It is well evident that the embryonic stem cells (ESCs) are pluripotent, can differentiate into all the three germ layers namely ectoderm, mesoderm and endoderm and into 200 odd cell types present in the body, are immortal, can expand in large numbers in vitro, and are genetically stable over long periods in culture. Three groups in the country have successfully derived well characterized human ES (hES) cell lines including Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), Bengaluru1, Reliance Life Sciences2 and National Institute for Research in Reproductive Health (NIRRH)3, Mumbai. However, hES cells have the associated issues of immune-rejection and risk of teratoma formation. On the other hand, the adult stem cells (ASCs) do not expand in culture but these can be isolated from the patients’ own bone marrow and thus are considered safe. Though studies/trials have been undertaken using autologus stem cells in India4, at the global level this approach has not shown the desired results5,6,7. The National Guidelines for Stem Cells Research and Therapy became available in 2007, and has been recently revised (http://icmr.nic.in/guidelines/NGSCR%202013.pdf) and are named National Guidelines for Stem Cells Research indicating that stem cells are not yet ready for therapy and more research is required before these are put to translational use. Simultaneously, Central Drugs Standard Control Organization (CDSCO), New Delhi has put up a draft guidance document for regulatory approval of Stem Cell and Cell Based Products (SCCPs) (http://www.cdsco.nic.in). Several companies/clinics have started culturing and using mesenchymal stem cells (MSCs) for treating various conditions in India. The situation is alarming as it is not clear whether these therapies would benefit the public. Are mesenchymal cells true stem cells or just stromal cells? Similar situation exists with cord blood banking to treat multiple diseases and claims that cord blood may serve as a future health insurance for the baby. It has become evident that (i) besides blood related disorders, cord blood stem cells may not regenerate other tissues as these do not possess trans-differentiation potential, and (ii) autologus cryopreserved cord blood sample will never suffice when the baby grows up as an adult8. Use of fresh unrelated donor cord blood sample may be a viable alternative as emerging studies suggest that mismatched allogeneic cord blood stem cell transplant is easily tolerated with 1-2 antigen mismatch and is associated with lower anticipated risk of graft versus host disease (GVHD)9,10. Moreover, the present article suggests that pluripotent stem cells exist in adult body organs and the need to bank cord blood as a source of stem cells may be a futile exercise. The concerns raised in a recently published editorial11 are timely. More research is required to understand how normal body stem cells interact with their niche, differentiate and get mobilized under disease conditions to bring about regeneration. Such information is crucial to exploit therapeutic potential of stem cells in the field of regenerative medicine. Here the author discusses the existing confusion as to what are the true stem cells in the body, how these are different from the tissue-specific progenitors and whether these may be implicated in initiating cancers. It also provides an interesting perspective to the stem cell field based on available literature and highlights the importance of microenvironment/niche in deciding the stem cell fate.
To begin with, we need to remind ourselves that the stem cells are basically cells that reside in a specialized somatic microenvironment (niche) and undergo asymmetric cell divisions, can self-renew and at the same time give rise to the tissue-specific progenitors. The progenitors (immediate descendants of stem cells) multiply in large numbers, differentiate into tissue-specific cells and thereby maintain homeostasis. The normal body stem cells are expected to get mobilized, bring about regeneration and also possibly transform into cancer stem cells under certain yet not well understood conditions (Fig. 1).
Fig. 1.
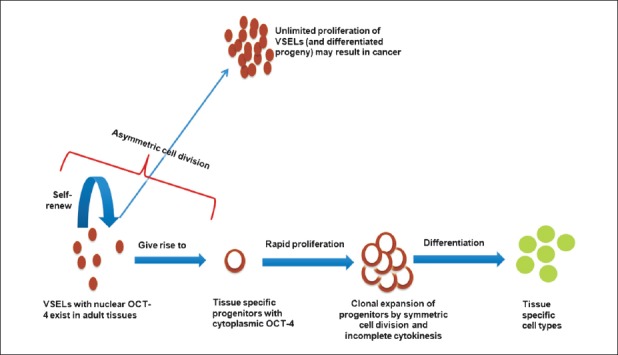
Proposed basic concepts in stem cell biology. VSELs, very small embryonic-like stem cells; OCT-4, a stem cell marker, nuclear OCT-4A isoforms responsible for pluripotent state; ASCs, adult stem cells which are tissue specific, actually progenitors; CSCs, cancer stem cells.
ES cells derived from the inner cell mass of the blastocyst, undergo symmetrical cell divisions ‘self-renewal’ in culture12 and have been correctly labelled as a tissue culture artifact13 as these do not reflect in vivo conditions. The inner cell mass from which the embryonic stem cells are derived is a transient phase during development and does not behave as stem cells in situ. The ES cells rather arise through selection and adaptation under in vitro conditions. These stem cells are pluripotent, produce teratoma on being injected in SCID (severe combined immunodeficiency) mice and integrate easily in a developing blastocyst to form a chimera. Differentiation of human ES cells into fully committed cell types in vitro is an inefficient process, thus attempts are made to transplant progenitors and allow in situ maturation. However, such efforts have resulted in short-term benefits14. These stem cells have the potential to differentiate into various cell types in vitro but whether these can sustain long-term regeneration in situ remains to be demonstrated. It is indeed fascinating that recently human ES cells were successfully differentiated into mini-brains15. Early success on safety and efficacy has been reported using human embryonic stem cells derived retinal epithelial cells but it is still a long way to go16. Tabar and Studer17 have discussed the existing challenges in translating ES based cell therapies to the clinic.
Best examples of ASCs are the haematopoietic stem cells (HSCs) and MSCs which have been extensively studied in the bone marrow, cord blood, Wharton's jelly, etc. and spermatogonial stem cells (SSCs) in the testes. HSCs undergo symmetric cell divisions to maintain themselves but whether they undergo asymmetric cell divisions remains elusive18. Ting et al19 have found that the endocytic protein Ap2a2 possibly regulates asymmetric cell renewal of HSCs since it enhances HSC function without any substantial increase in HSC numbers and that the protein was asymmetrically distributed during cell division. However, more work needs to be done to prove this conclusively18. Further, like HSCs, the testicular SSCs undergo symmetric cell divisions20. If both HSCs and SSCs undergo symmetric cell divisions, which are the stem cells in these tissues that undergo asymmetric cell division? Are we missing out on them? At the same time it has been reported that various adult body organs (gut epithelium, hair, bone marrow, skin, etc.) harbour two stem cell populations including a relatively quiescent and actively dividing population21,22,23.
There is yet another rapidly expanding body of literature that needs to be acknowledged. This is the presence of very small embryonic-like stem cells (VSELs) in adult body organs and have been described by different names such as ELH (elutriation, lineage depletion and the ability to home to bone marrow) cells, spore cells, MAPCs (multipotent adult progenitor cells), MIAMI (marrow-isolated adult multineage-inducible cells), Muse cells (multilineage-differentiating stress-enduring stem), etc24. VSELs first reported by Ratajczak's group25, are believed to be the primordial germ cells (or their precursors) that arise from the embryonic germ cells in the yolk sac and in addition to migrating along the dorsal mesentery and settling in the gonadal ridge to differentiate into germ cells these migrate and settle in various body organs and persist throughout life. VSELs are extremely quiescent in nature and studies done using mouse bone marrow VSELs have shown that IGF-1 mediated growth factor signaling pathways play a crucial role in their quiescence26, these are mobilized under disease condition, give rise to three germ layers in both mice27 as well as in humans28, and have been proposed to be the embryonic remnants that could result in various cancers29. We have reported spontaneous differentiation of ovary surface epithelial cells into oocyte-like structures30 and also that testicular cell suspension from a busulphan treated mouse testes spontaneously differentiating into sperm (unpublished data). Both had VSELs in the initial culture whereas similar success of differentiating hES cells into gametes in vitro has not yet been achieved. Reasons for this remarkable potential of VSELs over hES/iPS cells have been recently reviewed31. We have also demonstrated that VSELs regenerate the adult mouse pancreas after partial pancreatectomy32. However, the scientific community at large is not yet convinced by the existence of VSELs. This has resulted mainly because of their very small size and tendency to get discarded as debris during processing since these cells do not easily settle down on centrifugation33. The recent report34 casted serious doubts on the very presence of VSELs. However, Ratajczak's group explained the technical reasons that could lead to mistaken results by others23,35.
Initial work from our group resulted in the derivation of two hES cell lines KIND1 and KIND-23, we studied their propensity36, adapted both the cell lines to feeder-free conditions and established directed differentiation protocols to make pancreatic37 and tripotent cardiovascular progenitors38. At present, pre-clinical evaluation of safety, efficacy and feasibility of these progenitors is being studied in animal models. Working with ES cells taught us the meaning of pluripotency and importance of transcription factors OCT-4, NANOG and SOX2 as the ‘Triumvirate of Pluripotency’. Of the three, OCT-4 appears to be crucial (especially the OCT-4 transcript which is expressed in the nucleus) as it belongs to Octamer class of transcription factors that recognize 8bp DNA site with the consensus ATGCAAAT. Along with Pit and Unc protein, OCT defines the POU class of transcription factors that interact with DNA. OCT-4 is crucial for pluripotency and self-renewal and silencing OCT-4 results in differentiation of ES cells39,40. OCT-4 is also crucial to re-establish pluripotency in somatic cells as one of the main ‘Yamanaka factors’41. OCT-4 biology has confused stem cell biologists as they failed to discriminate between various transcripts of Oct-4 and this has led to a lot of mix-up42,43.
Using a polyclonal OCT-4 antibody and specific primers for Oct-4A and for OCT -4 (comprising OCT-4A, Oct-4B/B1) we have demonstrated the presence of two distinct cell types expressing OCT-4 in adult human testis44, ovary39,45, pancreas32, cord blood, cord tissue and bone marrow33 including nuclear expression in VSELs and cytoplasmic OCT-4 in slightly bigger cells. The slightly bigger cells are the tissue specific progenitors that arise from the VSELs and cytoplasmic OCT-4 gradually disappears as the cells undergo further differentiation. Thus pluripotent VSELs that exist in various adult body organs are expected to be similar but the progenitors that arise are tissue-specific. The VSELs are invariably discarded along with the red blood cells during cord blood banking and processing bone marrow samples for autologus use33.
Ratajczak's group has shown that total body irradiation completely destroys the HSCs in mice whereas the VSELs survive and have the ability to proliferate as evident from BrdU uptake46. Similarly, we have observed that chemotherapy destroys actively dividing germ cells in both ovary and testis; however, the VSELs persist in the gonads47,48. These results suggest that VSELs are relatively quiescent (dormant) stem cells in the body organs whereas the HSCs, OGSCs (ovarian germ stem cells) and SSCs are the actively dividing (restless) progenitors that arise from the VSELs. Then what are the adult stem cells? The existing terminology appears to be a misnomer! The adult body organs harbour nuclear OCT-4 positive, relatively quiescent VSELs that resist oncotherapy and actively dividing progenitors with cytoplasmic OCT-4. The progenitors are tissue specific and differ based on their location (somatic microenvironment or the niche), in testis these are the SSCs, in ovary these are the OGSCs, in bone marrow HSCs whereas in the Wharton's jelly these are the MSCs. Thus the understanding based on differential expression of nuclear OCT-4 in VSELs and cytoplasmic OCT-4 in the progenitors has led to better understanding of stem cells biology49. We have also observed that under normal conditions it is the cytoplasmic OCT-4 positive progenitors that expand in number to maintain tissue homeostasis whereas uncontrolled expansion of nuclear OCT-4 positive VSELs results in testicular tumour50. To summarize, a confusion exists in the basic terminology of stem cells. Are adult stem cells indeed stem cells or just progenitors? As suggested earlier33, we believe that the term ASC is a misnomer, these are progenitors that arise from VSELs which are the true stem cells existing in various adult body organs (Fig. 2).
Fig. 2.
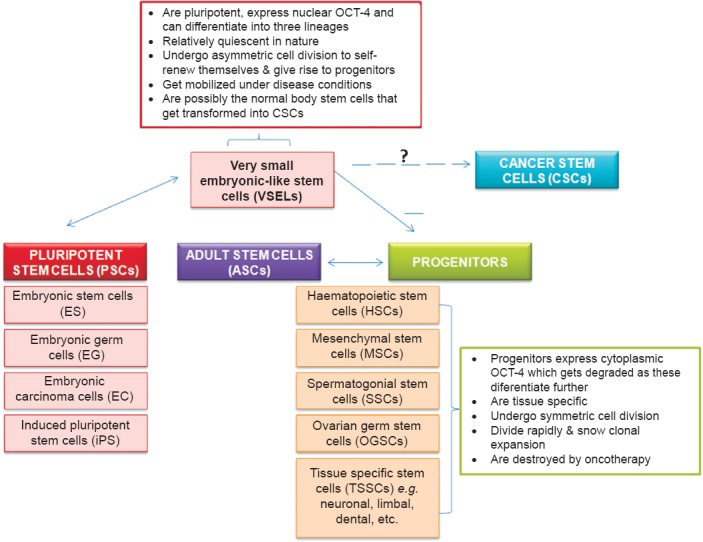
Basic understanding on stem cells and progenitors.
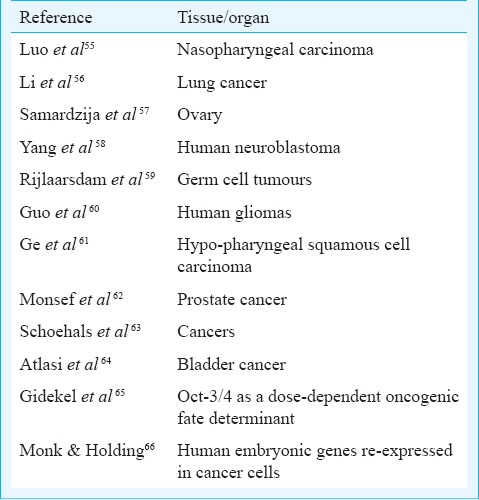
MSCs derived from adult bone marrow are considered to be pluripotent and several studies show their ability to trans-differentiate into all three germ layers51. But pluripotent properties are possibly due to a sub-population of VSELs that exists amongst MSCs49. We believe that the ubiquitous nature of MSCs is because these are the niche forming cells in various adult body organs and these are indeed multipotent rather than pluripotent. Similarly, the claims that testis is the only organ that can undergo spontaneous reprogramming to ES-like status in vitro52 are controversial and the pluripotent ES-like colonies reported in vitro could be because of the presence of VSELs as a sub-population53. Why is the efficiency of derivation of iPS cells so low? Why not all skin fibroblasts get reprogrammed to embryonic state? It is likely that VSELs in primary skin fibroblasts culture overcome quiescence and start growing when exposed to reprogramming factors. Although bone marrow transplant is a standard method of care for blood disorders, but it has failed to regenerate other organs during autologus bone marrow stem cell therapy5,6,7. The injected cells in various trials perhaps largely comprised progenitors which did not have the ability to trans-differentiate. This might be the reason why various multi-centre trials world-wide resulted in negative findings5,6,7. During these trials, regenerative potential of VSELs was never evaluated as these were invariably lost during processing33. Total nucleated cells are injected during autologous adult stem cell therapy and limbal epithelial cells are injected during limbal stem cell therapy54 as we still do not know the true identity and how to purify the stem cells in these tissues. Against this backdrop, presence of pluripotent VSELs in various adult body organs needs to be accepted as (i) these have the ability to self-renew and differentiate into various cell types, (ii) their presence can explain asymmetric cell division and (iii) these could be the elusive cancer initiating cells as nuclear OCT-4 has now been reported in several cancers (Fig. 1; Table). VSELs with nuclear OCT-4 per se are not carcinogenic but the changes in their microenvironment alter their behaviour from remaining quiescent to uncontrolled proliferation. Similar views have been published earlier27.
Table.
Expression of pluripotent markers including Oct-4 in various cancers
Our country needs to invest more into VSELs research. India has not yet heavily invested in any particular stem cell type, rather supported research on all of them but has a chance to now lead in the field of VSELs biology. Brain storming is required to facilitate both basic research as well as pilot trials targeting VSELs biology. Also VSELs biology in the field of cancer initiation needs to be deciphered as these stem cells could be the root cause of recurrence (because of their quiescent nature and cancer drugs generally target actively dividing cells) during remission period.
We need to think carefully before allowing mass culture of MSCs in the country. MSCs therapy should not suffer from similar outcome like autologus stem cells therapy5,6,7. Most perplexing is the observation in our laboratory that VSELs are mobilized and increased in numbers (more than 5-folds based on flow cytometry studies) in a streptozotocin treated mouse pancreas (unpublished observation). Then why these stem cells do not regenerate the diabetic pancreas? Do we really need to inject more stem cells in a diabetic pancreas during stem cell therapy or do we need to target the niche or both that will allow restoration of normal biology of endogenous stem cells (VSELs)? It is not easy to decipher well kept secrets of Mother Nature.
To conclude, there is a huge scope for basic research in the field of stem cells biology before translating from the bench to bedside.
References
- 1.Inamdar MS, Venu P, Srinivas MS, Rao K, VijayRaghavan K. Derivation and characterization of two sibling human embryonic stem cell lines from discarded grade III embryos. Stem Cells Dev. 2009;18:423–33. doi: 10.1089/scd.2008.0131. [DOI] [PubMed] [Google Scholar]
- 2.Mandal A, Bhowmik S, Patki A, Viswanathan C, Majumdar AS. Derivation, characterization, and gene expression profile of two new human ES cell lines from India. Stem Cell Res. 2010;5:173–87. doi: 10.1016/j.scr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Kumar N, Hinduja I, Nagvenkar P, Pillai L, Zaveri K, Mukadam L, et al. Derivation and characterization of two genetically unique human embryonic stem cell lines on in-house-derived human feeders. Stem Cells Dev. 2009;18:435–45. doi: 10.1089/scd.2008.0234. [DOI] [PubMed] [Google Scholar]
- 4.Bhansali A, Ashokumar P, Walia R, Bhansali S, Gupta V, Jain A, et al. Efficacy and safety of autologous bone marrow- derived stem cell transplantation in patients with type 2 diabetes mellitus: a randomized placebo-controlled study. Cell Transplant. 2014;23:1075–85. doi: 10.3727/096368913X665576. [DOI] [PubMed] [Google Scholar]
- 5.Francis DP, Mielewczik M, Zargaran D, Cole GD. Autologous bone marrow derived stem cell therapy in heart disease: discrepancies and contradictions. Int J Cardiol. 2013;168:3381–403. doi: 10.1016/j.ijcard.2013.04.152. [DOI] [PubMed] [Google Scholar]
- 6.Nowbar AN, Mielewczik M, Karavassilis M, Dehbi HM, Shun-Shin MJ, Jones S, et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. BMJ. 2014;348:g2688. doi: 10.1136/bmj.g2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freemantle N, Rait G. Trials of autologous bone marrow stem cells for heart disease. BMJ. 2014;348:g2750. doi: 10.1136/bmj.g2750. [DOI] [PubMed] [Google Scholar]
- 8.Chao NJ, Emerson SG, Weinberg KI. Stem cell transplantation (cord blood transplants) Hematology Am Soc Hematol Educ Program. 2004:354–71. doi: 10.1182/asheducation-2004.1.354. [DOI] [PubMed] [Google Scholar]
- 9.Oran B, Shpall EJ. Allele-level HLA cord blood matching matters. Blood. 2014;123:8–9. doi: 10.1182/blood-2013-11-534164. [DOI] [PubMed] [Google Scholar]
- 10.Stavropoulos-Giokas C, Dinou A, Papassavas A. The role of HLA in cord blood transplantation. Bone Marrow Res 2012. 2012 doi: 10.1155/2012/485160. doi: 10.1155/2012/485160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Advancing regenerative medicine. Nat Med. 2014;20:795. doi: 10.1038/nm.3658. [DOI] [PubMed] [Google Scholar]
- 12.Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, Biella G, et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3:e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zwaka TP, Thomson JA. Differentiation of human embryonic stem cells occurs through symmetric cell division. Stem Cells. 2005;23:146–9. doi: 10.1634/stemcells.2004-0248. [DOI] [PubMed] [Google Scholar]
- 14.Jiang FX, Morahan G. Pancreatic stem cells remain unresolved. Stem Cells Dev. 2014;23:2803–12. doi: 10.1089/scd.2014.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–9. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atala A. Human embryonic stem cells: early hints on safety and efficacy. Lancet. 2012;379:689–90. doi: 10.1016/S0140-6736(12)60118-4. [DOI] [PubMed] [Google Scholar]
- 17.Tabar V, Studer L. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet. 2014;15:82–92. doi: 10.1038/nrg3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson J. Do HSCs divide asymmetrically? Blood. 2012;119:2431–2. doi: 10.1182/blood-2012-01-400713. [DOI] [PubMed] [Google Scholar]
- 19.Ting SB, Deneault E, Hope K, Cellot S, Chagraoui J, Mayotte N, et al. Asymmetric segregation and self-renewal of hematopoietic stem and progenitor cells with endocytic Ap2a2. Blood. 2012;119:2510–22. doi: 10.1182/blood-2011-11-393272. [DOI] [PubMed] [Google Scholar]
- 20.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–98. [PubMed] [Google Scholar]
- 21.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–5. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Rosa L, De Luca M. Cell biology: Dormant and restless skin stem cells. Nature. 2012;489:215–7. doi: 10.1038/489215a. [DOI] [PubMed] [Google Scholar]
- 23.Mascré G, Dekoninck S, Drogat B, Youssef KK, Broheé S, Sotiropoulou PA, et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–62. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- 24.Suszynska M, Zuba-Surma EK, Maj M, Mierzejewska K, Ratajczak J, Kucia M, et al. The proper criteria for identification and sorting of very small embryonic-like stem cells, and some nomenclature issues. Stem Cells Dev. 2014;23:702–13. doi: 10.1089/scd.2013.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Ratajczak J, Kucia M. Very small embryonic-like stem cells: characterization, developmental origin, and biological significance. Exp Hematol. 2008;36:742–51. doi: 10.1016/j.exphem.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mierzejewska K, Heo J, Kang JW, Kang H, Ratajczak J, Ratajczak MZ, et al. Genome-wide analysis of murine bone marrow-derived very small embryonic-like stem cells reveals that mitogenic growth factor signaling pathways play a crucial role in the quiescence and ageing of these cells. Int J Mol Med. 2013;32:281–90. doi: 10.3892/ijmm.2013.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–69. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 28.Havens AM, Sun H, Shiozawa Y, Jung Y, Wang J, Mishra A, et al. Human and murine very small embryonic-like cells represent multipotent tissue progenitors, in vitro and in vivo. Stem Cells Dev. 2014;23:689–701. doi: 10.1089/scd.2013.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratajczak MZ, Shin DM, Liu R, Marlicz W, Tarnowski M, Ratajczak J, et al. Epiblast/germ line hypothesis of cancer development revisited: lesson from the presence of Oct-4+ cells in adult tissues. Stem Cell Rev. 2010;6:307–16. doi: 10.1007/s12015-010-9143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parte S, Bhartiya D, Telang J, Daithankar V, Salvi V, Zaveri K, et al. Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells Dev. 2011;20:1451–64. doi: 10.1089/scd.2010.0461. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Bhartiya D, Hinduja I, Patel H, Bhilawadikar R. Making gametes from pluripotent stem cells - a promising role for very small embryonic-like stem cells. Reprod Biol Endocrinol. 2014;12:114. doi: 10.1186/1477-7827-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhartiya D, Mundekar A, Mahale V, Patel H. Very small embryonic-like stem cells are involved in regeneration of mouse pancreas post-pancreatectomy. Stem Cell Res Ther. 2014;5:106. doi: 10.1186/scrt494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhartiya D, Shaikh A, Nagvenkar P, Kasiviswanathan S, Pethe P, Pawani H, et al. Very small embryonic-like stem cells with maximum regenerative potential get discarded during cord blood banking and bone marrow processing for autologous stem cell therapy. Stem Cells Dev. 2012;21:1–6. doi: 10.1089/scd.2011.0311. [DOI] [PubMed] [Google Scholar]
- 34.Abbott A. Doubt cast over tiny stem cells. Nature. 2013;499:390. doi: 10.1038/499390a. [DOI] [PubMed] [Google Scholar]
- 35.Ratajczak MZ, Zuba-Surma E, Wojakowski W, Suszynska M, Mierzejewska K, Liu R, et al. Very small embryonic-like stem cells (VSELs) represent a real challenge in stem cell biology: recent pros and cons in the midst of a lively debate. Leukemia. 2014;28:473–84. doi: 10.1038/leu.2013.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagvenkar P, Pethe P, Pawani H, Telang J, Kumar N, Hinduja I, et al. Evaluating differentiation propensity of in-house derived human embryonic stem cell lines KIND-1 and KIND-2. In Vitro Cell Dev Biol Anim. 2011;47:406–19. doi: 10.1007/s11626-011-9420-9. [DOI] [PubMed] [Google Scholar]
- 37.Pethe P, Nagvenkar P, Bhartiya D. Polycomb group protein expression during differentiation of human embryonic stem cells into pancreatic lineage in vitro. BMC Cell Biol. 2014;15:18. doi: 10.1186/1471-2121-15-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pawani H, Nagvenkar P, Pethe P, Bhartiya D. Differentiation of human ES cell line KIND-2 to yield tripotent cardiovascular progenitors. In Vitro Cell Dev Biol Anim. 2013;49:82–93. doi: 10.1007/s11626-012-9558-0. [DOI] [PubMed] [Google Scholar]
- 39.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–6. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Dai J. Isoforms of OCT4 contribute to the confusing diversity in stem cell biology. Stem Cells. 2010;28:885–93. doi: 10.1002/stem.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liedtke S, Stephan M, Kögler G. Oct4 expression revisited: potential pitfalls for data misinterpretation in stem cell research. Biol Chem. 2008;389:845–50. doi: 10.1515/BC.2008.098. [DOI] [PubMed] [Google Scholar]
- 44.Bhartiya D, Kasiviswanathan S, Unni SK, Pethe P, Dhabalia JV, Patwardhan S, et al. Newer insights into premeiotic development of germ cells in adult human testis using Oct-4 as a stem cell marker. J Histochem Cytochem. 2010;58:1093–106. doi: 10.1369/jhc.2010.956870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhartiya D, Unni S, Parte S, Anand S. Very small embryonic-like stem cells: implications in reproductive biology. Biomed Res Int 2013. 2013 doi: 10.1155/2013/682326. doi:10.1155/2013/682326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ratajczak J, Wysoczynski M, Zuba-Surma E, Wan W, Kucia M, Yoder MC, et al. Adult murine bone marrow-derived very small embryonic-like stem cells differentiate into the hematopoietic lineage after coculture over OP9 stromal cells. Exp Hematol. 2011;39:225–37. doi: 10.1016/j.exphem.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anand S, Bhartiya D, Sriraman K, Patel H, Manjramkar DD. Very small embryonic-like stem cells survive and restore spermatogenesis after busulphan treatment in mouse testis. J Stem Cell Res Ther. 2014;4:216. [Google Scholar]
- 48.Sriraman K, Bhartiya D, Anand S, Bhutda S. Mouse ovarian very small embryonic-like stem cells resist chemotherapy and retain ability to initiate oocyte-specific differentiation. Reprod Sci. 2015 Mar;:16. doi: 10.1177/1933719115576727. pii: 1933719115576727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhartiya D. Are mesenchymal cells indeed pluripotent stem cells or just stromal cells? OCT-4 and VSELs biology has led to better understanding. Stem Cells Int 2013. 2013:547501. doi: 10.1155/2013/547501. doi:10.1155/2013/547501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhartiya D, Sriraman K, Bhutda S, Mundekar A, Mulla S, Modak H. Neonatal exposure to estrogen affects very small ES-like stem cells (VSELs) leading to various pathologies in adults including cancer. J Cancer Stem Cell Res. 2013;1:e1003. [Google Scholar]
- 51.Mundra V, Gerling IC, Mahato RI. Mesenchymal stem cell-based therapy. Mol Pharm. 2013;10:77–89. doi: 10.1021/mp3005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mizrak SC, Chikhovskaya JV, Sadri-ardekani H, Van Daalen S, Korver CM, Hovingh SE, et al. Embryonic stem cell-like cells derived from adult human testis. Hum Reprod. 2010;25:158–67. doi: 10.1093/humrep/dep354. [DOI] [PubMed] [Google Scholar]
- 53.Bhartiya D, Kasiviswananthan S, Shaikh A. Cellular origin of testis-derived pluripotent stem cells: a case for very small embryonic-like stem cells. Stem Cells Dev. 2012;21:670–4. doi: 10.1089/scd.2011.0554. [DOI] [PubMed] [Google Scholar]
- 54.Mariappan I, Maddileti S, Savy S, Tiwari S, Gaddipati S, Fatima A, et al. In vitro culture and expansion of human limbal epithelial cells. Nat Protoc. 2010;5:1470–9. doi: 10.1038/nprot.2010.115. [DOI] [PubMed] [Google Scholar]
- 55.Luo W, Li S, Peng B, Ye Y, Deng X, Yao K. Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PLoS One. 2013;8:e56324. doi: 10.1371/journal.pone.0056324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X, Wang J, Xu Z, Ahmad A, Li E, Wang Y, et al. Expression of Sox2 and Oct4 and their clinical significance in human non-small-cell lung cancer. Int J Mol Sci. 2012;13:7663–75. doi: 10.3390/ijms13067663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samardzija C, Quinn M, Findlay JK, Ahmed N. Attributes of Oct4 in stem cell biology: Perspectives on cancer stem cells of the ovary. J Ovarian Res. 2012;5:37. doi: 10.1186/1757-2215-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang S, Zheng J, Ma Y, Zhu H, Xu T, Dong K, et al. Oct4 and Sox2 are overexpressed in human neuroblastoma and inhibited by chemotherapy. Oncol Rep. 2012;28:186–92. doi: 10.3892/or.2012.1765. [DOI] [PubMed] [Google Scholar]
- 59.Rijlaarsdam MA, van Herk HA, Gillis AJ, Stoop H, Jenster G, Martens J, et al. Specific detection of OCT3/4 isoform A/B/B1 expression in solid (germ cell) tumours and cell lines: Confirmation of OCT3/4 specificity for germ cell tumours. Br J Cancer. 2011;105:854–63. doi: 10.1038/bjc.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo Y, Liu S, Wang P, Zhao S, Wang F, Bing L, et al. Expression profile of embryonic stem cell associated genes Oct4, Sox2 and Nanog in human gliomas. Histopathology. 2011;59:763–75. doi: 10.1111/j.1365-2559.2011.03993.x. [DOI] [PubMed] [Google Scholar]
- 61.Ge N, Lin HX, Xiao XS, Guo L, Xu HM, Wang X, et al. Prognostic significance of Oct4 and Sox2 expression in hypopharyngeal squamous cell carcinoma. J Transl Med. 2010;8:94. doi: 10.1186/1479-5876-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monsef N, Soller M, Isaksson M, Abrahamsson PA, Panagopoulos I. The expression of pluripotency marker Oct 3/4 in prostate cancer and benign prostate hyperplasia. Prostate. 2009;69:909–16. doi: 10.1002/pros.20934. [DOI] [PubMed] [Google Scholar]
- 63.Schoenhals M, Kassambara A, De Vos J, Hose D, Moreaux J, Klein B. Embryonic stem cell markers expression in cancers. Biochem Biophys Res Commun. 2009;383:157–62. doi: 10.1016/j.bbrc.2009.02.156. [DOI] [PubMed] [Google Scholar]
- 64.Atlasi Y, Mowla SJ, Ziaee SA, Bahrami AR. OCT-4, an embryonic stem cell marker, is highly expressed in bladder cancer. Int J Cancer. 2007;120:1598–602. doi: 10.1002/ijc.22508. [DOI] [PubMed] [Google Scholar]
- 65.Gidekel S, Pizov G, Bergman Y, Pikarsky E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell. 2003;4:361–70. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 66.Monk M, Holding C. Human embryonic genes re-expressed in cancer cells. Oncogene. 2001;20:8085–91. doi: 10.1038/sj.onc.1205088. [DOI] [PubMed] [Google Scholar]


