Abstract
Background:
Both C-peptide and nicotinamide are known to reduce blood glucose in type 1 diabetes. In the present study, the effects of C-peptide alone or in combination with nicotinamide on glucose and insulin levels in streptozotocin-nicotinamide-induced type 2 diabetic mice.
Methods:
The study used 70 adult male NMARI mice, weighing 25–35 g, divided into seven groups: control; type 1 diabetic; type 2 diabetic; type 2 diabetic + C-peptide; type 2 diabetic + nicotinamide; type 2 diabetic + nicotinamide and C-peptide; type 2 diabetic + glyburide. Type 2 diabetes was induced with ip injection of streptozotocin–nicotinamide. Twenty eight days after the onset of diabetes, treatment with C-peptide, nicotinamide, nicotinamide + C-peptide, or glyburide were initiated. Glucose and insulin levels were evaluated. One-way ANOVA and Least Significant Difference (LSD) tests were used to test for significance.
Results:
Blood glucose significantly increased (P < 0.001) in all diabetic mice compared with control mice. Insulin resistance and blood glucose levels were significantly reduced (P < 0.05) in C-peptide and nicotinamide + C-peptide mice compared with type 2 diabetic mice.
Conclusion:
The present study supports the anti-diabetic effects of C-peptide, nicotinamide + C-peptide, and suggests that one of the anti-diabetic mechanisms of these compounds is mediated through the reduction of insulin resistance.
Keywords: C-peptide, nicotinamide, mice, insulin resistance, diabetes type 2
Introduction
Type 2 diabetes mellitus (T2DM) is an advanced and complicated metabolic disorder that is characterised by weakened insulin release and reduced insulin sensitivity in target tissues such as the liver, muscle, and adipose tissue; thus, the metabolic effects of insulin normally do not apply. High blood glucose is the principal cause associated with the complications of T2DM (1). All over the world, T2DM currently affects 246 million people, and is expected to increase to 380 million by 2025 (2). Long-term high blood glucose leads to complications related to T2DM, such as cardiovascular disease, neuropathy, kidney failure, and retinopathy (3). Novel compounds are successively being examined and novel strategies established to prevent and improve T2DM. In experimental studies, several appropriate animal models of diabetes have been applied. One of these models is streptozotocin (STZ)–nicotinamide (NA)-induced diabetes in the mouse. In this model, mice present mild hyperglycemia linked with the lack of first-phase insulin release, and reduction in mean insulin secreted from the pancreas. Therefore, STZ–NA-induced diabetic mice possess many pathological characteristics similar to T2DM (4). STZ is a common diabetogenic factor that has cytotoxic effects on pancreatic β-cells. NA has partially protective effects on pancreatic β-cells in mice; therefore, injection of STZ and NA leads to partial damage of pancreatic β-cells (5).
C-peptide is composed of 31 amino acids and has a half-life of nearly 30 min, and plays an important role in the biosynthesis of insulin. After separation from proinsulin in the pancreatic β-cells, C-peptide is released into the blood stream in the same amounts with insulin (6). C-peptide has long been regarded as an inert compound; however, several studies suggest that C-peptide has a physiological function in various tissues. C-peptide elevates nitric oxide (NO) generation and Na+/K+-ATPase activation in different tissues (7). C-peptide improves the complications caused by T2DM, including kidney disease and neuropathy (8).
NA is a water soluble vitamin belonging to the vitamin B group. It has been shown to act in four different ways: (1) it reduces the toxicity of free oxygen radical scavengers, (2) it can improve insulin release via increasing NA-adenine dinucleotide (NAD), (3) it can increase B-cell regeneration, and (4) it can prevent NO release. Nicotinamide is therapeutic in type 1 diabetes of experimental animal models and humans (9,10). The effects of nicotinamide, C-peptide, and C-peptide-nicotinamide combination in animal models of type 2 diabetes have not been studied. Therefore, in the present paper, we examined the effects of C-peptide alone and in combination with nicotinamide on glucose and insulin levels in STZ–NA-induced type 2 diabetes mice. Another aim of this study was to induce type 1 diabetes by STZ and compare with STZ–NA-induced type 2 diabetes.
Materials and Methods
Materials
Streptozotocin, nicotinamide, and glyburide were bought from Sigma-Aldrich Co. (St. Louis, MD, USA). C-peptide was bought from Bachem Company (Germany). IRMA kit for the measurement of insulin was purchased from Belgium Company.
Animals
70 normal male mice NMARI, five weeks of age, weighing approximately 25–35 g, were purchased from Ahvaz Jundishapur University of Medical Sciences Animal Care Center (Iran). The mice were housed in cages under standard conditions at 22 °C ± 2 °C with a 12 h day and 12 h night cycle, and 65% ± 10% relative humidity. Food pellets and water were available throughout the experiment. Our study was conducted in accordance with guidelines for ethical laboratory animal care from Ahvaz Jundishapur University of Medical Sciences.
Experimental Design
After one week of adaptation, the mice were divided principally into the following groups (in each group, n = 10)
Group 1: Control (0.5 mL/kg saline, intraperitoneal (ip))
Group 2: Untreated type 1 diabetic (65 mg/kg STZ, ip)
Group 3: Untreated type2 diabetic (110 mg/kg NA and 65 mg/kg STZ, ip)
Group 4: Type 2 diabetic + C-peptide (25 nmol/kg, ip)
Group 5: Type 2 diabetic + Nicotinamide (120 mg/kg, ip)
Group 6: Type 2 diabetic + combination C-peptide and Nicotinamide (25 nmol/kg C-peptide + 120 mg/kg Nicotinamide, ip)
Group 7: Type 2 diabetic + glyburide (10 mg/kg, ip)
Before the experiment, the mice were fasted for 12 h, but water was freely given. Streptozotocin (STZ) was dissolved in normal saline. Type 1 diabetes was induced in mice via a single administration of 65 mg/kg streptozotocin (11). During the first 24 h after injection of streptozotocin, to overcome the fatal hypoglycemia caused by STZ injection, the mice were given a glucose solution (12).
In the present study, the goal of inducing type 1 diabetes was to compare it with STZ–NAinduced type 2 diabetic mice. Nicotinamide was dissolved in normal saline. Type 2 diabetes was induced in mice via a single administration of 65 mg/kg streptozotocin, 15 min after the injection of 110 mg/kg of nicotinamide (13). Diabetes was made conclusive 14 days after administration by the positive identification of increased blood glucose levels (14). STZ-injected mice exhibited blood glucose greater than 250 mg/dL, regarded as type 1 diabetic, and STZ–NA-injected mice exhibited blood glucose greater than 200 mg/dl, regarded as type 2 diabetic (15,16). Blood glucose levels from tail vein samples were measured using a glucometer. Body weight was measured weekly during the experiment.
The doses of C-peptide (12), Nicotinamide (10), and glyburide (17) were obtained through prior studies. The compounds were injected once daily for 28 days after verification of diabetes in STZ–NA-induced type 2 diabetic mice. The control and untreated diabetic mice were given saline (0.5 mL/kg) daily during the treatment period. In this study, glyburide was utilized as the hypoglycemic factor (18). Before injections, C-peptide and nicotinamide were dissolved in normal saline. Glyburide was dissolved in distilled water. At 24 h after the final injections, the mice were killed under light ether anesthesia and blood samples were obtained from the heart. A portion of blood was used for glucose measurement, while the remaining blood was poured into centrifuge tubes and centrifuged at 3500 rpm for 20 min, and then the serum was used for the measurement of insulin (19).
Blood glucose, serum insulin levels, and fasting insulin resistance index (FIRI)
Serum insulin levels were measured via 50 μL of serum using immunoradiometric assay (IRMA) kit. Intra- and inter-assay coefficients of variation were 2% and 4%, respectively (20). Low-end sensitivity of insulin was 1 μU/mL. Blood glucose levels were determined using a glucometer. A drop of blood given from the heart was slowly placed on the test region of the glucometer and the blood glucose level was indicated in mg/dL. FIRI was computed using the formula, FIRI = fasting insulin (μU/mL) × fasting glucose (mg/dL) / 405 (21).
Statistical analysis
The results were presented as mean (SD), if normally distributed. Before statistical analysis was performed, the normal distribution and homogeneity of the variances were evaluated using Kolmogorov–Smirnov test and Levene’s test. Results were analysed by one-way analysis of variance (ANOVA), followed by post-hoc LSD tests. P < 0.05 was regarded as significant. Calculations were performed with SPSS version 15.0.
Results
Effect of STZ and combination of STZ–NA- in mice
Injection of STZ significantly enhanced blood glucose levels (P < 0.001) in STZ-induced diabetic mice compared to control and STZ–NA mice, and also decreased body weight (P < 0.05) and serum insulin (P < 0.001) in STZ-induced diabetic mice compared to control mice. Injection of ST–ZNA significantly enhanced blood glucose levels (P < 0.001) in STZ–NA-induced diabetic mice compared to control mice (Table 1).
Table 1.
Effect of streptozotocin (STZ) and combination of nicotinamide (NA)– with STZ in diabetic mice (n = 10)
| Control Group | STZ group | STZ-NA group | |
|---|---|---|---|
| Body weight (g) | 35.53 (0.87) | 30.83 (2.9)* | 32.66 (2.71) |
| Blood glucose (mg/dL) | 152.54 (4) | 346.5 (4.5) ***# | 256.53 (4)*** |
| Serum insulin (μIU/mL) | 39.24 (1) | 28.28 (1.31)*** | 34.46 (2) |
*P < 0.05, ***P < 0.001 versus control group, #P < 0.05 versus STZ-NA group. Mean (SD), one-way ANOVA and post hoc LSD tests.
Effect of C-peptide, nicotinamide, and combination of C-peptide with nicotinamide on blood glucose levels in STZ–NA-induced diabetic mice
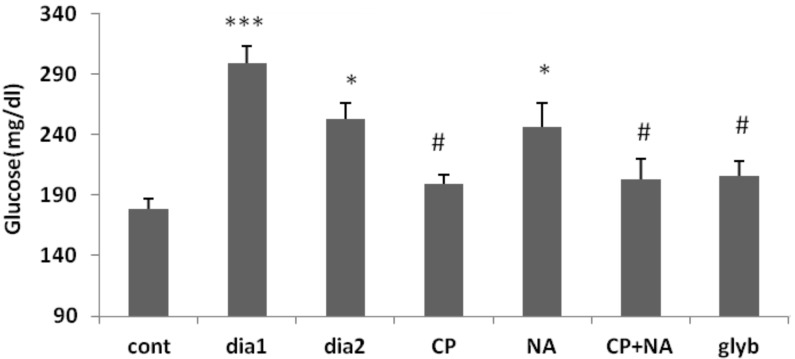
Administration of C-peptide and glyburide, and co-administration of C-peptide and nicotinamide, for 28 days in STZ–NA-induced diabetic mice when compared to type 2 diabetic mice, had significantly (P < 0.05) reduced blood glucose. Blood glucose in type 1 diabetic (P < 0.001), type 2 diabetic (P < 0.05), and nicotinamide-treated (P < 0.05) mice were significantly elevated compared to control mice (Figure 1).
Figure 1:
Blood glucose concentration of control (cont), type 1 diabetic (dia1), type 2 diabetic (dia2), C-peptide-treated (CP), Nicotinamide-treated (NA), C-peptide + nicotinamide-treated (CP+NA) and glyburide (glyb) treated mice (n = 10). *P < 0.05, ***P < 0.001 versus control group, #P < 0.05 versus dia2 group. Mean (SD). one-way ANOVA and post hoc LSD tests.
Effect of C-peptide, nicotinamide, and combination of C-peptide with nicotinamide on serum insulin levels in STZ–NA-induced diabetic mice
Administration of C-peptide and nicotinamide for 28 days in STZ–NA-induced diabetic mice did not significantly alter serum insulin compared to control mice. Serum insulin levels in type 1 diabetic (P < 0.001), C-peptide + nicotinamide-treated (P < 0.01), and glyburidetreated (P < 0.01) were significantly reduced compared with control mice.
Effect of C-peptide, nicotinamide, and combination of C-peptide with nicotinamide on fasting insulin resistance index (FIRI) in STZ–NA-induced diabetic mice
Administration of C-peptide and glyburide, and co-administration of C-peptide and nicotinamide, for 28 days in STZ–NA-induced diabetic mice compared to type 2 diabetic mice, significantly (P < 0.05) reduced FIRI. Nicotinamide-treated mice did not significantly alter FIRI compared to type 2 diabetic mice.
Discussion
The present study showed that STZ injection seriously enhanced blood glucose caused by loss of insulin, and decreased body weight as a consequence of glycosuria and serious destruction of tissue proteins in STZ-induced type 1 diabetic mice (11,22). STZ is a toxic compound for pancreatic beta cells and can release nitric oxide (NO), which NO plays a main role in beta cell destruction linked to STZ. STZ causes rapid death of beta cells. STZ has been shown to cause damage to beta cells and subsequent insulin deficiency (23–25). Previous investigations demonstrated that hyperglycemia-induced destruction of pancreatic beta cells could be a consequence of the oxidative stress; therefore, antioxidant compounds possess protective effects against the damage caused by hyperglycemia (26). NA and STZ administration mildly enhanced the blood glucose without affecting body weight in STZ–NA-induced type 2 diabetic mice, and this may be ascribed to NA inducing T2DM. NA functions as an antioxidant and inhibitor of NO release, protecting β-cells against the cytotoxic action of STZ (10). T2DM occurs through the downregulation of insulin receptors in target tissues and insulin resistance. Insulin is released but cannot activate receptors on target tissues (27). Glyburide is an anti-diabetes medication in which the mechanism of action is not properly understood (28). In this study, administration of glyburide for 28 days in STZ–NA-induced diabetic mice significantly decreased serum insulin, blood glucose, and FIRI compared to control and type 2 diabetic mice. Extended administration periods of glyburide reduces blood glucose associated with a progressive descent in insulin release. In type 2 diabetic patients, Hirshman et al. showed that glyburide administration decreased insulin resistance in rats (18). C-peptide is an effective endogenous compound that increases the expression of endothelial nitric oxide synthase (eNOS), Na+, K+-ATPase activity, glycogen synthesis, amino acid absorption, and the stimulation of glucose transport (6,29). Several experiments in animals and type 1 diabetic patients have shown that C-peptide administration exerts advantageous effects on diabetes-induced complications in kidneys, central and peripheral nerves (30). Results from the present study revealed that blood glucose and FIRI were significantly reduced without affecting serum insulin levels in type 2 diabetic mice that received C-peptide. Reduced blood glucose, the decrease of FIRI, and the reducing effect of C-peptide on FIRI may be because of stimulation of glucose transport in skeletal muscle. Wu and Sato et al. showed that C-peptide decreased blood glucose in type 1 diabetic rats (8,31); however, Rebsomen and Akihiro et al. showed that C-peptide did not affect blood glucose levels (11,32). Nicotinamide is a vitamin that is utilized for the prevention of type 1 diabetes in animal models and clinical trials (12). In this investigation, nicotinamide injection did not show an effect on blood glucose in type 2 diabetic mice. Yoshino et al. reported that nicotinamide injection improved glucose tolerance in female diabetic mice, but glucose tolerance was not completely improved in male diabetic mice (33). Alenzi, Stevens et al. revealed that nicotinamide administration decreased blood glucose in type 1 diabetic rats (10,34). Coadministration of C-peptide and nicotinamide in our study reduced blood glucose and FIRI similar to C-peptide administration alone. C-peptide, when associated with nicotinamide, was capable of decreasing serum insulin levels compared with control mice, although the mechanism of action is unclear. Further experiments are needed to understand the precise mechanisms by which C-peptide, nicotinamide, and the combination of C-peptide and nicotinamide reduce the increased blood glucose in type 2 diabetic mice.
Conclusion
Our study suggests that C-peptide administration, and co-administration of C-peptide and nicotinamide, may decrease blood glucose by reducing FIRI in type 2 diabetic mice.
Acknowledgments
This paper is No D-9106 of MSc thesis, Health research institute, Diabetes Research Center of Ahvaz Jundishapur Medical Sciences University, Ahvaz, Iran.
Footnotes
Conflict of interest
None.
Funds
This study supported by a grant from Health research institute, Diabetes Research Center of Ahvaz Jundishapur Medical Sciences University, Ahvaz, Iran.
Authors’ contributions
Conception and design, analysis and interpretation of the data, critical revision of the article for the important intellectual content, provision of study materials or patient, statistical expertise and obtaining of funding: AA
Drafting of the article, administrative, technical or logistic support collection and assembly of data: AA, FRA
Final approval of the article: AA, HFM
References
- 1.Rabbani SI, Devi K, Khanam S. Role of Pioglitazone with Metformin or Glimepiride on Oxidative Stressinduced Nuclear Damage and Reproductive Toxicity in Diabetic Rats. Malays J Med Sci. 2010;17(1):3–11. [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Maweri SA, Ismail NM, Ismail AR, Al-Ghashm A. Prevalence of Oral Mucosal Lesions in Patients with Type 2 Diabetes Attending Hospital Universiti Sains Malaysia. Malays J Med Sci. 2013;20(4):38–45. [PMC free article] [PubMed] [Google Scholar]
- 3.Kante K, Reddy CS. Anti diabetic activity of Dolichos lablab (seeds) in Streptozotocin- Nicotinamide induced diabetic rats. Hygeia. J D Med. 2013;5(1):32–40. [Google Scholar]
- 4.Tahara A, Matsuyama-Yokono A, Shibasaki M. Effects of antidiabetic drugs in high-fat diet and streptozotocin-nicotinamide-induced type 2 diabetic mice. Eur J Pharmacol. 2011;1–3:108–116. doi: 10.1016/j.ejphar.2011.01.015. doi: 10.1016/j.ejphar.2011.01.015 . [DOI] [PubMed] [Google Scholar]
- 5.Szkudelski T. Streptozotocin–nicotinamide-induced diabetes in the rat Characteristics of the experimental model. Exp Biol Med. 2012;237(5):481–490. doi: 10.1258/ebm.2012.011372. doi: 10.1258/ebm.2012.011372 . [DOI] [PubMed] [Google Scholar]
- 6.Wahren J, Kallas Å, Sima A. The clinical potential of C-Peptide replacement in type 1 Diabetes. Diabetes. 2012;61(4):1–12. doi: 10.2337/db11-1423. doi: 10.2337/db11-1423 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasic D, Walcher D. Proinflammatory Effects of C-Peptide in Different Tissues. Int J Inflam. 2012;2012:932725. doi: 10.1155/2012/932725. doi: 10.1155/2012/932725 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato Y, Oshida Y, Han YQ, Han YQ, Morishita Y, Li L, et al. C-peptide fragments stimulate glucose utilization in diabetic rats. Cell Mol Life Sci. 2004;61(6):727–732. doi: 10.1007/s00018-003-3460-6. doi: 10.1007/s00018-003-3460-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamal M, Abbasy AJ, Muslemani A, Bener A. Effect of nicotinamide on newly diagnosed type 1 diabetic children. Acta Pharmacol Sin. 2006;27(6):724–727. doi: 10.1111/j.1745-7254.2006.00313.x. doi: 10.1111/j.1745-7254.2006.00313.x . [DOI] [PubMed] [Google Scholar]
- 10.Alenzi FQ. Effect of nicotinamide on experimental induced diabetes. Iran J Allergy Asthma Immunol. 2009;8(1):11–18. doi: 08.01/ijaai.1118 . [PubMed] [Google Scholar]
- 11.Rebsomen L, Pitel S, Boubred F, Buffat C, Feuerstein JM, Raccah D, et al. C-peptide replacement improves weight gain and renal function in diabetic rats. Diabetes Metab. 2006;32(3):223–228. doi: 10.1016/s1262-3636(07)70272-0. doi: DM-06-2006-32-3-1262-3636-101019-200518716 . [DOI] [PubMed] [Google Scholar]
- 12.Safinaz SI, Sherine MR. Nicotinamide: A cytoprotectant against streptozotocin-induced diabetic damage in Wister rat brains. Afr J Biochem Res. 2008;2(8):174–180. [Google Scholar]
- 13.Lee J, Yee ST, Kim JJ, Choi MS, Kwon EY, Seo KI, et al. Ursolic acid ameliorates thymic atrophy and hyperglycemia in streptozotocin-nicotinamideinduced diabetic mice. Chem Biol Interact. 2010;188(3):635–642. doi: 10.1016/j.cbi.2010.09.019. doi: 10.1016/j.cbi.2010.09.019 . [DOI] [PubMed] [Google Scholar]
- 14.Novelli M, Bonamassa B, Masini M, Funel N, Canistro D, Tata De, et al. Persistent correction of hyperglycemia in streptozotocin-nicotinamideinduced diabetic mice by a non-conventional radical scavenger. Naunyn Schmiedebergs Arch Pharmacol. 2010;382(2):127–137. doi: 10.1007/s00210-010-0524-7. doi: 10.1007/s00210-010-0524-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palsamy P, Subramanian S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed Pharmacother. 2008;62(9):598–605. doi: 10.1016/j.biopha.2008.06.037. doi: 10.1016/j.biopha.2008.06.037 . [DOI] [PubMed] [Google Scholar]
- 16.Torres-Piedra M, Ortiz-Andrade R, Villalobos-Molina R, Singh N, Medina-Franco JL, Webster SP, et al. A comparative study of flavonoid analogues on streptozotocin-nicotinamide induced diabetic rats: quercetin as a potential antidiabetic agent acting via 11beta-hydroxysteroid dehydrogenase type 1 inhibition. Eur J Med Chem. 2010;45(6):2606–2612. doi: 10.1016/j.ejmech.2010.02.049. doi: 10.1016/j.ejmech.2010.02.049 . [DOI] [PubMed] [Google Scholar]
- 17.Grimsby J, Sarabu R, Corbett WL, Haynes NE, Bizzarro FT, Coffey JW, et al. Allosteric activators of glucokinase: potential role in diabetes therapy. Science. 2003;301(5631):370–374. doi: 10.1126/science.1084073. [DOI] [PubMed] [Google Scholar]
- 18.Hirshman MF, Horton ES. Glyburide increases insulin sensitivity and responsiveness in peripheral tissues of the rat as determined by the glucose clamp technique. Endocrinology. 1990;126(5):2407–2412. doi: 10.1210/endo-126-5-2407. doi http://dx.doi.org/10.1210/endo-126-5-2407 . [DOI] [PubMed] [Google Scholar]
- 19.Mousavi SE, Shahriari A, Ahangarpour A, Vatanpour H, Jolodar A. Effects of Teucrium polium Ethyl acetate Extract on Serum, Liver and Muscle Triglyceride Content of Sucrose-Induced Insulin Resistance in Rat. Iran J Pharm Res. 2012;11(1):347–355. [PMC free article] [PubMed] [Google Scholar]
- 20.Ahangarpour A, Mohammadian M, Dianat M. Antidiabetic Effect of Hydroalcholic Urtica dioica Leaf Extract in Male Rats with Fructose-Induced Insulin Resistance. Iran J Med Sci. 2012;37(3):181–186. [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. doi: 10.2337/diacare.27.6.1487 . [DOI] [PubMed] [Google Scholar]
- 22.Amin KA, Awad EM, Nagy MA. Effects of panax quinquefolium on streptozotocin- induced diabetic rats: role of C-peptide, nitric oxide and oxidative stress. Int J Clin Exp Med. 2011;4(2):136–147. [PMC free article] [PubMed] [Google Scholar]
- 23.Arora S, Kumar Sh, Vohora O, Vohora D. Characterisation of Streptozotocin Induced Diabetes Mellitus in Swiss Albino Mice. Global J Pharmacol. 2009;3(2):81–84. [Google Scholar]
- 24.Guyton AC, Hall JE. 11th ed. Philadephia (PA): Elsevier Saunders; 2006. Textbook of medical physiology; pp. 961–976. [Google Scholar]
- 25.Gireesh G, Thomas SK, Joseph B, Paulose CS. Antihyperglycemic and insulin secretory activity of Costus pictus leaf extract in streptozotocin induced diabetic rats and in vitro pancreatic islet culture. J Ethnopharmacol. 2009;123(3):470–474. doi: 10.1016/j.jep.2009.03.026. doi: 10.10 16/j.jep.2009.03.026 . [DOI] [PubMed] [Google Scholar]
- 26.Singh SK, Kesari AN, Gupta RK, Jaiswal D, Watal G. Assessment of antidiabetic potential of Cynodon dactylon extract in streptozotocin diabetic rats. J Ethnopharmacol. 2007;114(2):174–179. doi: 10.1016/j.jep.2007.07.039. doi: 10.1016/ j.jep.2007.07.039 . [DOI] [PubMed] [Google Scholar]
- 27.Matsuyama-Yokono A, Nakano R, Someya Y, Hayakawa M, Shibasaki M. Effects of the combination of dipeptidyl peptidase-IV inhibitor ASP8497 and antidiabetic drugs in streptozotocin nicotinamide-induced mildly diabetic mice. Eur J Pharmacol. 2009;605(1–3):170–176. doi: 10.1016/j.ejphar.2008.12.040. doi: 10.1016/j.ejphar.2008.12.040 . [DOI] [PubMed] [Google Scholar]
- 28.Abel ED, Shepherd PR, Kahn BB. Glucose transporters and pathophysiologic states. Diabetes Mellitus a Fundamental and Clinical Text. In: Leroith D, Olefsky JM, Taylor S, editors. Philadelphia (PA): JB Lipincott; 2003. pp. 917–938. [Google Scholar]
- 29.Grunberger G, Qiang X, Li Z, Mathews ST, Sbrissa D, Shisheva A, et al. Molecular basis for the insulinomimetic effects of C-peptide. Diabetologia. 2001;44(10):1247–1257. doi: 10.1007/s001250100632. doi: 10.1007/s001250100632 . [DOI] [PubMed] [Google Scholar]
- 30.Sima AA, Zhang W, Grunberger G. Type 1 diabetic neuropathy and C-peptide. Exp Diabesity Res. 2004;5(1):65–77. doi: 10.1080/15438600490424541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu L, Olverling A, Huang Z, Jansson L, Chao H, Gao X, et al. GLP-1, exendin-4 and C-Peptide regulate pancreatic islet microcirculation, insulin secretion and glucose tolerancein rats. Clin Sci (Lond) 2011;122(8):375–384. doi: 10.1042/CS20090464. doi: 10.1042/CS20090464 . [DOI] [PubMed] [Google Scholar]
- 32.Kamikawa A, Ishii T, Shimada K, Makondo K, Inanami O, Sakane N, et al. Proinsulin C-peptide abrogates type-1 diabetes-induced increase of renal endothelial nitric oxide synthase in rats. Diabetes Metab Res Rev. 2008;24(4):331–338. doi: 10.1002/dmrr.810. doi: 10.1002/dmrr.810 . [DOI] [PubMed] [Google Scholar]
- 33.Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD (+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528–536. doi: 10.1016/j.cmet.2011.08.014. doi: 10.1016/j.cmet.2011.08.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens MJ, Li F, Drel VR, Abatan OI, Kim H, Burnett D, et al. Nicotinamide Reverses Neurological and Neurovascular Deficits in Streptozotocin Diabetic Rats. J Pharmacol Exp Ther. 2007;320(1):458–464. doi: 10.1124/jpet.106.109702. doi: 10.1124/jpet.106.109702 . [DOI] [PubMed] [Google Scholar]