Abstract
An embryonic stem cell (ESC) is a good tool to generate neurons in vitro and can be used to mimic neural development in vivo. It has been widely used in research to examine the role of cell signalling during neuronal development, test the effects of drugs on neurons, and generate a large population of functional neurons. So far, a number of protocols have been established to promote the differentiation of ESCs, such as direct and indirect differentiation. One of the widely used protocols to generate neurons is through the spontaneous formation of multicellular aggregates known as embryonic bodies (EBs). However, for some, it is not clear why EB protocol could be the protocol of choice. EB also is known to mimic an early embryo; hence, knowing the similarities between EB and an early embryo is essential, particularly the information on the players that promote the formation of EBs or the aggregation of ESCs. This review paper focuses on these issues and discusses further the generation of neural cells from EBs using a well-known protocol, the 4−/4+ protocol.
Keywords: embryonic stem cells, embryoid bodies, neural differentiation, retinoic acid, cell aggregation
Introduction
One of the unique characteristics of embryonic stem cells (ESCs) is that they can differentiate into the three primary germ layer derived cells (1,2). This together with another characteristic, their ability to self-divide makes ESCs a reliable tool to generate a large number of functional cells and a good model to study the development of early embryos. However, the balance between self-renewal and differentiation of ESCs is controlled by a complex network of genes and signalling pathways. This complex network, at a certain level, complicates the control of the differentiation process and thus, generates a large number of specific cell types. Many protocols have been established to differentiate ESCs into specific cell types such as neurons, islet cells, cardiomyocytes, and so on (3,4). Although the supplements used and the procedures of these protocols are very different from each other, in general, there are two types of differentiation protocols; indirect and direct differentiation. Different from direct differentiation, in which differentiation of ESCs is “directed” to certain cell types by using specific exogenous chemicals and factors, indirect differentiation is more dependent on endogenous factors and the specific signalling pathways involved. Indirect differentiation is also called spontaneous differentiation. Spontaneous differentiation means to withdraw the pluripotent-dependent factors (such as leukemia inhibitory factor (LIF) and feeder layers), therefore making the balance to favour differentiation. The spontaneous differentiation of ESCs is demonstrated through the formation of embryoid bodies (EBs). EBs are multicellular 3D aggregates that contain the cells of the three primary germ layers. EB is widely used in examining mammalian development in vitro (5–7).
In contrast, directed differentiation can occur without going through the formation of EBs but directly generate the specific cells from ESCs. Instead of the formation of EBs, some growth factors and chemicals such as fibroblast growth factor (FGF) (8), noggin (9), N2, and B27 (8) are added to trigger the neural differentiation of ESCs as monolayer-adherent cells. In addition, culturing the ESCs in low density under defined conditions (without serum and feeder layer) could also trigger neural differentiation (9).
Although both direct and spontaneous differentiation methods are efficient and widely used, an advantage of spontaneous differentiation through the formation of EBs is that it can be used as one of the important “golden rules” to check the pluripotency of any pluripotent stem cells (including iPSCs and ESCs). For example, human amniotic fluid stem cells (AFSCs) have been found to form EBs spontaneously, in which the study provides evidence of pluripotency in AFSCs (10). Hence, knowing the essential information about the formation of EBs might be useful to those who have decided on checking the pluripotency of stem cells or generating neural cells under a condition mimicking the embryo development in vivo.
This review focuses on applying a spontaneous differentiation protocol to generate neural lineage from ESCs. It starts with the introduction of EBs and summarises the techniques used in generating EBs, followed by the introduction of the players behind the formation of EBs, particularly those that promote the neural differentiation. The similarities between EBs and early embryos are also discussed in this review.
Embryoid Body (EB) and its Formation
EBs are made from ESCs; each EB is a multicellular 3D aggregate that contains partly differentiated ESCs and a cavity caused by cell death (7) (Figure 1). During spontaneous differentiation, the cells inside the EB keep dividing and interacting with each other, which then lead to the generation of the three primary germ layer–derived cells. The formation of EBs starts from the aggregation of ESCs. In this process, the balance between non differentiated and differentiated ESCs can be broken by withdrawing the pluripotent-related factors. Differentiation of ESCs through the formation of EBs generally follows three steps: (1) culturing of ESCs in suspension without LIF for mouse ESCs or without a feeder layer for human ESCs, (2) spontaneous differentiation of EBs in a noncoated dish, and (3) directed differentiation of mature EBs into specific cell lineages with the addition of growth factors/chemicals. At the first step of EB formation, the density of ESCs is crucial for the quality of EBs (11). The quality of EBs also influences the differentiation efficiency of ESCs toward specific cell lineages (11). Simple ways to judge the quality of EBs normally depend on the size/shape and morphology of EBs during spontaneous differentiation (11,12). The size of EBs has been found to influence the differentiation efficiency of human and mouse ESCs (12,13). For example, a smaller EB around 150–300 μm in diameter is suitable for endothelial cell differentiation, but bigger EBs around 450 μm in diameter can promote the cardiogenesis of ESCs (14). In addition, the shape of EBs is also affected by the environment surrounding the EBs such as the cell signals, extracellular matrix, and the material of the plate/dish (15).
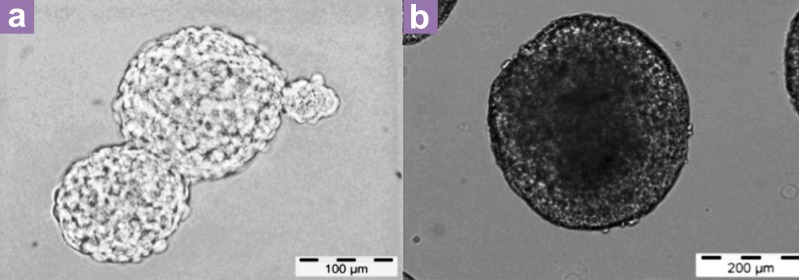
Figure 1.
The phase contrast images of ESC aggregates and EBs. (a) shows the aggregates of ESCs and (b) shows the morphology of EBs.
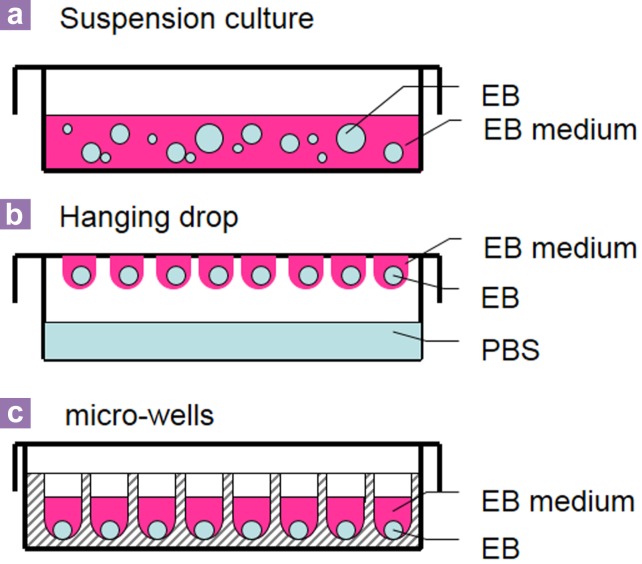
In order to obtain good-quality EBs, the spontaneous differentiation method has been optimized. Optimized protocols have been established to control the size and the shape of EBs. These include the suspension culture in the petri dish, hanging drop, and microwells/capsule methods (Figure 2).
Figure 2.
Methods to form embryoid bodies.
Suspension culture in the petri dish
The suspension culture in the petri dish (Figure 2a) is the simplest method to make a large amount of EBs at the same time. Generally, ESCs are harvested from a feeder layer and counted and suspended inside a non-coated petri dish to allow them to aggregate and form EBs. This method can generate a large number of EBs in one petri dish, but the size/shape of each EB cannot be controlled (11,12). The material of a petri dish is also crucial for the formation of an EB. Only a non-surfacetreated petri dish (normally a bacteriologicalgrade petri dish) can be used in this process to prevent uncontrollable attachment of ESCs to the bottom of the dish instead of just the cellto-cell attachment. The appropriate materials of the petri dish/well are also important for the culturing of EBs. Inappropriate dishes/wells might cause the attachment of EBs and reduce the efficiency of differentiation (16,17). Petri dishes made of different materials, such as polystyrene (PS), polypropylene (PP), and phosphorylcholine were tested during the formation of EBs. PP has been found to be better than other materials for making EBs (16). In addition, petri dishes coated with chemicals may also enhance the formation of EBs and reduce the attachment. For example, a 2-methacryloyloxyethyl phosphorylcholine–coated surface has been found to protect cells from attaching to the bottom of dishes, and silicon-coated glass petri dishes are good for the formation of EBs (17,18). Therefore, for the formation of EBs, the suitable petri dish or the specific type of dish is very important for the differentiation efficiency of EBs.
Hanging drop
Hanging drop is a method (Figure 2b) to generate homogenous-size EBs by suspending single cells in drops, which hang on the cover of a petri dish, and each hanging drop contains a certain number of ESCs. The ESCs aggregate at the bottom of droplets upon being affected by gravity (19). The hanging drop method successfully prevents EBs from attaching to the surface of the container and controls the size and the shape of EBs. However, this method has some disadvantages. First, it cannot produce a high number of EBs at the same time because each drop only contains a few EBs. Second, changing the medium and treatment of EBs is very difficult to carry out.
Microwell/microcapsule
Microwell/capsule methods (Figure 2c) were adopted to control the size, shape, and homogeneity of EBs. Round-bottom 96-well plates have been used to form EBs because these plates were found to be better than the flatbottom 96-well plates for the formation of EBs (17). Polyacrylamide hydrogel made microwells good for culturing EBs in 3D. Polyacrylamide hydrogel microwells are stable in an EB medium, and the surface of polyacrylamide hydrogel can prevent ESCs from attaching to the surface of the well. In addition, they can create a hydrated niche condition for ESC differentiation (20). The size of EBs generated in the microwell method depends on the initial cell density in each well as well as the dimension of the wells. Normally, the optimal size of EBs ranges from 100 to 500 μm (14,20,21). Compared with the hanging drop method, changing the medium, treatment, and collection of EBs is easier done with the microwell method. Moreover, EBs trapped in hydrogel microwells can be protected from the stresses of fluid flow (15).
The players behind aggregation of embryonic stem cells
There are several important factors that regulate attachment of ESCs and the formation of EBs. At the early stage of EB formation, ESCs cultured in suspension attach to each other and form ESC aggregates (Figure 3a). The surface of the EB becomes smoother and rounder compared to early cell aggregates because of the differentiation of ESCs on the outer layer of the EB (Figure 3b) (7,22). However, not all ESC aggregates will differentiate into mature EBs; a report found that culturing mouse ESC aggregates under microgravity conditions has managed to maintain the pluripotency of ESCs even in the absence of LIF (23). Therefore, finding the best players behind the differentiation of ESCs is important in order to understand more about neural development and generate more neuronal cells (Figure 4).
Figure 3.
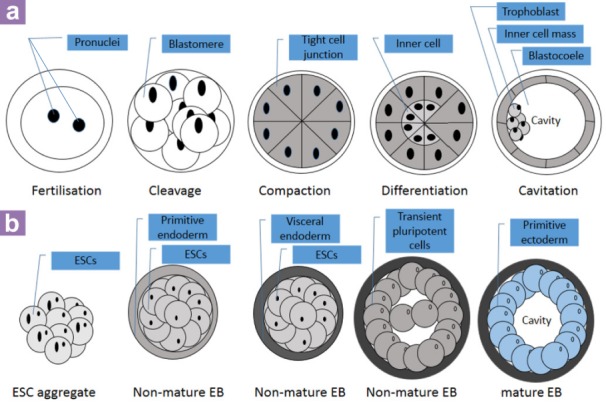
The development of an early embryo (a) and the formation of an EB in vitro (b). (Images are adapted and modified from: http://en.wikipedia.org/wiki/File: HumanEmbryogenesis.svg,September 2013, 33 and 7).
Figure 4.
The players behind the formation of EBs.
Cell signalling pathway and growth factors
Some factors that are used to maintain the undifferentiated status of ESCs, such as LIF for mouse ESCs (mESCs) and mouse embryonic fibroblast (MEF) feeder layer for human ESCs (hESCs), have to be removed during the formation of EBs (24–26). LIF/Stat3 is one of the main LIF pathways that have been found to regulate pluripotency of mESCs. Pluripotent-related genes such as Nanog, Gdf3, Rex1, Rest, Socs3, CD9, and Tdgf1 have been found to be upregulated by the LIF/Stat3 signalling pathway in mESCs, but Brachyury, Eomes, Foxa2, Gata6, and Lhx1 were downregulated (27). Different from mESCs, a LIF/Stat3 signalling pathway is not sufficient for maintaining the self-renewal and pluripotency of hESCs (2,28).
The Wnt signalling pathway, which is known to regulate the self-renewal and differentiation of ESCs, has also been found to be crucial for the development of the central nervous system (CNS). Nordin et al. (2008) found that Wnt3, 5b, 6, 7a, 7b, 8a, 9a, 10b, and 16 were expressed in ESCs, and upon screening 19 mouse Wnt genes during neural differentiation of mESCs through the formation of EBs, Wnt3, 3a, 5a, 5b, 7b, 8a, 9b, and 10b were found to be expressed in early EBs, but the expression of Wnt6, 7a, 7b, and 8a decreased dramatically during the spontaneous differentiation of mESCs and the formation of EBs (29). Wnt signalling pathways also regulate the proliferation and differentiation of hESCs. Wnt3a was found to improve the self-renewal and “stemness” of hESCs, but the activity of Wnt/β-catenin signalling only increased during differentiation of hESCs (30).
Bone morphogenetic proteins (BMPs) are another important factor for early differentiation of an embryo in vivo. Different from the function that Wnt signalling plays during neural differentiation, BMP signals can promote endodermal and mesodermal differentiation (31–35). Together with fibroblast growth factors (FGFs), BMPs are able to promote the primitive endodermal differentiation both in vivo and in vitro (7,36). Moreover, FGFs and BMPs regulate the formation of visceral endoderm, which provides an important source of cellular signals during the gastrulation of an embryo and cavitation of EB (7,37–40).
The changes in the expression of certain genes happen rapidly during the spontaneous differentiation of mESCs. Research on the early differentiation process of mESCs found that within the first 12 hours after spontaneous differentiation, Pim1, Pim3, SOCS3, Anxa3, Mras (signalling-related proteins), Fblim1, Vim, Tagln, Mapt, Brca2, Bhlbh2, Bcl3, Klf4, Klf5, and Nr0b1 (nuclear proteins) were downregulated; meanwhile, TAPP2 (signalling-related protein), Wdr1, Arpc5, and Myl9 (cytoskeleton-related proteins) and Smn1, Phf21a, Myb, and Otx2 (nuclear proteins) were upregulated (41).
Cell adhesion factors
Some cell surface proteins called adhesion molecules regulate the cell-cell adhesion of ESCs. These factors play important roles in the aggregation of ESCs and differentiation of EBs (42). E-cadherin is one of the cell-cell adhesion molecules; it regulates the aggregation of hESCs and mESCs (42–44). The cell-cell adhesion defect was found to increase the proportion of single cells and decrease the size of EBs (42). An increasing number of studies have suggested that β-catenin may act as a cell-cell adhesion factor during the formation of EBs (45–47). Previous studies found that β-catenin is crucial for the differentiation of neuroepithelia, the formation of telencephalon, and the development of the nervous system in vivo (45,47,48).
Embryoid Body versus Early Embryo: The Similarities
At around embryonic day 6.5 (E6.5) of a mouse embryo, the inner cell mass inside the blastocyst will differentiate into the epiblast and subsequently into the primitive ectoderm (39, 49). The primitive ectoderm of an early embryo further develops into mesoderm, ectoderm, and endoderm during gastrulation (50). Similar to the gastrulation process in vivo, inner cell massderived ESCs are able to differentiate into the three germ layers through a gastrulation-like process in vitro (Figure 3a) (7). In EBs, the formation of a primitive ectoderm is followed by the visceral endoderm on the surface of EBs (Figure 3b) (7,49). The formation of epiblast cells and visceral endoderm can be found in both embryos and EBs (7). Although both EBs and embryo possess the same ability to differentiate into the three germ layers, primordial germ cells (PGCs), which further develop into sperm and egg, can only be derived from the embryo before the gastrulation. Moreover, because of the same structure of the cavity in both the early embryo and EBs, the ESC-derived EBs have been used as an in vitro model to examine the process of cavitation during embryo development (39) (Figure 5).
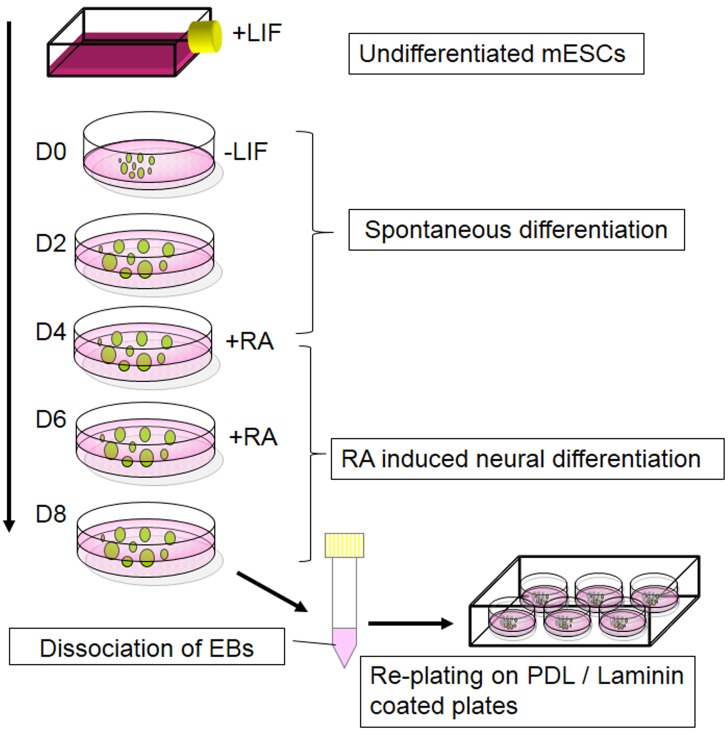
Figure 5.
4-/4+ protocol.
Embryoid Body Protocol of Neural Differentiation (4−/4+ protocol)
The 4−/4+ protocol is one of the widely used protocols to differentiate ESCs into neurons through the formation of EBs (52). To induce differentiation of ESCs by the 4−/4+ protocol, spontaneous differentiation of EBs is essential during the first four days of EB culture. During this period, EBs develop into mature EBs, where they are exposed to all-trans retinoic acid (RA) for another four days to promote the differentiation toward neural lineages (Figure 5). This method has been successfully used in neural differentiations of mESCs and hESCs (53,54).
RA has been found to be expressed in early embryos as a morphogen that regulates the development of the neural tube in vivo and triggers neural differentiation during the early development of the CNS (55). When RA is present, it binds to the receptor complex, which is composed of retinoic acid receptors (RARs) and retinoid X receptors (RXRs) in the cell nucleus, and activates the transcription of target genes, including RARs, Hox genes, HNF-3α, and Cdx1 (56,57). The RA signalling pathway regulates anteroposterior patterning of the CNS and the migration of hindbrain neural crest through the expression of Hox genes (58–60). It can also be used as a supplement to trigger neuroectodermal differentiation in vitro (52). Previous research found that the neural differentiation of EBs could be increased up to six times compared with spontaneous differentiation without in RA (61), suggesting that the effect of RA is efficient during neural differentiation in vitro. However, RA not only promotes neural differentiation of the CNS but also differentiation toward other organs, such as skeleton, forelimb, heart, somites, eyes, pancreas, and lungs (62–65). The effect of RA is concentration-dependent during the embryonic development in vivo (66). Therefore, the concentration of RA is crucial for EB differentiation. Studies found that high concentrations of RA (10−7 M) have led to neural differentiation (56), while low concentrations of RA (10−8 M) triggered differentiation toward vascular smooth muscle (68). Lower concentrations of RA (10−9–10−8 M) can trigger differentiation toward cardiogenic cells (68). Furthermore, for the neural differentiation of EBs, a higher concentration of RA (around 2 × 10−6 M) is better than a low concentration of RA in generating more postmitotic neurons and glia, but a lower concentration of RA (around 2 × 10−8 M) can increase the population of neural progenitor cells (69).
The EB protocol can be used to differentiate ESCs into motor neurons and oligodendrocytes of the ventricular region and dorsalises neural progenitors (69). A study found that the appearance of neural precursor cells (NPCs) in day 6 EBs and the population of NPCs reached the peak around day 8 (29).
Although the EB-based 4−/4+ protocol is widely used, some disadvantages were observed during RA-induced neural differentiation (69–71). Studies found that RA treatment causes the caudalisation of the neural tube and reduces cell proliferation of the chick embryo (69,70). In some cases, RA-treated progenitors show a limited capacity to differentiate after transplanting into the embryonic chick neural tube (71).
Besides the EB-based, RA-induced neural differentiation (4−/4+) protocol, there are some other chemicals and cell signalling proteins that can be used to trigger the neural differentiation of EBs. Cell signalling pathways such as FGF, Wnts, BMP, and Sonic hedgehog (SHH), which are involved in neural differentiation of ESCs (69,72–74), are potential candidates to be used to establish more therapeutic-promising neural differentiation protocols in the future.
Conclusion
In this review, we focused on the mechanism of the spontaneous differentiation method, which can be used to differentiate ESCs through the formation of EBs toward neural lineages. Understanding the mechanisms of EB formation processes could allow us to use them as a suitable tool to study embryo development and neurogenesis in vitro. A number of EB-based protocols have been established to differentiate ESCs into different cell types, but the basic principles behind these methods are more or less similar. Because of the similarities of EB and pregastrulation embryo, spontaneous differentiation (EB method) is widely used in examining the effect of the cell signalling pathway and the effect of chemicals during embryogenesis. The presence of the three primary germ layer–derived cells in EBs under certain conditions has made it a valuable tool in providing a standard mean to examine the pluripotency of stem cells or generate specific cell types from certain pluripotent stem cells (such as ESCs and iPSCs). In contrast, directed differentiation via monolayer-adherent cells is more straightforward and generally faster in getting certain cell types than spontaneous differentiation. However, the method does not really mimic the in vivo embryonic development. The 4−/4+ neural differentiation protocol was discussed in the last part of this review as an example of how this protocol can be used to produce specific cell lineages (neural cells) from EBs. Hopefully, this review may provide some useful information for those who are interested in cell signalling pathways and mammalian development.
Acknowledgments
We would like to express our gratitude to Graduate Research Fellowship (GRF) from Universiti Putra Malaysia, Malaysia, ScienceFund (project no: 02-01-04-SF1651) from Ministry of Science, Technology and Inovation, Malaysia and Dr. John Mason of University of Edinburgh for his valuable and constructive comments on the contents and the English language of the paper.
Footnotes
Conflict of interest
None.
Funds
Science Fund (project no: 02-01-04-SF1651) from Ministry of Science, Technology and Inovation, Malaysia.
References
- 1.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. National Acad Sciences. 1981;78(12):7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. doi: 10.1126/science.282.5391.1145 . [DOI] [PubMed] [Google Scholar]
- 3.Jing Y, Machon O, Hampl A, Dvorak P, Xing Y, Krauss S. In Vitro Differentiation of Mouse Embryonic Stem Cells into Neurons of the Dorsal Forebrain. Cell Mol Neurobiol. 2011;31(5):715–727. doi: 10.1007/s10571-011-9669-2. doi: 10.1007/s10571-011-9669-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pal R, Totey S, Mamidi MK, Bhat VS, Totey S. Experimental Biology and Medicine Propensity of Human Embryonic Stem Cell Differentiation into Mature Cell Types. Expr Biol Medincine (Maywood) 2012;234(10):1230–1243. doi: 10.3181/0901-RM-38. doi: 10.3181/0901-RM-38 . [DOI] [PubMed] [Google Scholar]
- 5.Vallier L, Pedersen RA. Human embryonic stem cells: an in vitro model to study mechanisms controlling pluripotency in early mammalian development. Stem Cell Rev. Humana Press Inc. 2005;1(2):119–130. doi: 10.1385/SCR:1:2:119. doi: 10.1385/SCR:1:2:119 . [DOI] [PubMed] [Google Scholar]
- 6.Kong XB, Zhang C. Dickkopf (Dkk) 1 promotes the differentiation of mouse embryonic stem cells toward neuroectoderm. Vitr Cell Dev Biol Anim. 2009;45(3-4):185–93. doi: 10.1007/s11626-008-9157-2. [DOI] [PubMed] [Google Scholar]
- 7.Rodda SJ, Kavanagh SJ, Rathjen J, Rathjen PD. Embryonic stem cell differentiation and the analysis of mammalian development. Int J Dev Biol. 2002;46(4):449–458. [PubMed] [Google Scholar]
- 8.Ying Q-L, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21(2):183–186. doi: 10.1038/nbt780. doi: 10.1038/nbt780 . [DOI] [PubMed] [Google Scholar]
- 9.Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30(1):65–78. doi: 10.1016/s0896-6273(01)00263-x. doi: 10.1016/S0896-6273(01)00263-X . [DOI] [PubMed] [Google Scholar]
- 10.Valli A, Rosner M, Fuchs C, Siegel N, Bishop CE, Dolznig H, et al. Embryoid body formation of human amniotic fluid stem cells depends on mTOR. Oncogene. 2010;29(7):966–977. doi: 10.1038/onc.2009.405. doi: 10.1038/onc.2009.405 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J-M, Xing F-Y, Shi J-J, Fang Z-F, Chen X-J, Chen F. Quality of embryonic bodies and seeding density effects on neural differentiation of mouse embryonic stem cells. Cell Biol Int. 2008;32(9):1169–1175. doi: 10.1016/j.cellbi.2008.04.025. doi: 10.1016/j.cellbi.2008.04.025 . [DOI] [PubMed] [Google Scholar]
- 12.Mohr JC, Zhang J, Azarin SM, Soerens AG, de Pablo JJ, Thomson J a, et al. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials. 2010;31(7):1885–1893. doi: 10.1016/j.biomaterials.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messana JM, Hwang NS, Coburn J, Elisseeff JH, Zhang Z. Size of the embryoid body influences chondrogenesis of mouse embryonic stem cells. J Tissue Eng Regen Med. 2008;2(8):499–506. doi: 10.1002/term.125. [DOI] [PubMed] [Google Scholar]
- 14.Hwang Y shik, Bhung BG, Ortmann D, Hattori N, Moeller HC, Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of Wnt5a and Wnt11. PNAS. 2009;106(40):16978–16983. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moeller H, Mian M, Shrivastava S, Geun B, Khademhosseini A, Supérieure É, et al. A microwell array system for stem cell culture. Biomaterials. 2009;29(6):752–763. doi: 10.1016/j.biomaterials.2007.10.030. doi: 10.1016/j.biomaterials.2007.10.030 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dang SM, Kyba M, Perlingeiro R, Daley GQ, Zandstra PW. Efficiency of Embryoid Body Formation and Hematopoietic Development from Embryonic Stem Cells in Different Culture System. Biotechnol Biog. 2002;78(4):443–453. doi: 10.1002/bit.10220. [DOI] [PubMed] [Google Scholar]
- 17.Koike M, Kurosawa H, Amano Y. A roundbottom 96-well polystyrene plate coated with 2-methacryloyloxyethyl phosphorylcholine as an effective tool for embryoid body formation. Cytotechnology. 2005;47(1-3):3–10. doi: 10.1007/s10616-005-3743-x. doi: 10.1007/s10616-005-3743-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fathi F, Altiraihi T, Mowla SJ, Movahedin M. Formation of embryoid bodies from mouse embryonic stem cells cultured on silicon-coated surfaces. Cytotechnology. 2009;59(1):11–16. doi: 10.1007/s10616-009-9188-x. doi: 10.1007/s10616-009-9188-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerdan C, Hong SH, Bhatia M. Formation and hematopoietic differentiation of human embryoid bodies by suspension and hanging drop cultures. Curr Protoc Stem Cell Biol. 2007;Chapter 1:Unit 1D.2 doi: 10.1002/9780470151808.sc01d02s3. doi: 10.1002/9780470151808.sc01d02s3 . [DOI] [PubMed] [Google Scholar]
- 20.Giobbe GG, Zagallo M, Riello M, Serena E, Masi G, Barzon L, et al. Confined 3D Microenvironment Regulates Early Differentiation in Human Pluripotent Stem Cells. Biotechnol Bioeng. 2012;109(12):1–14. doi: 10.1002/bit.24571. doi: 10.1002/bit.24571 . [DOI] [PubMed] [Google Scholar]
- 21.Xu F, Sridharan B, Wang S, Gurkan UA, Syverud B, Demirci U. Embryonic stem cell bioprinting for uniform and controlled size embryoid body formation. Biomicrofluidics. 2011;5(2):22207. doi: 10.1063/1.3580752. doi: 10.1063/1.3580752 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Xia Y. Formation of Embryoid Bodies with Controlled Sizes and Maintained Pluripotency in Three-Dimensional Inverse Opal Scaffolds. Adv Funct Mater. 2011;22(1):121–129. [Google Scholar]
- 23.Kawahara Y, Manabe T, Matsumoto M, Kajiume T, Matsumoto M, Yuge L. LIF-free embryonic stem cell culture in simulated microgravity. PLoS One. 2009;4(7):e6343. doi: 10.1371/journal.pone.0006343. doi: 10.1371/journal.pone.0006343 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conley BJ, Denham M, Gulluyan L, Olsson F, Cole TJ, Mollard R. Mouse embryonic stem cell derivation, and mouse and human embryonic stem cell culture and differentiation as embryoid bodies. Curr Protoc cell Biol. 2005;Chapter 23(Unit 23):2. doi: 10.1002/0471143030.cb2302s28. doi: 10.1002/0471143030.cb2302s28 . [DOI] [PubMed] [Google Scholar]
- 25.Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103(5):389–98. doi: 10.1263/jbb.103.389. doi: 10.1263/jbb.103.389 . [DOI] [PubMed] [Google Scholar]
- 26.Murray P, Edgar D. The regulation of embryonic stem cell differentiation by leukaemia inhibitory factor (LIF) Differentiation. 2001;68(4-5):227–234. doi: 10.1046/j.1432-0436.2001.680410.x. doi: 10.1046/j.1432-0436.2001.680410.x . [DOI] [PubMed] [Google Scholar]
- 27.Kidder BL, Yang J, Palmer S. Stat3 and c-Myc Genome-Wide Promoter Occupancy in Embryonic Stem Cells. Najbauer J, editor. Public Library of Science. PLoS One. 2008;3(12):14. doi: 10.1371/journal.pone.0003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahéron L, Opitz SL, Zaehres H, Lensch MW, Andrews PW, Itskovitz-Eldor J, et al. LIF/STAT3 signalling fails to maintain self-renewal of human embryonic stem cells. Stem Cells. 2004;22(5):770–8. doi: 10.1634/stemcells.22-5-770. doi: 10.1634/stemcells.22-5-770 . [DOI] [PubMed] [Google Scholar]
- 29.Nordin N, Li M, Mason JO. Expression profiles of Wnt genes during neural differentiation of mouse embryonic stem cells. Cloning Stem Cells. 2008;10(1):37–48. doi: 10.1089/clo.2007.0060. doi: 10.1089/clo.2007.0060 . [DOI] [PubMed] [Google Scholar]
- 30.Dravid G, Ye Z, Hammond H, Chen G, Pyle A, Donovan P, et al. Defining the role of Wnt/beta-catenin signalling in the survival, proliferation, and selfrenewal of human embryonic stem cells. Stem Cells. Wiley Online Library. 2005;23(10):1489–501. doi: 10.1634/stemcells.2005-0034. [Internet] doi: 10.1634/stemcells.2005-0034 . [DOI] [PubMed] [Google Scholar]
- 31.Baker JC, Beddington RSP, Harland RM. Wnt signalling in Xenopus embryos inhibits Bmp4 expression and activates neural development. Genes Dev. 1999;13(23):3149–3159. doi: 10.1101/gad.13.23.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyd NL, Dhara SK, Rekaya R, Godbey EA, Hasneen K, Rao RR, et al. BMP4 promotes formation of primitive vascular networks in human embryonic stem cell-derived embryoid bodies. Exp Biol Med Maywood NJ. 2007;232(6):833–843. [PubMed] [Google Scholar]
- 33.Kraushaar DC, Rai S, Condac E, Nairn A, Zhang S, Yamaguchi Y, et al. Heparan sulfate facilitates FGF and BMP signalling to drive mesoderm differentiation of mouse embryonic stem cells. J Biol Chem. 2012;29(287):22691–2700. doi: 10.1074/jbc.M112.368241. doi: 10.1074/jbc.M112.368241 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finley MF, Devata S, Huettner JE. BMP-4 inhibits neural differentiation of murine embryonic stem cells. J Neurobiol. 1999;40(3):271–287. [PubMed] [Google Scholar]
- 35.Baker KD, Ramel M-C, Lekven AC. A direct role for Wnt8 in ventrolateral mesoderm patterning. Dev Dyn an Off Publ Am Assoc Anat. 2010;239(11):2828–2836. doi: 10.1002/dvdy.22419. [DOI] [PubMed] [Google Scholar]
- 36.Lima MJ, Docherty HM, Chen Y, Vallier L, Docherty K. Pancreatic Transcription Factors Containing Protein Transduction Domains Drive Mouse Embryonic Stem Cells towards Endocrine Pancreas. PLoS One. 2012;7(5):e36481. doi: 10.1371/journal.pone.0036481. doi: 10.1371/journal.pone.0036481 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lake J, Rathjen J, Remiszewski J, Rathjen PD. Reversible programming of pluripotent cell differentiation. J Cell Sci. 2000;113(Pt3):555–566. doi: 10.1242/jcs.113.3.555. [DOI] [PubMed] [Google Scholar]
- 38.Rathjen J, Dunn S, Bettess MD, Rathjen PD. Lineage specific differentiation of pluripotent cells in vitro: a role for extraembryonic cell types. Reprod Fertil Dev. 2001;13(1):15–22. doi: 10.1071/rd00079. [DOI] [PubMed] [Google Scholar]
- 39.Coucouvanis E, Martin GR. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83(2):279–287. doi: 10.1016/0092-8674(95)90169-8. doi: 10.1071/RD00079 . [DOI] [PubMed] [Google Scholar]
- 40.Coucouvanis E, Martin GR. BMP signalling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo. Development. 1999;126(3):535–546. doi: 10.1242/dev.126.3.535. [DOI] [PubMed] [Google Scholar]
- 41.Hailesellasse Sene K, Porter CJ, Palidwor G, Perez-Iratxeta C, Muro EM, Campbell P a, et al. Gene function in early mouse embryonic stem cell differentiation. BMC Genomics. 2007;8:85. doi: 10.1186/1471-2164-8-85. doi: 10.1186/1471-2164-8-85 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Bennett SAL, Wang L. Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cell. Cell Adh Migr. 2012;6(1):59–70. doi: 10.4161/cam.19583. doi: 10.4161/cam.19583 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larue L, Antos C, Butz S, Huber O, Delmas V, Dominis M, et al. A role for cadherins in tissue formation. Development. 1996;122(10):3185–3194. doi: 10.1242/dev.122.10.3185. [DOI] [PubMed] [Google Scholar]
- 44.Dasgupta A, Hughey R, Lancin P, Larue L, Moghe P V. E-Cadherin Synergistically Induces Hepatospecific Phenotype and Maturation of Embryonic Stem Cells in Conjunction With Hepatotrophic Factors. Biotechnol Bioeng. 2005;92(3):257–266. doi: 10.1002/bit.20676. [DOI] [PubMed] [Google Scholar]
- 45.Junghans D, Hack I, Frotscher M, Taylor V, Kemler R. Beta-catenin-mediated cell-adhesion is vital for embryonic forebrain development. Dev Dyn. 2005;233(2):528–539. doi: 10.1002/dvdy.20365. doi: 10.1002/dvdy.22075 . [DOI] [PubMed] [Google Scholar]
- 46.Hierholzer A, Kemler R. β-Catenin-Mediate Signalling and Cell Adhesion in Postgastrulation Mouse Embryos. Dev Dyn. 2010;239(1):191–199. doi: 10.1002/dvdy.22075. doi: 10.1002/dvdy.22075 . [DOI] [PubMed] [Google Scholar]
- 47.Lyashenko N, Winter M, Migliorini D, Biechele T, Moon RT, Hartmann C. Differential requirement for the dual functions of β-catenin in embryonic stem cell self-renewal and germ layer formation. Nat Cell Biol. 2011;13(7):753–761. doi: 10.1038/ncb2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore R, Tao W, Meng Y, Smith ER, Xu X. Cell adhesion and sorting in embryoid bodies derived from N-or E-cadherin deficient murine embryonic stem cells. Biol Open. 2014;3(2):121–128. doi: 10.1242/bio.20146254. doi: 10.1242/bio.20146254 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vassilieva S, Goh HN, Lau KX, Hughes JN, Familari M, Rathjen PD, et al. A System to Enrich for Primitive Streak-Derivatives, Definitive Endoderm and Mesoderm, from Pluripotent Cells in Culture. PLoS One. 2012;7(6):1–10. doi: 10.1371/journal.pone.0038645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gardner RL, Rossant J. Investigation of the fate of 4-5 day post-coitum mouse inner cell mass cells by blastocyst injection. J Embryol Exp Morphol. 1979;52:141–152. [PubMed] [Google Scholar]
- 51.Wild AE. 1. Vol. 33. [Place of publication uknown]: John Wiley & Sons, Ltd; 2001. Cleavage and Gastrulation in Mouse Embryos; Encyclopedia of Life Science; pp. 1–7. [Google Scholar]
- 52.Yao M, Bain G, Gottlieb DI. Neuronal differentiation of P19 embryonal carcinoma cells in defined media. J Neurosci Res. 1995;41(6):792–804. doi: 10.1002/jnr.490410610. [DOI] [PubMed] [Google Scholar]
- 53.Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27(4):806–11. doi: 10.1002/stem.31. doi: 10.1002/stem.31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Du Z-W, Zhang S-C. Neural differentiation from embryonic stem cells: which way? Stem Cells Dev. 2004;13(4):372–381. doi: 10.1089/scd.2004.13.372. doi: 10.1089/scd.2004.13.372 . [DOI] [PubMed] [Google Scholar]
- 55.Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev. 2000;80(3):1021–1054. doi: 10.1152/physrev.2000.80.3.1021. [DOI] [PubMed] [Google Scholar]
- 56.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43(11):1773–808. doi: 10.1194/jlr.r100015-jlr200. doi: 10.1194/jlr.R100015-JLR200 . [DOI] [PubMed] [Google Scholar]
- 57.Napoli JL. Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochimica Biophysica Acta. 1999;1440(2–3):139–162. doi: 10.1016/s1388-1981(99)00117-1. doi: 10.1016/S1388-1981(99)00117-1 . [DOI] [PubMed] [Google Scholar]
- 58.Grandel H, Lun K, Rauch G-J, Rhinn M, Piotrowski T, Houart C, et al. Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development. 2002;129(12):2851–2865. doi: 10.1242/dev.129.12.2851. [DOI] [PubMed] [Google Scholar]
- 59.Niederreither K, Vermot J, Le Roux I, Schuhbaur B, Chambon P, Dollé P. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development. 2003;130(11):2525–25234. doi: 10.1242/dev.00463. [DOI] [PubMed] [Google Scholar]
- 60.Lee YM, Osumi-Yamashita N, Ninomiya Y, Moon CK, Eriksson U, Eto K. Retinoic acid stage-dependently alters the migration pattern and identity of hindbrain neural crest cells. Development. 1995;121(3):825–837. doi: 10.1242/dev.121.3.825. [DOI] [PubMed] [Google Scholar]
- 61.Strübing C, Ahnert-Hilger G, Shan J, Wiedenmann B, Hescheler J, Wobus AM. Differentiation of pluripotent embryonic stem cells into the neuronal lineage in vitro gives rise to mature inhibitory and excitatory neurons. Mech Dev. 1995;53(2):275–287. doi: 10.1016/0925-4773(95)00446-8. [DOI] [PubMed] [Google Scholar]
- 62.Cvekl A, Wang W-L. Retinoic acid signalling in mammalian eye development. Exp Eye Res. 2009;89(3):280–291. doi: 10.1016/j.exer.2009.04.012. doi: 10.1016/j.exer.2009.04.012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chazaud C, Chambon P, Dollé P. Retinoic acid is required in the mouse embryo for left-right asymmetry determination and heart morphogenesis. Development. 1999;126(12):2589–2596. doi: 10.1242/dev.126.12.2589. [DOI] [PubMed] [Google Scholar]
- 64.Tehrani Z, Lin S. Antagonistic interactions of hedgehog, Bmp and retinoic acid signals control zebrafish endocrine pancreas development. Development. 2011;138(4):631–640. doi: 10.1242/dev.050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duester G. Retinoic Acid Synthesis and Signalling during Early Organogenesis. Cell. 2008;134(6):921–931. doi: 10.1016/j.cell.2008.09.002. doi: 10.1016/j.cell.2008.09.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruiz i Altaba a, Jessell T. Retinoic acid modifies mesodermal patterning in early Xenopus embryos. Genes Dev. 1991;5(2):175–187. doi: 10.1101/gad.5.2.175. [DOI] [PubMed] [Google Scholar]
- 67.Bain G, Kitchens D, Tao M, Huettner EJ, Gottlieb ID. Embryonic Stem Cells Express Neuronal Properties in vitro. Dev Biol. 1995;168(2):342–357. doi: 10.1006/dbio.1995.1085. doi: 10.1006/dbio.1995.1085 . [DOI] [PubMed] [Google Scholar]
- 68.Guan K, Rohwedel J, Wobus AM. Embryonic stem cell differentiation models: cardiogenesis, myogenesis, neurogenesis, epithelial and vascular smooth muscle cell differentiation in vitro. Cytotechnology. 1999;30(1–3):211–226. doi: 10.1023/A:1008041420166. doi: 10.1023/A:1008041420166 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okada Y, Shimazaki T, Sobue G, Okano H. Retinoicacid-concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev Biol. 2004;275(1):124–142. doi: 10.1016/j.ydbio.2004.07.038. doi: 10.1016/j.ydbio.2004.07.038 . [DOI] [PubMed] [Google Scholar]
- 70.Modak SP, Ghatpande SK, Rane RK, Mulherkar L. Caudalization by retinoic acid is correlated with inhibition of cell population growth and expansion of chick. Int J Dev Biol. 1993;37(4):601–607. [PubMed] [Google Scholar]
- 71.Plachta N, Bibel M, Tucker KL, Barde Y-A. Developmental potential of defined neural progenitors derived from mouse embryonic stem cells. Development. 2004;131(21):5449–5456. doi: 10.1242/dev.01420. doi: 10.1242/dev.01420 . [DOI] [PubMed] [Google Scholar]
- 72.Sarkar SA, Sharma RP. All-trans-retinoic acidmediated modulation of p53 during neural differentiation in murine embryonic stem cells. Cell Biol Toxicol. 2002;18(4):243–257. doi: 10.1023/a:1016003027850. [DOI] [PubMed] [Google Scholar]
- 73.Linker C, Stern CD. Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development. 2004;131(22):5671–5681. doi: 10.1242/dev.01445. doi: 10.1242/dev.01445 . [DOI] [PubMed] [Google Scholar]
- 74.Falk S, Wurdak H, Ittner LM, Ille F, Sumara G, Schmid M-T, et al. Brain area-specific effect of TGFbeta signalling on Wnt-dependent neural stem cell expansion. Cell Stem Cell. 2008;2(5):472–483. doi: 10.1016/j.stem.2008.03.006. doi: 10.1016/j.stem.2008.03.006 . [DOI] [PubMed] [Google Scholar]