Excluded infants were not significantly different from the study infants in GA at birth, birth weight, APGAR scores at one and five minutes, and gender, but were significantly different by race (, p < .04; n = 118) with proportionally more Black infants included in the sample analyzed here.
The infants received care in a level IV neonatal intensive care unit (NICU) and two transitional care nurseries in a medical center in the southeastern U.S. between 2003 and 2006. The neonatology team, consisting of neonatologists, nurses, neonatal nurse practitioners, speech and language pathologists, and occupational therapists—used a consistent approach to manage feeding difficulties across each of the nurseries, as follows. Enteral feedings were initiated by 48 hours of life and advanced at a rate of 15–30 cc/kg/day to the goal of 120–150 cc/kg/day—if prefeeding residuals were under 25% of the prior feeding volume with the presence of normal stooling patterns and the absence of abnormal abdominal/gastrointestinal signs and symptoms (progressive abdominal distention, absence of bowel sounds, and/or bilious aspirates). Oral feedings (breast and/or bottle) were initiated at 32–34 weeks PMA once infants demonstrated oral-motor cues and physiologic stability (no longer required positive airway pressure or manifested respiratory distress), and were able to receive full enteral feedings of 100–130 kcal/kg/day. Oral feedings were advanced based on cardiorespiratory stability, gastrointestinal tolerance, and appropriate weight gain. When feeding intolerance was present, standard evaluation and management were performed as deemed necessary. The standardized feeding protocol has remained essentially unchanged since the data were collected.
Measures
Feeding progression
Feeding progression was defined as the infant’s PMA in weeks at each of five milestones:
first enteral feeding;
first full (100cc/kg/day) enteral feeding;
first attempt at oral feeding at breast or bottle;
first half (50% of total nutritional intake) oral feeding; and
first full (100%) oral feeding followed by two consecutive days of full exclusively oral feeding.
Infant’s PMA was calculated as GA at birth plus postnatal age; PMA was used to account for variation in maturity level at birth.
Medical complications and maturity level
Medical complications included neurological risk score, severity of lung disease, and the diagnoses of NEC, PDA, and GERD. Neurological risk score was based on the presence of periventricular leukomalacia (PVL) and grade of intraventricular hemorrhage (IVH) (no risk = no PVL or IVH grade 1–2; risk = PVL or IVH grade 3–4) (Payne et al., 2013). Severity of lung disease was identified using diagnostic criteria for BPD (none, mild, moderate, or severe), depending on the duration and degree of supplemental oxygen required when the infant reached 36 weeks of PMA (Jobe & Bancalari, 2001). Diagnosis of NEC was classified as no NEC, NEC with medical treatment, or NEC with surgical treatment; PDA was classified as no PDA, PDA with medical treatment, or PDA with surgical treatment. GERD was based on need for anti-reflux medications as determined by the physician. Maturity level was measured as GA in weeks at birth, which was determined by physical examination using the New Ballad Score (Ballard et al., 1991). Milk type was assessed on the day when infants reached each milestone as either having some breast milk or having formula, and used as a time-varying covariate in the analysis because of its potential to affect the feeding progression.
Procedures
Data of the PMA for feeding progression, medical complications, and level of maturity were determined from the parent study data, as well as confirmatory retrospective chart reviews conducted by the first author. To assess reliability, n = 9 charts were randomly selected and reviewed again separately three weeks later. There was 100% agreement between the first and second reviews of the nine charts.
Data Analysis
Data were analyzed using SAS Version 9.3. Descriptive statistics were used to describe the sample. Linear mixed modeling was used to examine feeding progression in EP infants by assessing the timing (infant’s PMA) of the acquisition of five early feeding milestones, and to examine if the progression differed by intervention groups, immaturity at birth, and each of five medical conditions. Linear mixed models were generated with PROC MIXED and estimated with maximum likelihood. The covariance structure (see below) accounted for correlation and variance of infant’s PMA at each feeding milestone within the same infant. Milk type (breast milk versus formula) was included in all analyses as a time-varying covariate. Linear mixed models allow for parameter estimation without having to impute any missing outcome values and without losing partial data (e.g., for infants who were discharged prior to attaining all milestones).
First a linear mixed model was used to examine patterns of infants’ PMA across the feeding milestones with a fixed ANOVA effect of infants’ PMA. An unstructured covariance matrix was used in all analyses because it generated the best Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) scores (two well-established model selection criteria) for this analysis among a variety of directly specified structures, as well as random effects models (Knafl, Beeber, & Schwartz, 2012). All fixed effects tests were conducted with denominator degrees of freedom determined by the default approach (i.e., based on dividing the residual degrees of freedom into between-subjects and within-subjects portions).
Then, linear mixed models were used to examine whether patterns of infant’s PMA across the feeding milestones differed by each of seven factors separately, including intervention groups, immaturity at birth, and each of five medical conditions (neurologic risk score, BPD, NEC, PDA, GERD). For each factor, fixed effects were based on a full factorial model, including infant’s PMA at feeding milestone, each of seven factors, their interaction, and milk type as a covariate. Then, each full factorial fixed effects model was reduced by removing the interaction term—as long as AIC and BIC scores improved.
Results
Sample Characteristics
Table 1 describes sample characteristics. Infants were fairly evenly divided between the treatment conditions in the parent study; early cycled (47.9%) and late cycled light intervention (52.1%). Males made up 55.3% of the sample and 72.3% were non-White infants. Infants’ mean GA at birth was 26.2 weeks (range = 22.4 – 28.6), mean birth weight was 868.1 g (range = 460–1450), APGAR score was 4 at one minute (range = 0–9) and 6.5 at five minutes (range = 2–9), and mean length of hospitalization was 103 days (range = 36–268). Growth for gestational age was appropriate for 80.9% of the infants; 10.6% were small for gestational age and 8.5% were large for gestational age. A neurologic risk (PVL or IVH grade 3–4) was shown for 14% of the infants and 75.6% had a diagnosis of BPD (47.9% met the definition of mild BPD, 14.9% moderate, and 12.8% severe). A NEC diagnosis occurred for 28.7% of the infants; 19.1% were treated medically, and 9.6% were treated surgically. A PDA diagnosis occurred for 64.5% of the infants; 47.3% required medical treatment and 17.2% surgical treatment. There were 57 infants (61.3%) diagnosed with GERD and received anti-reflux medications during hospitalization. Almost all infants had at least one medical complication (95.7%), and 79.6% were diagnosed with more than one medical complication during hospitalization (31.2% had two, 31.2% had three, 12.9% had four, and 4.3% had five). During hospitalization, 64.9% of the infants had received some breast milk (either exclusive breast milk or breast milk supplement with formula). Enteral feedings began with breast milk for 57.5% of the infants and 18.4% continued to receive some breast milk when they reached full oral feeding.
TABLE 1.
Sample Characteristics
| Characteristic | M | (SD) |
|---|---|---|
| Gestational age (weeks) | 26.2 | (1.4) |
| Birth weight (grams) | 868.1 | (228.5) |
| APGAR at 1 minute | 4.0 | (2.3) |
| APGAR at 5 minutes | 6.5 | (1.8) |
| Length of hospitalization (days) | 103.1 | (37.7) |
| n | (%) | |
| Gender (male) | 52 | (55.3) |
| Race (Black) | 68 | (72.3) |
| Breast milk during hospitalization (yes) | 61 | (64.9) |
| Neurologic risk (none)a | 80 | (86.0) |
| GERD (yes)a | 57 | (61.3) |
| Size for gestational ageb | ||
| Small for gestational age | 10 | (10.6) |
| Appropriate for gestational age | 76 | (80.9) |
| Large for gestational age | 8 | (8.5) |
| Intervention | ||
| Early cycled light | 45 | (47.9) |
| Late cycled light | 49 | (52.1) |
| BPD severity | ||
| None | 23 | (24.4) |
| Mild | 45 | (47.9) |
| Moderate | 14 | (14.9) |
| Severe | 12 | (12.8) |
| Patent ductus arteriosusa | ||
| None | 33 | (35.5) |
| Medical treatment | 44 | (47.3) |
| Surgical treatment | 16 | (17.2) |
| Necrotizing enterocolitis | ||
| None | 67 | (71.3) |
| Medical treatment | 18 | (19.1) |
| Surgical treatment | 9 | (9.6) |
| Coexisting medical complicationsa,c | ||
| 0 | 4 | (4.3) |
| 1 | 15 | (16.1) |
| 2 | 29 | (31.2) |
| 3 | 29 | (31.2) |
| 4 | 12 | (12.9) |
| 5 | 4 | (4.3) |
| Breast milk at each milestone | ||
| First enteral feeding (yes) | 54 | (57.5) |
| Full enteral feeding (yes) | 45 | (47.8) |
| First oral feeding (yes) | 24 | (25.5) |
| Half oral feeding (yes) | 18 | (20.2) |
| Full oral feeding (yes) | 16 | (18.4) |
Note. N = 94. BPD = bronchopulmonary dysplasia GERD = gastroesophageal reflux disease; IVH = intraventricular hemorrhage; PVL = periventricular leukomalacia.
n = 93; one subject had missing data in three of the five comorbidities and was not included in this descriptive statistic.
Small for gestational age is birth weight below the 10th percentile for gestational age; appropriate for gestational age is birth weight between the 10th and 90th percentile for gestational age; large for gestational age is birth weight above the 90th percentile for gestational age;
Coexisting medical complications was the total number of neurologic risk, BPD, PDA, NEC, and GERD present.
Feeding Progression in EP Infants
Detailed statistical tables are available in Supplemental Digital Content 1. Overall, 90.4% of the infants (n = 85) achieved full oral feeding by discharge; 9.6% (n = 9) were discharged on gastrostomy feeding as the main feeding method. As expected, infants significantly progressed in oral feeding competence across the milestones after controlling for milk type (F4,93 = 224.19; p < .001). Predicted mean PMA for the case when infants were fed with breast milk was 27.8 weeks at first enteral feeding, 30.3 weeks at full enteral feeding, 35.5 weeks at first oral feeding, 38.0 weeks at half oral feeding, and 39.2 weeks at full oral feeding. Estimated values were shifted up by 0.60 weeks for the infants fed with formula (F1,93 = 10.86; p = .001), indicating infants fed with breast milk are younger consistently across the milestones compared to formula-fed infants.
Factors Associated with Feeding Progression
Intervention effect
In this subsample of infants from the parent RCT, patterns of feeding progression were not different by intervention group (early vs. late cycled light) (F1,92 = 0.70, p = .41).
Gestational age
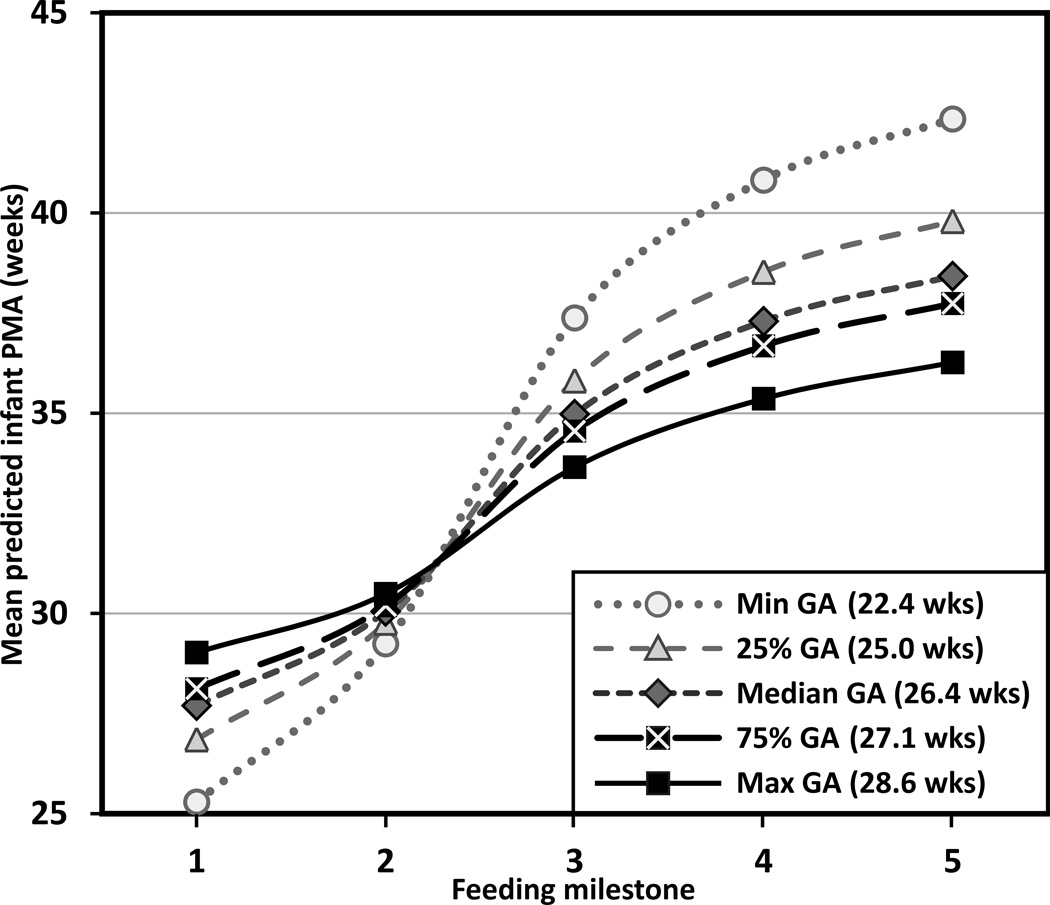
Compared to those of older GA, infants of younger GA exhibited a significant delay in progress toward oral feedings across the milestones (F1,92 = 4.84, p = .03), with an interaction effect between GA and PMA at each feeding milestone (F4,92 = 22.91, p < .001). The significant interaction effect showed that infants of younger GA at birth commenced enteral feeding earlier because all infants initiated enteral feeding within 48 hours of life, but achieved subsequent oral feeding milestones at a later PMA (Steps 3–5). Figure 1 depicts how feeding progression differed by GA using minimum, 25% quartile, median, 75% quartile, and maximum GA for the case when infants are fed with breast milk. Estimated values were shifted up by 0.60 weeks for formula fed infants (F1,92 = 14.02; p < .001).
FIGURE 1.
Feeding progression by gestational age at birth for breast milk-fed Infants. Feeding milestones: (1) first enteral feeding, (2) full enteral feeding, (3) first oral feeding, (4) half oral feeding, and (5) full oral feeding. PMA = postmenstrual age, GA = gestational age, min = minimum, max = maximum, wks = weeks. Trajectories are significantly shifted up by 0.60 weeks for formula-fed infants (p < .001) (not shown).
Neurologic risk
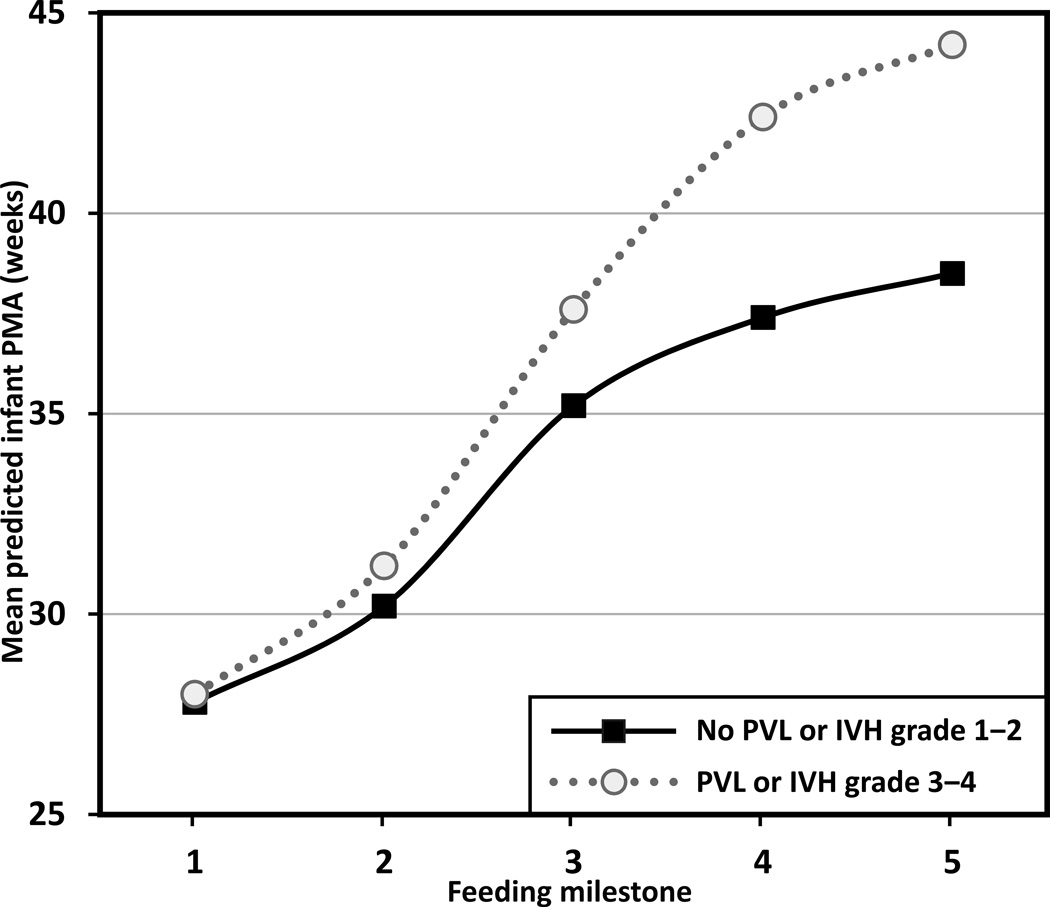
Infants with neurologic risk were significantly delayed in achieving the five feeding milestones compared to those without neurologic risk (F1,91 = 21.72, p < .001). There was also a significant interaction effect between neurologic risk and PMA at each feeding milestone (F4,91 = 6.63, p < .001), suggesting the delays in progress toward full oral feedings were greater at later feeding milestones (see Figure 2 for the case when infants are fed with breast milk; estimates are shifted up by 0.60 weeks for formula-fed infants [F1,91 = 10.41; p = .002]). More specifically, infants with neurologic risk had no delay in achieving first enteral feeding but delayed achievement of full enteral feeding by 1 week, first oral feeding by 2.4 weeks, half oral feeding by 5.0 weeks, and full oral feeding by 5.7 weeks, compared to those without neurological risk (based on differences in mean predicted PMA between the neurological risk groups).
FIGURE 2.
Feeding progression by neurologic risk for breast milk-fed infants. Feeding milestones: (1) first enteral feeding, (2) full enteral feeding, (3) first oral feeding, (4) half oral feeding, and (5) full oral feeding. PMA = postmenstrual age, PVL = periventricular leukomalacia, IVH = intraventricular hemorrhage. Trajectories are significantly shifted up by 0.60 weeks for formula-fed infants (p = .002) (not shown).
Severity of lung disease
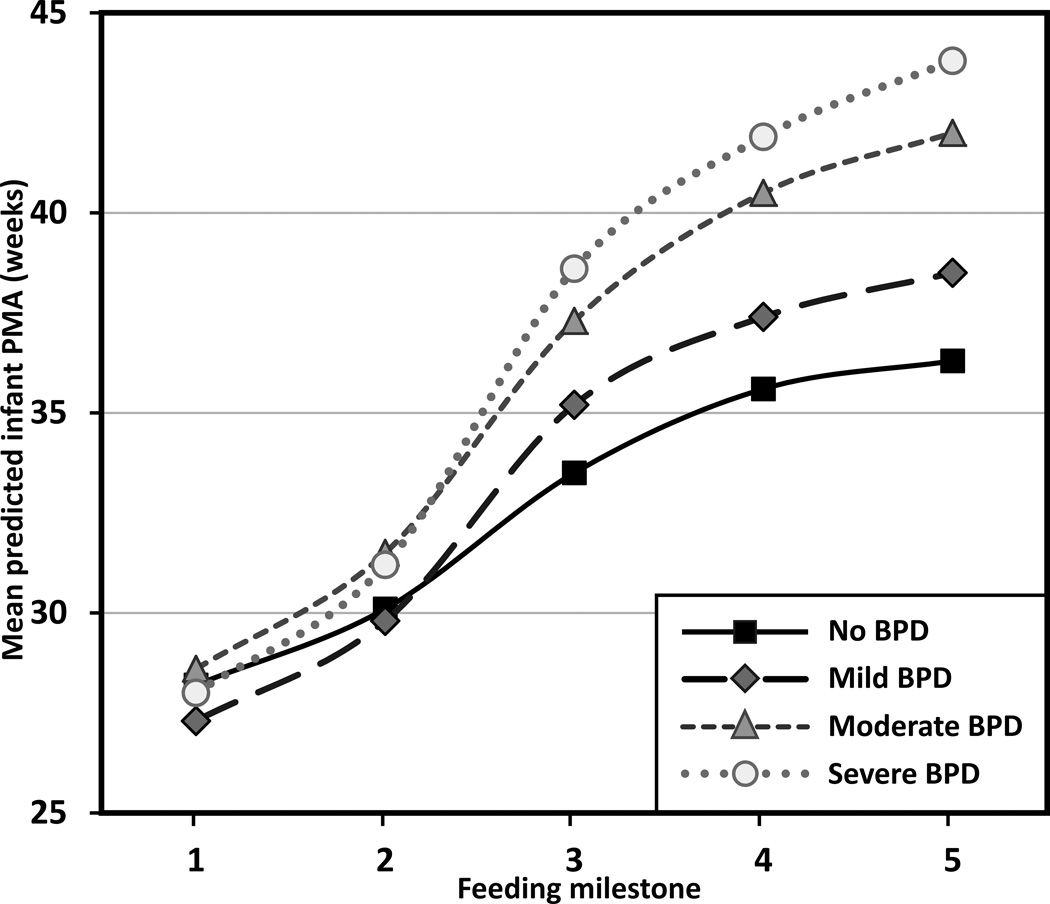
Infants with BPD showed a significant delay in progression to oral feedings across the milestones (F3,90 = 23.11, p < .001), compared to those without BPD. There was also a significant interaction effect between BPD severity and PMA at each feeding milestone (F12,90 = 7.12, p < .001), which suggested infants began enteral feedings and reached full enteral feeds around the same time—regardless of the severity of BPD—however, oral feeding milestones (Steps 3–5) were more delayed for those infants with more severe BPD (see Figure 3 for the case when infants are fed with breast milk; estimates are shifted up by 0.60 weeks for formula-fed infants [F1,90 = 11.65; p = .001]). More specifically, compared to infants without BPD, infants with mild BPD delayed achievement of first oral feeding by 1.7 weeks, half oral feeding by 1.8 weeks, and full oral feeding by 2.2 weeks. Infants with moderate BPD delayed achievement of full enteral feeding by 1.4 weeks, first oral feeding by 3.8 weeks, half oral feeding by 5 weeks, and full oral feeding by 5.7 weeks. Infants with severe BPD delayed achievement of full enteral feeding by 1.1 weeks, first oral feeding by 5.1 weeks, half oral feeding by 6.3 weeks, and full oral feeding by 7.5 weeks (based on differences in mean predicted PMA between the no BPD and BPD group).
FIGURE 3.
Feeding progression by severity of lung disease for breast milk-fed infants. Feeding milestones: (1) first enteral feeding, (2) full enteral feeding, (3) first oral feeding, (4) half oral feeding, and (5) full oral feeding. PMA = postmenstrual age. Trajectories are significantly shifted up by 0.60 weeks for formula-fed infants (p = .001) (not shown).
Necrotizing entercolitis
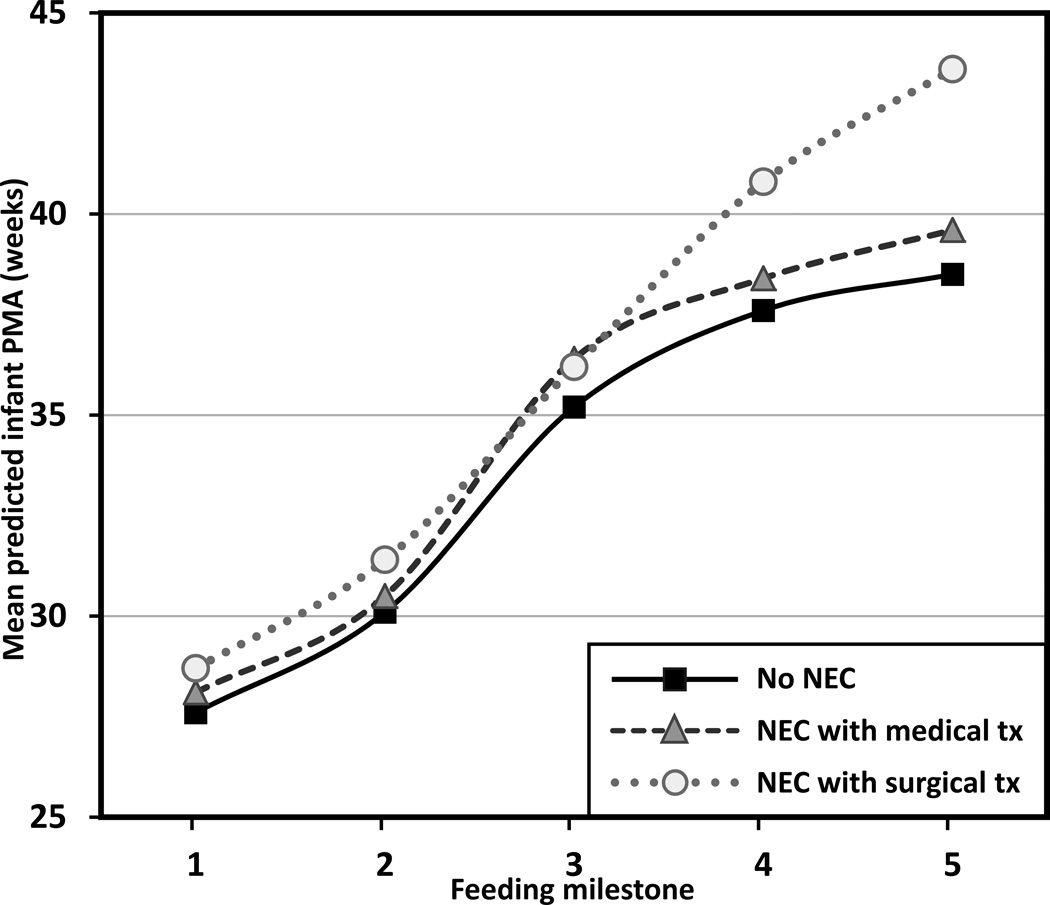
Infants with NEC were significantly delayed in progression to oral feedings across the milestones (F2,91 = 5.66, p = .005) with an interaction effect between NEC group and PMA at each feeding milestone (F8,91 = 3.28, p = .003). The significant interaction effect suggested the delay was greater in those with surgically managed NEC, especially as they advanced to half and full oral feedings (see Figure 4 for the case when infants are fed with breast milk; estimates are shifted up by 0.59 weeks for formula-fed infants [F1,91 = 10.37; p = .002]). More specifically, compared to infants without NEC, the infants with surgically managed NEC delayed achievement of first enteral feeding by 1.1 weeks, full enteral feeding by 1.3 weeks, first oral feeding by 1 week, half oral feeding by 3.2 weeks, and full oral feeding by 5.1 weeks. Infants with medically managed NEC delayed achievement of first oral feeding by 1.2 weeks, but less than one week delay for other feeding milestones (based on differences in mean predicted PMA between the no NEC and NEC group).
FIGURE 4.
Feeding progression by necrotizing enterocolitis for breast milk-fed infants. Feeding milestones: (1) first enteral feeding, (2) full enteral feeding, (3) first oral feeding, (4) half oral feeding, and (5) full oral feeding. PMA = postmenstrual age, NEC = necrotizing enterocolitis, tx = treatment. Trajectories are significantly shifted up by 0.59 weeks for formula-fed infants (p = .002) (not shown).
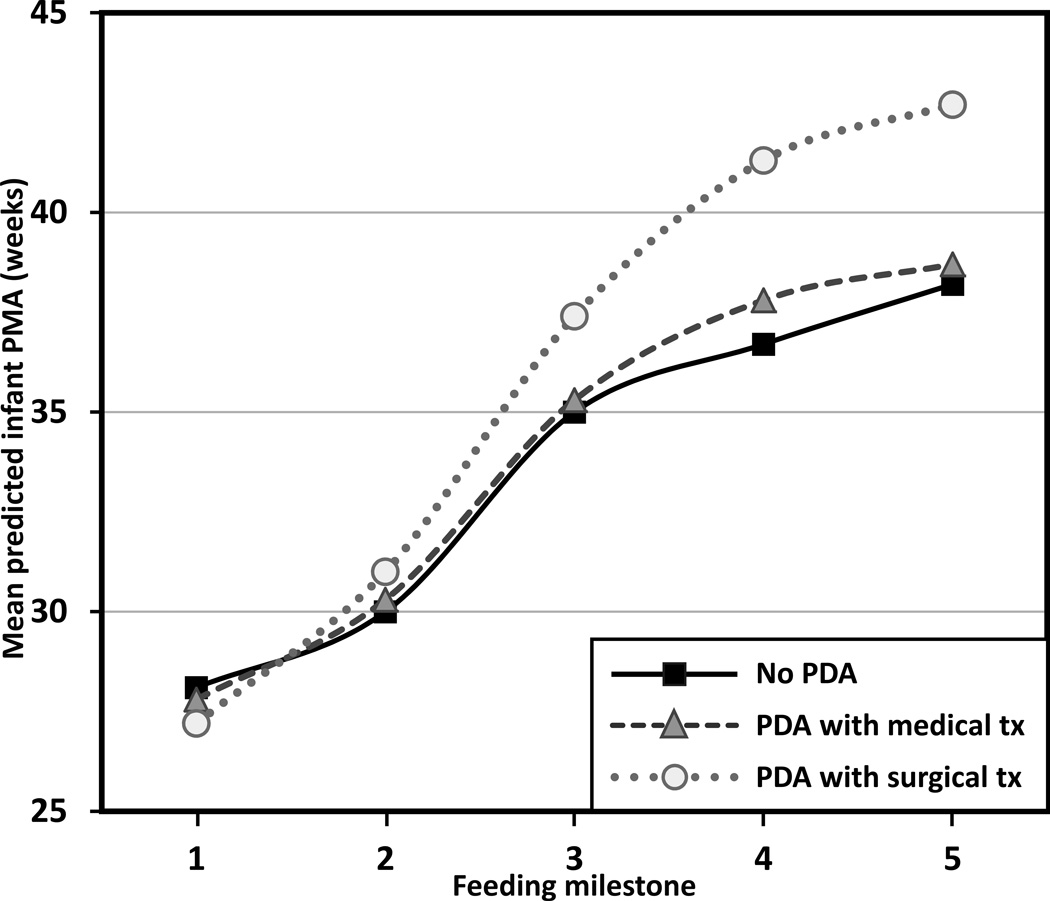
Patent ductus arteriosus
Infants with PDA exhibited a significant delay in progression to oral feedings across the milestones (F2,90 = 7.21, p = .001) with an interaction effect between PDA group and PMA at each feeding milestone (F8,90 = 5.53, p < .001). The significant interaction effect suggested feeding delays were associated with surgical treatment as these infants initiated and advanced through oral feedings (see Figure 5 for the case when infants are fed with breast milk; estimates are shifted up by 0.57 weeks for formula-fed infants [F1,90 = 9.78; p = .002]). More specifically, compared to infants without PDA, the infants with surgically treated PDA showed no delay in achieving first enteral feeding, but delayed achievement of full enteral feeding by 1 week, first oral feeding by 2.4 weeks, half oral feeding by 4.6 weeks, and full oral feeding by 4.5 weeks. Those with PDA treated medically were delayed by 1.1 weeks in achieving half oral feeding, but were delayed less than one week for other feeding milestones (based on differences in mean predicted PMA between the no PDA and PDA group).
FIGURE 5.
Feeding progression by patent ductus arteriosus for breast milk-fed infants. Feeding milestones: (1) first enteral feeding, (2) full enteral feeding, (3) first oral feeding, (4) half oral feeding, and (5) full oral feeding. PMA = postmenstrual age, PDA = patent ductus arteriosus, tx = treatment. Trajectories are significantly shifted up by 0.57 weeks for formula-fed infants (p = .002) (not shown).
Gastroesophageal reflux disease
Patterns of feeding progression did not differ by GERD status (F1,91 = 0.11, p = .74).
Discussion
This exploratory descriptive study was the first to examine feeding progression by assessing the timing of acquisition of five early feeding milestones among EP infants during their initial hospitalization, and the impact of immaturity at birth and five medical complications on the feeding progression. EP infants gradually achieved feeding milestones; however, the attainment of the feeding milestones slowed significantly for infants with younger GA at birth and the presence of medical complications, including neurological risk, BPD, NEC, and PDA, but not GERD. Milk type—either receiving breast milk or formula—was a significant covariate for all analyses, indicating infants fed with breast milk achieved each of five milestones consistently earlier than formula-fed infants. Earlier achievement of feeding milestones for infants fed with breast milk may be attributed to advantages of breast milk over formula to the development of premature infants’ immature host defense, gastrointestinal maturation, and reduced feeding intolerance (Arslanoglu et al., 2010; Eidelman et al., 2012; Quigley & McGuire, 2014).
The current findings are consistent with other work that showed younger GA at birth impeding attainment of full enteral feeding, and transition time from initiation of oral feeding to full oral feeding in premature infants (Dodrill et al., 2008; Hwang et al., 2013; Jadcherla et al., 2010). As well, this study showed that infants of younger GA at birth had a significant delay in feeding progress across feeding milestones in general. However, this was the first study to show a more pronounced delay as EP infants moved from enteral to oral feeding milestones. The delay may be because of maturational delays in the neurologic, gastrointestinal, cardiorespiratory, and oral-motor control systems or the impact of various medical complications on the functionality of these systems. More importantly, although infants of younger GA at birth were likely to have more difficulty in initiating oral feeding, once they began oral feeding, they were able to transition to full oral feeding within about three to five weeks, which is consistent with previous studies (Dodrill et al., 2008; Hwang et al., 2013). Therefore, the timing when infants are able to initiate oral feeding may be a red flag for inadequate development of feeding skills, i.e., if infants are not able to initiate oral feeding by the time skills necessary to oral feed are commonly present, i.e., 32–34 weeks PMA (Delaney & Arvedson, 2008), it may be considered delayed development of feeding skills.
This study also found that infants with neurologic risk—defined as having PVL or IVH grade 3–4—had a significant delay in the progression of feeding competence, especially once they began to attain oral feeding milestones. For safe and successful oral feeding, appropriate neurodevelopmental maturation is necessary to coordinate sucking, swallowing, and breathing with well-timed reconfigurations of the airway for air and nutrient passage (Barlow, 2009; Goldfield, 2007). However, maturational neurodevelopment is constrained by neurologic morbidity, such as the presence of PVL or IVH grade 3–4 (O'Shea et al., 2012), and this may impede adequate development of oral feeding skills.
The adverse effects of BPD on the oral feeding capacity of preterm infants have been documented in several studies (Gewolb & Vice, 2006; Mizuno et al., 2007). The current study further supported earlier findings that BPD is a significant medical complication that prolongs oral feeding progression in EP infants, such that infants with BPD have significant delays in initiating and advancing oral feedings, compared to those without BPD. The current study is the first to show that feeding progression is further slowed as severity of BPD increases. These findings are consistent with previous findings that moderate to severe BPD is a significant factor associated with delay of attainment of full oral feeding (Hwang et al., 2013), and that duration of ventilation and continuous positive airway pressure are positively associated with delayed attainment of full enteral and oral feeding (Jadcherla et al., 2010). The current study extended those findings by examining feeding progression that includes a full range of the early feeding milestones that are necessary to achieve competent oral feeding skills.
In this study, infants with NEC delayed initiating oral feeding by about one week, compared to those without NEC, and infants with surgically treated NEC had a further delay in achieving half and full oral feedings, compared to those with medically treated NEC. These findings could be expected because once NEC is suspected infants are placed on bowel resting for a minimum of 7–10 days, which may delay in advancing to initiation of oral feeding. Moreover, infants who undergo a surgical treatment for NEC often suffer from short gut syndrome (Cole, Hansen, Higgins, Ziegler, & Stoll, 2008)—which may extend the length of time to feed by tube, thereby impeding development of oral feeding competence. Our findings are also consistent with previous findings that presence of NEC is a significant factor associated with delay of attainment of full oral feeding (Hwang et al., 2013), and extended the previous findings by demonstrating a more significant delay for infants with surgically treated NEC.
Infants with surgically treated PDA had a significant delay in the progression to oral feedings, compared to those with medically treated PDA or without PDA—which was more noticeable as they attained oral feeding milestones. Surgical treatment for PDA is usually elected when medical treatment has failed or is contraindicated; therefore, the timing of a closure of PDA is often delayed for infants with surgically treated PDA. The prolonged patency of ductus arteriosus is often related to cardiorespiratory failure, prolonged mechanical ventilation, and subsequent BPD (Ehrenkranz et al., 2005; Lee, Tillett, Tulloh, Yates, & Kelsall, 2006; Vida et al., 2009). As a result, the complications of the prolonged closure of PDA may have resulted in delays in attaining oral feeding milestones for the group of infants with surgically treated PDA.
In the current study, oral feeding milestone progression was not affected by presence of GERD, which is not consistent with the previous study that GERD was one of the significant factors impeding attainment of both full enteral and oral feedings (Jadcherla et al., 2010). This discrepancy may be because different care decisions to treat GERD across the hospitals or use of different analysis strategy (i.e., consideration of all milestones as a continuous process). In the study hospital, GERD is not typically considered a pathological condition, and infants with GERD are usually allowed to proceed to oral feedings with supplements of anti-reflux medications and other nonpharmacological management, including body positioning, modification of feeding methods, or milk thickening (Corvaglia et al., 2013), rather than being prohibited from feeding for a certain period as for other medical complications, such as NEC.
Strengths and Limitations
The strength of this study included the assessment of the full range of the early feeding milestones that are necessary to achieve competent feeding skills during the initial hospitalization with a relatively large number of EP infants. There are also some limitations to this study. First, all data were collected via chart review, so recording errors by nursing staff or physicians may have confounded the findings. Second, this study included information from one NICU and two transitional care nurseries in one large perinatal center. Especially given high variability in feeding practices across hospitals, our findings cannot be generalized to other centers with different strategies to manage feeding. Finally, this study considered a separate model for each medical condition to specifically examine the effects of each medical complication on the feeding progression. Therefore, the findings should be interpreted with a caution of confounding effects of either individual variability within the conditions or coexisting medical conditions, especially given 78.7% of the study infants were diagnosed with more than one medical complication during hospitalization. Additional research with multiple sites and a larger sample would permit better understanding on the process of attaining feeding milestones in EP infants.
Conclusions
EP infants underwent the expected essential feeding milestones as they matured during the initial hospitalization, but the timing of milestone achievements was delayed according to the types and degrees of medical complications and degree of prematurity at birth, after controlling for milk type. Milk type was a significant covariate affecting the feeding progression such that infants fed with breast milk achieved each of five milestones earlier than formula-fed infants. This expanded evidence of the timing of essential feeding milestones among EP infants and the contribution of specific medical conditions to the acquisition of these feeding milestones may allow for a more thorough clinical assessment of these factors, and development of individualized feeding plans to support their feeding skill development in the clinical setting.
Supplementary Material
Acknowledgments
The authors acknowledge the preparation of this paper was supported by the National Institute of Nursing Research, 5 R01 NR008044 (PI: Brandon, DH).
The parent randomized trial is registered at clinicaltrials.gov, identifier NCT02146287.
Footnotes
The authors have no conflicts of interest to report.
Supplemental Digital Content 1. Detailed statistical tables are provided. .doc
Contributor Information
Jinhee Park, Postdoctoral Associate, Duke University School of Nursing, Durham, NC..
George Knafl, University of North Carolina at Chapel Hill School of Nursing, Chapel Hill, NC..
Suzanne Thoyre, University of North Carolina at Chapel Hill School of Nursing, Chapel Hill, NC..
Debra Brandon, Duke University School of Nursing, Durham, NC..
References
- Amaizu N, Shulman RJ, Schanler RJ, Lau C. Maturation of oral feeding skills in preterm infants. Acta Paediatrica. 2008;97:61–67. doi: 10.1111/j.1651-2227.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics. Hospital discharge of the high-risk neonate. Pediatrics. 2008;122:1119–1126. doi: 10.1542/peds.2008-2174. [DOI] [PubMed] [Google Scholar]
- Arslanoglu S, Ziegler EE, Moro GE World Association of Perinatal Medicine (WAMP) Working Group On Nutrition. Donor human milk in preterm infant feeding: Evidence and recommendations. Journal of Perinatal Medicine. 2010;38:347–351. doi: 10.1515/jpm.2010.064. [DOI] [PubMed] [Google Scholar]
- Bakewell-Sachs S, Medoff-Cooper B, Escobar GJ, Silber JH, Lorch SA. Infant functional status: The timing of physiologic maturation of premature infants. Pediatrics. 2009;123:e878–e886. doi: 10.1542/peds.2008-2568. [DOI] [PubMed] [Google Scholar]
- Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature-infants. Journal of Pediatrics. 1991;119:417–423. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- Barlow SM. Oral and respiratory control for preterm feeding. Current Opinion in Otolaryngology & Head and Neck Surgery. 2009;17:179–186. doi: 10.1097/MOO.0b013e32832b36fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon DH, Silva S, Goldstein R, Malcolm W, Barns A, Ryan D. Timing for introduction of cycled light in NICU. Under review for publication [Google Scholar]
- Breton A, Steinwender S. Timing introduction and transition to oral feeding in preterm infants: Current trends and practice. Newborn & Infant Nursing Reviews. 2008;8:153–159. [Google Scholar]
- Cole CR, Hansen NI, Higgins RD, Ziegler TR, Stoll BJ for the Eunice Kennedy Shriver NICHD Neonatal Research Network. Very low birth weight preterm infants with surgical short bowel syndrome: Incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics. 2008;122:e573–e582. doi: 10.1542/peds.2007-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvaglia L, Martini S, Aceti A, Arcuri S, Rossini R, Faldella G. Nonpharmacological management of gastroesophageal reflux in preterm infants. BioMed Research International. 2013;2013 doi: 10.1155/2013/141967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: Comparison of two birth cohorts in 1995 and 2006 (the EPICure studies) British Medical Journal. 2012;345:e7976. doi: 10.1136/bmj.e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney AL, Arvedson JC. Development of swallowing and feeding: Prenatal through first year of life. Developmental Disabilities Research Reviews. 2008;14:105–117. doi: 10.1002/ddrr.16. [DOI] [PubMed] [Google Scholar]
- Dodrill P, Donovan T, Cleghorn G, McMahon S, Davies PSW. Attainment of early feeding milestones in preterm neonates. Journal of Perinatology. 2008;28:549–555. doi: 10.1038/jp.2008.56. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Poole K. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- Eidelman AI, Schanler RJ, Johnston M, Landers S, Noble L, Szucs K, Viehmann L. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- Gewolb IH, Vice FL. Abnormalities in the coordination of respiration and swallow in preterm infants with bronchopulmonary dysplasia. Developmental Medicine & Child Neurology. 2006;48:595–599. doi: 10.1017/S0012162206001241. [DOI] [PubMed] [Google Scholar]
- Goldfield EC. A dynamical systems approach to infant oral feeding and dysphagia: From model system to therapeutic medical device. Ecological Psychology. 2007;19:21–48. [Google Scholar]
- Hwang Y-S, Ma M-C, Tseng Y-M, Tsai W-H. Associations among perinatal factors and age of achievement of full oral feeding in very preterm infants. Pediatrics & Neonatology. 2013;54:309–314. doi: 10.1016/j.pedneo.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Jadcherla SR, Wang M, Vijayapal AS, Leuthner SR. Impact of prematurity and co-morbidities on feeding milestones in neonates: A retrospective study. Journal of Perinatology. 2010;30:201–208. doi: 10.1038/jp.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- Klingenberg C, Embleton ND, Jacobs SE, O'Connell LAF, Kuschel CA. Enteral feeding practices in very preterm infants: An international survey. Archives of Disease in Childhood–Fetal and Neonatal Edition. 2011;97 doi: 10.1136/adc.2010.204123. [DOI] [PubMed] [Google Scholar]
- Knafl GJ, Beeber L, Schwartz TA. A strategy for selecting among alternative models for continuous longitudinal data. Research in Nursing & Health. 2012;35:647–658. doi: 10.1002/nur.21508. [DOI] [PubMed] [Google Scholar]
- Lee LCL, Tillett A, Tulloh R, Yates R, Kelsall W. Outcome following patent ductus arteriosus ligation in premature infants: A retrospective cohort analysis. BMC Pediatrics. 2006;6:15. doi: 10.1186/1471-2431-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Nishida Y, Taki M, Hibino S, Murase M, Sakurai M, Itabashi K. Infants with bronchopulmonary dysplasia suckle with weak pressures to maintain breathing during feeding. Pediatrics. 2007;120:e1035–e1042. doi: 10.1542/peds.2006-3567. [DOI] [PubMed] [Google Scholar]
- O'Shea TM, Allred EN, Kuban KCK, Hirtz D, Specter B, Durfee S, Leviton A. Intraventricular hemorrhage and developmental outcomes at 24 months of age in extremely preterm infants. Journal of Child Neurology. 2012;27:22–29. doi: 10.1177/0883073811424462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AH, Hintz SR, Hibbs AM, Walsh MC, Vohr BR, Bann CM …for the Eunice Kennedy Shriver NICHD Neonatal Research Network. Neurodevelopmental outcomes of extremely low-gestational-age neonates with low-grade periventricular-intraventricular hemorrhage. JAMA Pediatrics. 167:451–459. doi: 10.1001/jamapediatrics.2013.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickler RH, Best A, Crosson D. The effect of feeding experience on clinical outcomes in preterm infants. Journal of Perinatology. 2009;29:124–129. doi: 10.1038/jp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database of Systematic Reviews. 2014 doi: 10.1002/14651858.CD002971.pub3. [DOI] [PubMed] [Google Scholar]
- Russell RB, Green NS, Steiner CA, Meikle S, Howse JL, Poschman K, Petrini JR. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics. 2007;120:e1–e9. doi: 10.1542/peds.2006-2386. [DOI] [PubMed] [Google Scholar]
- Samara M, Johnson S, Lamberts K, Marlow N, Wolke D. Eating problems at age 6 years in a whole population sample of extremely preterm children. Developmental Medicine & Child Neurology. 2010;52:e16–e22. doi: 10.1111/j.1469-8749.2009.03512.x. [DOI] [PubMed] [Google Scholar]
- Underwood MA. Human milk for the premature infant. Pediatric Clinics of North America. 2013;60:189–207. doi: 10.1016/j.pcl.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida VL, Lago P, Salvatori S, Boccuzzo G, Padalino MA, Milanesi O, Stellin G. Is there an optimal timing for surgical ligation of patent ductus arteriosus in preterm infants? Annals of Thoracic Surgery. 2009;87:1509–1516. doi: 10.1016/j.athoracsur.2008.12.101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.