Abstract
Background:
Prediction of biological behavior in patients of prostate cancer (CaP) is a major challenge as current parameters only partially meet the need for prognostication. p53 as a prognostic indicator has been studied in several human cancers, including breast, lung, and colorectal carcinoma. However, its significance as a predictive biomarker for CaP is less well-studied.
Materials and Methods:
This study included 125 cases of CaP, 27 cases of prostatic intraepithelial neoplasia and 25 cases of benign prostatic hyperplasia. Immunohistochemical assessment for p53 nuclear protein was performed. Assessment for apoptotic index and DNA ploidy status by flow cytometry were also done.
Results:
p53 immunoreactivity was low in organ confined CaP cases having Gleason score ≤3 (P < 0.003). More hormone resistant cases 37 (83%) were aneuploid when compared with hormone sensitive cases 26 (33%) (P < 0.005). 93% of p53 positive cases and none of the p53 negative patient were aneuploid suggesting a significant relation between p53 immunoreactivity and aneuploidy. p53 positivity and DNA aneuploidy, independently, were also predictors of progression and relapse.
Conclusion:
DNA ploidy and p53 positivity go hand in hand and together yield additional prognostic information in CaP. p53 positivity is possibly a late event in carcinogenesis in CaP and a marker of change in biological behavior of CaP.
Keywords: p53, prostate cancer, prostatic intraepithelial neoplasia
Introduction
Prostate cancer (CaP) is the second commonest solid tumor in males. Prostate specific antigen (PSA) based early detection, means, and more patients being diagnosed early, with clinically localized CaP amenable to curative treatment. Many may have small organ confined nodules with an indolent course and may be left untreated. Identifying markers responsible for progression of CaP can thus aid clinical decision-making in these cases.
p53 is a tumor suppressor gene protecting the cell from DNA damage, triggering programmed cell death and thus a key regulator of cell cycle. Mutations in p53 that inactivate it, therefore, will lead to extensive changes in genetic characteristics of cells.
We aimed to examine the relationship of p53 protein expression and tumor characteristics of primary CaP and its association with tumor progression. We also studied the correlation of p53 protein expression with DNA ploidy and apoptotic index (AI) in these tumors.
Materials and Methods
This study was conducted on 177 patients with prostate enlargement admitted in the Department of Urology, Safdarjung Hospital, New Delhi, India. Approval of the Institutional Ethics Committee was taken. Prostate tissue samples were obtained from prostatectomy as clinically indicated. These included-125 cases of CaP, 27 cases of prostatic intraepithelial neoplasia (PIN) and 25 cases of benign prostatic hyperplasia (BPH). All samples were subjected to gross and histopathological examination. Cases of CaP were staged as per modified Whitemore-Jewett system and histopathologically graded by Gleason scoring. Immunohistochemical staining using monoclonal antibody against p53 protein (Dako, Clone DO-7) by standard avidin-biotin complex method was done and strong, distinct brown nuclear staining in >10% of tumor cells was defined as p53 positivity. AI and DNA ploidy status were analyzed. Androgen ablation therapy in the form of bilateral orchiectomy followed by low dose of antiandrogen (flutamide 50 mg) was given to patients with CaP as per institute policy. They were then followed for a mean period of 3 years. Serum PSA values were assessed before and after prostatectomy and at every 3 months interval. Patients with the rise in serum PSA values and/or clinical complaints were considered recurrences and were subjected to a repeat biopsy. Repeat biopsies were also processed for histopathological, immunohistochemical, DNA ploidy and AI analysis.
Apoptosis
Apoptotic bodies were counted in foci of CaP and quantitated using image acquisition software. Images of different fields of interest were grabbed and saved on hard disc. A grid of 100 squares was used and apoptotic bodies in each square in the images captured were counted. AI was expressed as the mean number of apoptotic bodies per 1000 prostate carcinoma cells.
DNA ploidy
A total of 50 μm sections of formalin fixed paraffin-embedded tissue were processed by standard protocol. At least 20,000 cells were measured on a standard ICP-22 flow cytometer. DNA histograms were analyzed and tumors displaying >10% aneuploidy were termed aneuploid and those displaying <10% aneuploidy were termed diploid [Figure 1].
Figure 1.
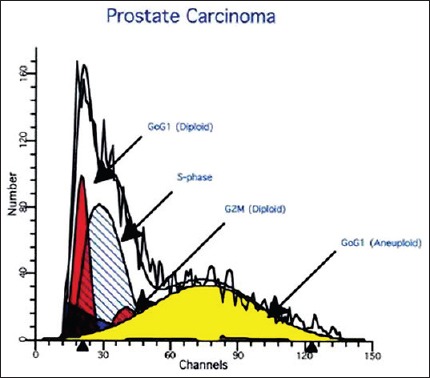
Flow cytometer analysis shows significant number of aneuploid tumor cells in carcinoma prostate
Results
A total of 177 cases (125-CaP, 27-PIN, 25-BPH) were studied. 25 (20%) had localized carcinoma (12-Stage A,13-Stage B), 25 were in Stage C and remaining 75 patients (60%) were in Stage D. Histologically, all CaP cases had adenocarcinoma. Tumors were graded according to Gleason score ≤3: 25 (20%), 4-6: 40 (32%) and ≥7: 60 (48%). Eighty patients (64%) were initially hormone sensitive and 45 patients (36%) were hormone resistant. During follow-up, another 35 patients who were initially hormone sensitive showed hormone resistance.
p53 status
p53 immunoreactivity was positive in 29.6% (8/27) cases of PIN and 35% (44/125) cases of CaP respectively, suggesting that p53 immunoreactivity significantly increased as the disease progressed from PIN to malignant state (P < 0.05). In CaP, significantly low p53 immunoreactivity was found in Stage A-8.3% and Stage B-7.7%, compared with Stage C-28% and Stage D-46.7% (P = 0.005). p53 positivity increased with increasing Gleason score (P < 0.003), being strikingly low in tumors with Gleason score ≤3. 24% (19/80) of hormone sensitive category and 57% (26/45) cases of hormone resistant type were p53 positive (P < 0.05). p53 positivity was significantly higher (29.6%) in cases of PIN as compared with localized cancer (8%).
DNA ploidy
Aneuploidy was seen in none of the BPH, 6/27 (22%) of PIN and 41/125 (33%) of CaP cases. None of the early Stages (A and B) tumor showed aneuploidy while 6/25 cases (24%) in Stage C and 35/75 cases (46.7%) of Stage D were aneuploid, suggesting statistically significant (P < 0.05) correlation. Aneuploidy was seen only in those with Gleason score >3, viz., 11% with score 4-6 and 30% with score >7, thereby revealing significant relation with the grade (P < 0.05) [Figure 1]. There was a significant relation (P < 0.005) between DNA ploidy and hormonal responsiveness, 37/45 (83%) hormone resistant being aneuploid versus 26/80 (33%) hormone sensitive cases. 93% of p53 positive cases were aneuploid (P < 0.005) compared with none of the p53 negative, suggesting a significant correlation between p53 immunoreactivity and aneuploidy.
Apoptotic index
Average apoptotic indices were 0.14, 0.26, and 0.34, respectively in BPH, PIN and CaP, respectively suggesting positive correlation between AI and progression of disease. Within CaP, relation between stage, Gleason score and AI was not statistically significant. A higher AI was found in hormone resistant patients (0.42) when comp7red to the hormone sensitive (0.26) patients (P = 0.001) [Figure 2].
Figure 2.
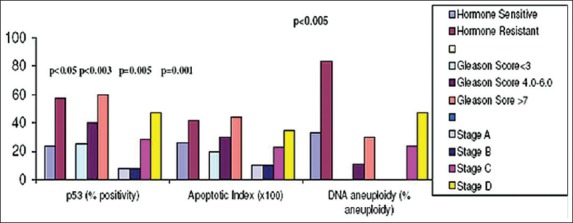
p53 immunoreactivity (%), apoptotic index and DNA aneuploidy in carcinoma prostate cases in relation with hormone response, clinical stage, and Gleason score
Follow-up was done for 12-48 months. Repeat biopsy was done in 40 patients who showed rise in serum PSA levels or had clinical features indicating progression of disease and presence of metastasis on bone scan. Five localized cases (Stages A and B) showed evidence of clinical progression while 35 advanced cases (Stages C and D) who were initially hormone sensitive became hormone resistant later. Three of the five patients in the first group (Stages A and B) transformed to p53 positive and showed a transition from diploid to aneuploid state. Twenty-seven out of 35 patients in the latter group (Stages C and D) transformed to p53 positive and showed a transition to aneuploidy. No significant change was found in AI in the repeat biopsy samples of these patients.
Discussion
In this study, we analysed three biological indices viz.; p53, AI and DNA ploidy in cases of BPH, PIN and CaP, and significance of these parameters as prognostic markers and predictors of tumor recurrence/progression.
Earlier studies, like ours have shown that p53 positivity increases as disease progresses from PIN to CaP.[1,2] However, in organ confined cancer cases with Gleason score ≤3 it is lower than PIN. This suggests that PIN progresses to high grade cancers, while low grade malignancies are possibly a separate entity.
In carcinoma cases other studies too,[3,4] as ours, showed that a high level of p53 accumulation was associated with a high histologic grade, advanced stage, and metastasis.[5] It can be suggested that clonal expansion of p53 mutated tumor cells is responsible for progression of CaP to metastatic/advanced stage. It further appears that p53 mutations in prostate tumor cells make them resistant to hormone withdrawal therapy. Like us, p53 overexpression and poor prognosis were correlated by Koivisto et al.[6] They also suggested, that mutation of p53 gene is associated with hormone refractory recurrent CaP. This study suggests that p53 mutation could be used as a marker of this change in biological behavior.
Apoptotic index showed a statistically significant increase (P < 0.005) as the disease progressed from BPH-PIN-CaP. Häussler et al.,[1] and Voelkel-Johnson et al.,[7] found that AI was generally low in BPH although it was higher in PIN and cancer. Our findings are similar; however, AI did not correlate with the clinical stage and Gleason score in cancer patients. AI was higher in hormone resistant cases when compared with hormone responsive cases as reported by Stapleton et al.,[8] and Staunton and Gaffney.[9]
DNA aneuploidy was statistically correlated with higher stage, Gleason grades, adverse prognosis and recurrence after prostatectomy. DNA ploidy combined with p53 immunostaining yields additional prognostic information in CaP.
Accurate preoperative prediction of progression in localized tumor and recurrence is urgently needed to select the right patients for curative therapy, enable appropriate counseling and select patients for adjuvant therapy and has been the goal of many studies over the past two decades. This study has shown that p53 positivity and DNA aneuploidy can be taken as independent predictors of progression and recurrence. Data on repeat biopsy in studies similar to ours are scanty. Quinn et al. also showed that p53 positivity was an independent predictor of relapse in patients of localized CaP.[10] These authors hypothesized that p53 dysfunction within CaP may exist in foci of tumor cells and clonal expansion of these cells results in metastases. A clear progression from untreated primary CaP to hormone refractory disease was seen with p53 overexpression and poor prognosis in terms of survival.
Our observations suggest that clinical progression in early stages is associated with transition to p53 positive state and from diploid to aneuploid state. Biopsy p53 status significantly predicts chances of recurrence. From this study, a patient with negative p53 immunostaining is likely to have a good prognosis on prolonged follow-up. Further, DNA aneuploidy correlated with adverse prognosis including recurrence.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Häussler O, Epstein JI, Amin MB, Heitz PU, Hailemariam S. Cell proliferation, apoptosis, oncogene, and tumor suppressor gene status in adenosis with comparison to benign prostatic hyperplasia, prostatic intraepithelial neoplasia, and cancer. Hum Pathol. 1999;30:1077–86. doi: 10.1016/s0046-8177(99)90226-5. [DOI] [PubMed] [Google Scholar]
- 2.Kallakury BV, Figge J, Ross JS, Fisher HA, Figge HL, Jennings TA. Association of p53 immunoreactivity with high Gleason tumor grade in prostatic adenocarcinoma. Hum Pathol. 1994;25:92–7. doi: 10.1016/0046-8177(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 3.Navone NM, Troncoso P, Pisters LL, Goodrow TL, Palmer JL, Nichols WW, et al. p53 protein accumulation and gene mutation in the progression of human prostate carcinoma. J Natl Cancer Inst. 1993;85:1657–69. doi: 10.1093/jnci/85.20.1657. [DOI] [PubMed] [Google Scholar]
- 4.Tamboli P, Amin MB, Xu HJ, Linden MD. Immunohistochemical expression of retinoblastoma and p53 tumor suppressor genes in prostatic intraepithelial neoplasia: Comparison with prostatic adenocarcinoma and benign prostate. Mod Pathol. 1998;11:247–52. [PubMed] [Google Scholar]
- 5.Yang G, Stapleton AM, Wheeler TM, Truong LD, Timme TL, Scardino PT, et al. Clustered p53 immunostaining: A novel pattern associated with prostate cancer progression. Clin Cancer Res. 1996;2:399–401. [PubMed] [Google Scholar]
- 6.Koivisto P, Visakorpi T, Rantala I, Isola J. Increased cell proliferation activity and decreased cell death are associated with the emergence of hormone-refractory recurrent prostate cancer. J Pathol. 1997;183:51–6. doi: 10.1002/(SICI)1096-9896(199709)183:1<51::AID-PATH1092>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 7.Voelkel-Johnson C, Voeks DJ, Greenberg NM, Barrios R, Maggouta F, Kurtz DT, et al. Genomic instability-based transgenic models of prostate cancer. Carcinogenesis. 2000;21:1623–7. [PubMed] [Google Scholar]
- 8.Stapleton AM, Zbell P, Kattan MW, Yang G, Wheeler TM, Scardino PT, et al. Assessment of the biologic markers p53, Ki-67, and apoptotic index as predictive indicators of prostate carcinoma recurrence after surgery. Cancer. 1998;82:168–75. doi: 10.1002/(sici)1097-0142(19980101)82:1<168::aid-cncr21>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Staunton MJ, Gaffney EF. Apoptosis: Basic concepts and potential significance in human cancer. Arch Pathol Lab Med. 1998;122:310–9. [PubMed] [Google Scholar]
- 10.Quinn DI, Henshall SM, Head DR, Golovsky D, Wilson JD, Brenner PC, et al. Prognostic significance of p53 nuclear accumulation in localized prostate cancer treated with radical prostatectomy. Cancer Res. 2000;60:1585–94. [PubMed] [Google Scholar]


