Abstract
Context:
Inducible nitric oxide synthase (iNOS) is a cytoplasmic enzyme which plays a crucial role in the pathogenesis of oral carcinomas and sarcomas.
Aims:
The objective of this study was to analyze the immunohistochemical expression of iNOS in carcinomas and sarcomas affecting the oral cavity in order to understand the possible role of iNOS in their biologic behavior and to correlate iNOS expression with lymph node metastasis in carcinomas and sarcomas.
Settings and Design:
Patients, who attended the oral diagnosis department of Vinayaka Missions Sankarachariyar Dental College, were screened, for the purpose of the study. Besides these, paraffin-embedded tissue blocks were also retrieved from archives of the Oral and Maxillofacial Pathology Department. A total of 40 cases (20 carcinomas and 20 sarcomas) were selected for the study.
Subjects and Methods:
A total of 40 cases (20 carcinomas and 20 sarcomas) were selected for the study. Five apparently normal tissues were obtained from the tumor adjacent normal tissue to be used as a control. These were subjected to immunohistochemical staining using antibody to iNOS and evaluated.
Statistical Analysis Used:
The results were analyzed using the Chi-square test.
Results:
Among the 20 carcinomas 19 showed a positive immunoreactivity for iNOS and 1 case was negative. Among the 19 immunopositive iNOS cases of carcinomas, 15 cases showed positive lymph node metastasis. Among the sarcomas, positive immunoreactivity for iNOS was seen in 10 hard tissue sarcomas, while the remaining 10 soft tissue sarcomas were negative for iNOS expression. The results were analyzed using the Chi-square test.
Conclusions:
iNOS is a reliable marker for lymph node metastasis in carcinomas irrespective of the histologic grade. The high expression in carcinomas shows that the carcinomas elaborate more angiogenesis for growth compared with the sarcomas with the exception of hard tissue sarcomas.
Keywords: Carcinoma, lymph node, nitric oxide synthase, sarcoma
Introduction
Nitric oxide (NO) has been called a “double edged sword” with beneficial antiviral, microbicidal, antiparasital, immunomodulatory and antitumoral effect and deleterious effects like inhibition of enzyme functions, alteration of DNA, induction of lipid peroxidation, mutation of tumor suppressor genes, cytotoxicity, inhibition of mitochondrial respiration, depletion of antioxidant stores and hypoxia induced angiogenesis in cancer depending on the amounts and conditions under which it is processed. In neoplastic tissue, NO is implicated as having a positive role in permitting tumor growth, including mutagenecity, angiogenesis, and metastasis.[1]
Nitric oxide is synthesized by a complex family of enzymes called nitric oxide synthases (NOS). It exists as three isoforms, the calcium dependent endothelial NOS, neuronal NOS, and calcium independent inducible NOS (iNOS).
Inducible NOS is an important molecule showing close relation to not only inflammation, but also carcinogenesis and angiogenesis. Angiogenesis is defined as the formation of new blood vessels from existing vasculature. It is necessary for tumor growth and progression and also involved in metastasis.[2]
Considering iNOS-derived NO as a multifactorial transmitter of tumorigenesis and tumor progression, it is tempting to speculate on therapeutical interference with iNOS activity, especially in tumors where metastatic activity, host defense mechanisms and the level of differentiation seem to be correlated to iNOS expression.
Expression of iNOS has been studied much in pathology and reports have emphasized the significance of iNOS expression to correlate with tumor growth, metastasis, and angiogenesis.
The purpose of the current study was to assess the immunohistochemical expression of iNOS in oral carcinomas and oral sarcomas and to correlate the expression with their unique biologic behavior, angiogenesis, and lymph node metastasis.
Subjects and Methods
Patients who attended the Oral Diagnosis Department of Vinayaka Missions Sankarachariyar Dental College were screened, for the purpose of the study. Besides these, paraffin embedded tissue blocks were also retrieved from archives of the Oral and Maxillofacial Pathology Department. A total of 40 cases (20 carcinomas and 20 sarcomas) were selected for the study. The 20 carcinomas comprised of well, moderate and poorly differentiated grades. Among the 20 sarcomas, 10 were soft tissue sarcomas, and 10 were hard tissue sarcomas, osteosarcomas (6) + chondrosarcomas (4) respectively as previously diagnosed by the Oral and Maxillofacial Pathology Department. Five apparently normal tissues were obtained from the tumor adjacent normal tissue to be used as a control.
Immunohistochemical procedure
Sections of 3 μm thick were cut on a semi-automatic microtome and carefully fixed on the positively charged poly-L-lysine coated microscopic slides. The sections were dried at room temperature (37°C) followed by 1 h incubation at 50°C. The tissue were de-paraffinized by giving two dips lasting 10 min each in fresh xylene. Rehydration of tissue were carried out by giving two dips for 10 min each in absolute alcohol and placed in distilled water bath and not allowed to dry.
Antigen retrieval
The slides were placed in a coplin jar, with Tris-ethylenediaminetetraacetic acid buffer solution, which in turn was kept in a pressure cooker containing water.
Timing was noted down after full pressure was reached and was kept for 10 min duration. The pressure cooker was allowed to cool down to room temperature before removal of slides. Excess water from the slides was drained. The tissue sections were then covered with peroxidase block (3% hydrogen peroxide), incubated for 10 min and gently washed with two changes of phosphate buffer for 3 min each following which tissue sections were covered with 0.4% casein in phosphate buffered saline and incubated for 10 min. Slides were then washed with two changes of phosphate buffer of pH 7.4 for 3 min each.
Primary antibody Santa Cruz genetix (Rabbit polyclonal primary antibody against iNOS in 1:50 dilutions in phosphate buffered saline) was added and the slides were incubated for 1 h at room temperature in a moist chamber. Slides were then washed with two changes of phosphate buffer of pH 7.4 for 3 min each. Excess buffer from slides was drained. The tissue sections were covered with post primary block, incubated for 30 min at room temperature in a moist chamber followed by treatment with horseradish peroxidase polymer, incubated for 30 min at room temperature in a moist chamber, secondary antibody staining was done. Slides were then washed with two changes of phosphate buffer of pH 7.4 for 3 min each. Freshly prepared 3,3′-diaminobenzidine – substrate working solution was added and incubated for 5 min at room temperature in a moist chamber. Slides were washed with distilled water for 5 min. Counter staining was done with Mayer's hematoxylin for 3–5 min and washed under tap water for 5 min. Dehydration was done in increasing grades of alcohol for 10 min each. Sections were mounted with DPX – distrene dibutylphthalate xylol (Distrene Company) and observed for positivity under × 10 and × 40 magnifications and recorded with a high power photomicrograph (14 MP).
Interpretation of staining
Presence of brown colored end product at the site of target antigen was considered as positive immunoreactivity. Cytoplasmic staining was observed which was considered as positive immunoreaction.
The expression of iNOS was determined to be either positive or negative. Based on the intensity of staining it was categorized into mild staining, moderate staining and strong staining respectively.
Based on the pattern of distribution the staining was categorized as focal distribution and diffuse.
Results
A total of 40 cases (n = 40) comprising of 20 carcinomas (n = 20) and 20 sarcomas (n = 20) were evaluated for immunohistochemical expression, correlation with lymph node metastasis, intensity and distribution pattern of inducible NOS (iNOS). Five control tissues were taken. The statistical analysis was carried out using Chi-square test, and the statistical significance was evaluated.
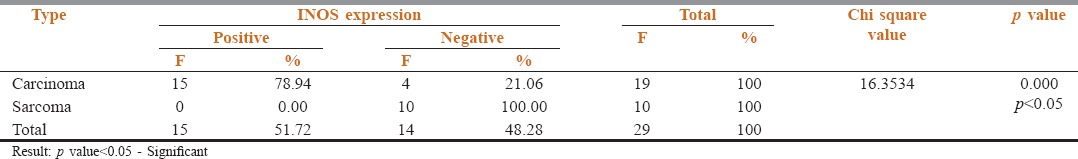
Among the 20 carcinomas, 19 showed a positive immunoreactivity for iNOS [Figures 1a and b, 2a and b] and 1 case was negative. Among the 20 sarcomas, positive immunoreactivity for iNOS was seen in 10 hard tissue sarcomas [Figure 3a and b] while the 10 soft tissue sarcomas were negative for iNOS expression [Figure 4a and b]. The iNOS expression was more in carcinomas compared with sarcomas. This result was statistically significant [Table 1, Graph 1].
Figure 1.
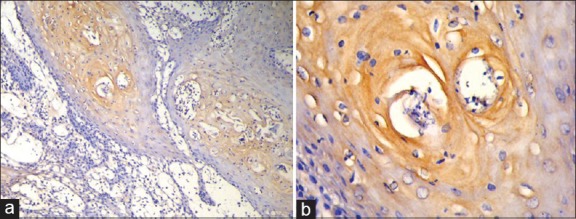
(a) Histologic section showing mild expression of inducible nitric oxide synthase (iNOS) in a well-differentiated squamous cell carcinoma (IHC stain at ×10 magnification). (b) Mild expression of iNOS in a well differentiated squamous cell carcinoma (IHC stain at ×40 magnification)
Figure 2.
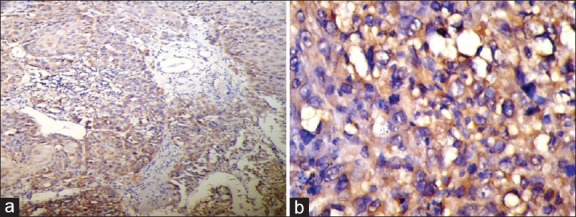
(a) Histologic section showing strong expression of inducible nitric oxide synthase (iNOS) in a poorly differentiated squamous cell carcinoma (IHC stain at ×10 magnification). (b) Strong expression of iNOS in a poorly differentiated squamous cell carcinoma (IHC stain at ×40 magnification)
Figure 3.
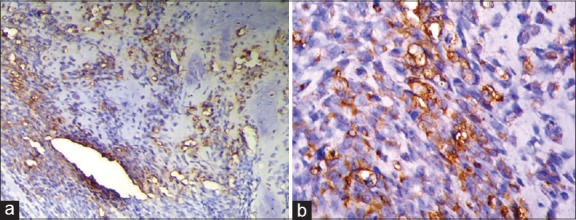
(a) Histologic section showing strong expression of inducible nitric oxide synthase (iNOS) in osteosarcoma (IHC stain at ×10 magnification). (b) Histologic section showing strong expression of iNOS in osteosarcoma (IHC stain at ×40 magnification)
Figure 4.
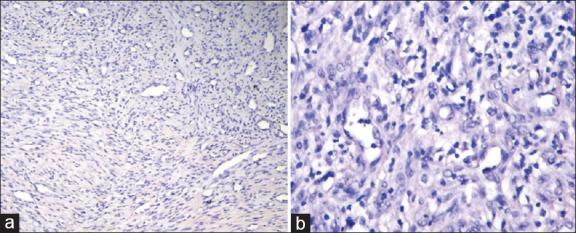
(a) Histologic section showing negative inducible nitric oxide synthase (iNOS) expression in fibrosarcoma (IHC stain at ×10 magnification). (b) Negative iNOS expression in fibrosarcoma (IHC stain at ×40 magnification)
Table 1.
iNOS Expression between Carcinomas and Sarcomas
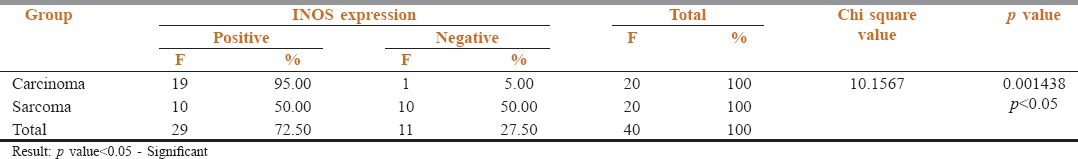
Graph 1.
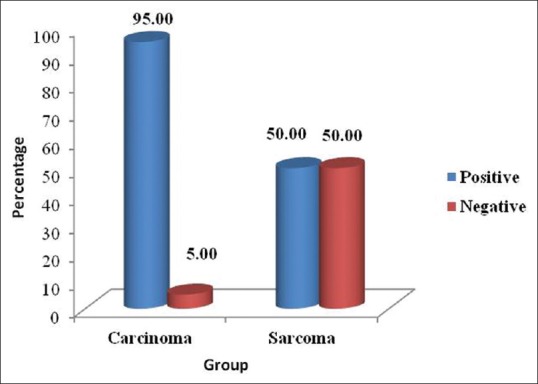
Inducible nitric oxide synthase expression between carcinomas and sarcomas
Weak, moderate, and strong expression was seen among the carcinomas whereas the intensity of iNOS expression was relatively strong in the hard tissue sarcomas. This difference was also statistically significant [Table 2, Graph 2].
Table 2.
Intensity of iNOS expression between Carcinomas and Sarcomas
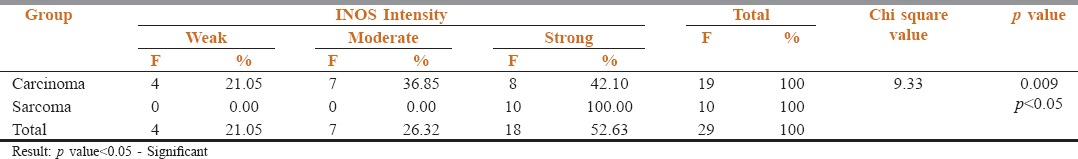
Graph 2.
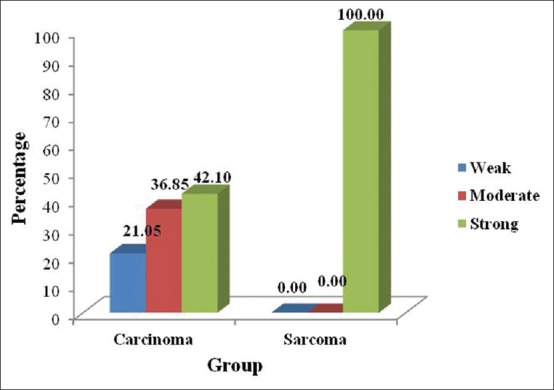
Intensity of inducible nitric oxide synthase expression between carcinomas and sarcomas
Out of the 19 immunopositive iNOS cases of carcinomas, 15 cases showed positive lymph node metastasis. The expression of iNOS in carcinomas correlated significantly with lymph node metastasis which was statistically proven with a significant P value [Table 3, Graph 3].
Table 3.
Correlation of iNOS expression with Lymph Node Metastasis
Graph 3.
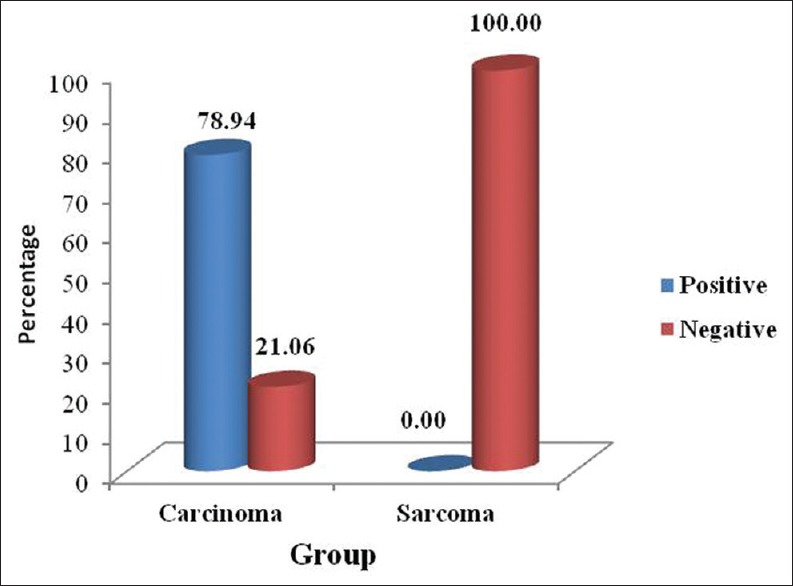
Correlation of inducible nitric oxide synthase expression with lymph node metastasis
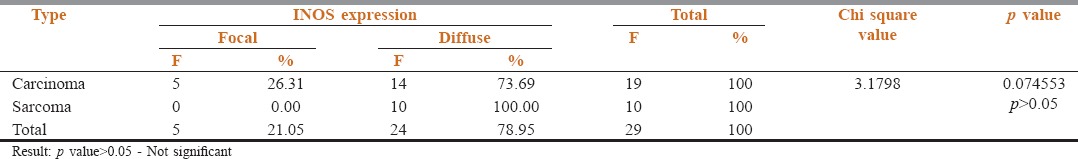
The pattern of expression was diffuse in the 10 positive cases of sarcomas while it was both focal (26.31%) and diffuse (73.69%) in carcinomas. The diffuse expression pattern was predominant [Table 4].
Table 4.
Pattern of iNOS expression between Carcinomas and Sarcomas
Discussion
Inducible NOS is an enzyme that has been implicated in the tumorigenesis of various neoplasms. The role of NO generated by the iNOS is very complex. However, induced at the wrong place or at the wrong time, iNOS has detrimental consequences and seems to be involved in the pathophysiology of different human diseases. The pathways regulating iNOS expression seem to vary in different cells or different species.[3]
Numerous studies have proved that there is an increase in the expression of iNOS in a variety of neoplasms and this pathway is involved in various stages of tumorigenesis such as cellular proliferation, evasion from p53 dependent apoptosis and tumor induced angiogenesis.[4]
In oral squamous cell carcinoma (OSCC), the iNOS expression may be positively associated with p53 mutation, suggesting that iNOS could cause the mutation of p53 gene, which might further lead to the progression of tumor.[5]
Yet another role of iNOS in many of the tumors is its pivotal role in the tumor induced angiogenesis, a process central to tumor growth, progression and metastasis.
Inducible NOS dependent NO controls angiogenesis by modulating the expression of vascular endothelial growth factor (VEGF), the prime growth factor involved in endothelial cell proliferation and sprouting of blood vessels. The relationship among iNOS, VEGF and vascular density in tumors is established in many recently reported studies.[6]
It is believed that iNOS stimulates tumor angiogenesis via its product NO, which acts mainly during VEGF-regulated angiogenesis, such as occurring in matrix degradation, endothelial cell migration and proliferation, new capillary network construction, and lumen formation. Since tumor angiogenesis is the pathological basis of biological actions such as tumor growth, invasion and metastasis, iNOS/NO probably have a definitive effect on tumor clinicopathology.
In addition, iNOS can influence the occurrence and progression of tumors by interacting with tumor oncogenes or tumor suppressor genes.[7]
In the present study, a positive correlation was seen in lymph node metastasis and iNOS expression in carcinomas, the association of lymph node metastasis with positive iNOS expression was statistically significant [Table 3, Graph 3]. This was in accordance with the study conducted by Sappayatosok et al.[2] who analyzed expression of pro-inflammatory protein, iNOS, VEGF and COX-2 in OSCC, relationship with angiogenesis and their clinico-pathological correlation and suggested that the expression of iNOS, VEGF and COX-2 exists in OSCC and showed that iNOS correlated with lymph node metastasis. The results were also in correlation with a study done by Brennan et al.[8]
None of the sarcomas that were positive for iNOS showed nodal metastasis.
Brennan et al. studied the relationship of iNOS activity and vascularity in head and neck squamous cell carcinoma, the results of which showed an increase in the iNOS activity and micro vessel count in these tumors.[9]
Continuous generation of NO by iNOS in the stroma of solid tumors contribute to the activation of matrix metalloproteinases (MMPs), which assist in the breakdown of basement membrane, the loss of integrity and degradation of collagenous and noncollagenous proteins of stromal tissue and bone, which in turn preconditions the stroma and facilitate the process of invasion.[10]
Matrix metalloproteinases may also indirectly influence the tumor microenvironment by enhancing tumor induced angiogenesis and destroying local tissue architecture. A huge variety of proteases has been associated with cancer progression. In carcinoma cell invasion, MMPs in particular, are considered to be the most significant proteases.[11]
As the results the present study were evaluated, 19 out of 20 carcinomas were positive whereas only 10 sarcomas out of 20 were positive, the expression of iNOS in carcinomas was significantly higher compared with the sarcomas, and this was proved by the Chi-square test which showed a significant P value [Table 1, Graph 1].
This is probably due to the reason that carcinomas are epithelial tumors hence needs to express more iNOS for inducing neo-angiogenesis for tumor growth. Whereas the sarcomas are connective tissue tumors, which express less iNOS to induce neo-angiogenesis, since they are already in the vicinity of the blood vessels in the connective tissue.
When the intensity of staining was compared between carcinomas and sarcomas, the carcinomas showed all three staining intensities (weak, moderate, and poor), whereas all the 10 sarcomas which were positive showed strong intensity [Table 2, Graph 2].
Among the 20 sarcomas, the 10 soft tissue sarcomas were negative for iNOS expression, while the 10 hard tissue sarcomas were positive for iNOS expression. This showed 100% positivity for the hard tissue sarcomas.
This result was in accordance with the study conducted by Chen et al.[12] who reported the expression and pathological relevance of iNOS in osteosarcoma of the jaws and stated that osteosarcoma of the jaw was associated with over expression of iNOS.
This shows that iNOS plays a major role in the development and growth of osteosarcomas. iNOS may promote tumor angiogenesis in osteosarcoma of the jaw, and so may represent an important target in antitumor therapy.
When the pattern of staining was evaluated in the 19 positive carcinomas, the pattern of staining was diffuse (73.69%) and focal areas were also seen (26.31%), in the 10 sarcomas that were positive the pattern was 100% diffuse [Table 4].
Apart from tumorigenesis, the role of iNOS in inflammation is also commendable as iNOS is secreted by endothelial cells and most of the inflammatory cells including macrophages.
It has been shown that various inflammatory cytokines can help in the stimulation of iNOS induction which can modulate many aspects of cellular growth, differentiation and activation, and might be associated with initiation of inflammation.[13]
In the present study, we also found iNOS expression in the inflammatory cells of most of the carcinomas, which shows the role of iNOS in the zone of inflammation as a modulator.
Conclusion
The results of this study show that iNOS is a reliable marker for lymph node metastasis in carcinomas irrespective of the histologic grade. This mandates removal of neck nodes irrespective of their involvement by tumor cells when the primary tumor is positive for iNOS expression.
The high expression of iNOS in carcinomas shows that the carcinomas need more angiogenesis for growth compared to the sarcomas with the exception of hard tissue sarcomas.
Inducible NOS may promote tumor angiogenesis in osteosarcoma of the jaw, and so may represent an important target in antitumor therapy.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Connelly ST, Macabeo-Ong M, Dekker N, Jordan RC, Schmidt BL. Increased nitric oxide levels and iNOS over-expression in oral squamous cell carcinoma. Oral Oncol. 2005;41:261–7. doi: 10.1016/j.oraloncology.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Sappayatosok K, Maneerat Y, Swasdison S, Viriyavejakul P, Dhanuthai K, Zwang J, et al. Expression of pro-inflammatory protein, iNOS, VEGF and COX-2 in oral squamous cell carcinoma (OSCC), relationship with angiogenesis and their clinico-pathological correlation. Med Oral Patol Oral Cir Bucal. 2009;14:E319–24. [PubMed] [Google Scholar]
- 3.Kleinert H, Pautz A, Linker K, Schwarz PM. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol. 2004;500:255–66. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Kitajima I, Kawahara K, Nakajima T, Soejima Y, Matsuyama T, Maruyama I. Nitric oxide-mediated apoptosis in murine mastocytoma. Biochem Biophys Res Commun. 1994;204:244–51. doi: 10.1006/bbrc.1994.2451. [DOI] [PubMed] [Google Scholar]
- 5.Calmels S, Hainaut P, Ohshima H. Nitric oxide induces conformational and functional modifications of wild-type p53 tumor suppressor protein. Cancer Res. 1997;57:3365–9. [PubMed] [Google Scholar]
- 6.Jablonska E, Puzewska W, Grabowska Z, Jablonski J, Talarek L. VEGF, IL-18 and NO production by neutrophils and their serum levels in patients with oral cavity cancer. Cytokine. 2005;30:93–9. doi: 10.1016/j.cyto.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Zeng SG, Chen WL, Zeng SG, Li HG, Huang HZ, Pan CB. Expression of iNOS mRNA in oral squamous cell carcinoma and its significance. Ai Zheng. 2002;21:314–8. [PubMed] [Google Scholar]
- 8.Brennan PA, Palacios-Callender M, Zaki GA, Spedding AV, Langdon JD. Type II nitric oxide synthase (NOS2) expression correlates with lymph node status in oral squamous cell carcinoma. J Oral Pathol Med. 2001;30:129–34. doi: 10.1034/j.1600-0714.2001.300301.x. [DOI] [PubMed] [Google Scholar]
- 9.Brennan PA, Thomas GJ, Langdon JD. The role of nitric oxide in oral diseases. Arch Oral Biol. 2003;48:93–100. doi: 10.1016/s0003-9969(02)00183-8. [DOI] [PubMed] [Google Scholar]
- 10.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: A review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 11.Folgueras AR, Pendás AM, Sánchez LM, López-Otín C. Matrix metalloproteinases in cancer: From new functions to improved inhibition strategies. Int J Dev Biol. 2004;48:411–24. doi: 10.1387/ijdb.041811af. [DOI] [PubMed] [Google Scholar]
- 12.Chen WL, Feng HJ, Li JS, Li HG. Expression and pathological relevance of inducible nitric oxide synthase in osteosarcoma of the jaws. Int J Oral Maxillofac Surg. 2007;36:541–4. doi: 10.1016/j.ijom.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Kröncke KD, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clin Exp Immunol. 1998;113:147–56. doi: 10.1046/j.1365-2249.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


