Abstract
Background & objectives:
Since the 2006 massive outbreaks, chikungunya (CHIK) is a major public health concern in India. The aim of this study was to assess envelope specific immune responses in patients with chikungunya infection.
Methods:
This study included 46 hospitalized patients with chikungunya virus infection (encephalitis, n=22, other systemic involvement, OSI, n=12, classical, n=12) and six controls from Ahmedabad city, Gujarat, India. T cell responses and the levels of Th1, pro/ anti-inflammatory cytokines against the CHIK virus envelope antigens were assessed by lymphocyte proliferation assay and by cytometric bead array in flow cytometry, respectively.
Results:
Lymphoproliferative response was uniform among the patients. Comparisons of cytokines revealed significantly higher levels of interleukin (IL)-4 and IL-5 in encephalitis, OSI and classical patients versus controls. The levels of tumour necrosis factor (TNF)-α were higher in classical patients categories compared to the controls. Interferon (IFN)-γ levels were lower in encephalitis patients versus control.
Interpretation & conclusions:
Our findings showed recognition of T cell epitopes on the envelope region of chikungunya virus by all patient categories. Lower level of IFN-γ may be associated with the severity of disease in these patients.
Keywords: Atypical/severe chikungunya infection, cytokines, E2 specific response
Since 2006, chikungunya (CHIK) virus infection has been re-emerging in explosive epidemic forms in Indian Ocean islands and in India1,2, and is rapidly spreading to new regions including Central Africa and Europe3. Chikungunya fever is becoming a public health problem4. The causative agent is an alphavirus belonging to the family Togaviridae5. During the epidemics, a variety of complications and mortality were recorded in both the Reunion islands6 as well as in India7. An estimated 1.38 million people from 11 States in India were severely affected by chikungunya infection by the end of 20068. Mavlankar et al8,9 estimated deaths in India based on mortality experience from Réunion Island using conservative and full estimates. During September to October 2006, it was observed that patients at Ahmedabad, Gujarat, experienced clinical complications of chikungunya infection requiring hospitalizations7. These included mostly the severe neurological syndrome including encephalitis in some cases among adults and elderly and non-neurological other systemic involvement presenting clinically as multi-organ dysfunction syndrome or multi-organ failure7. These atypical clinical presentations could be attributed to the host factors and/or critical mutations in the viral genome. In classical chikungunya infection, the dynamics and role of both humoral as well as T cell immune responses have not been studied in detail. In a study from the Gabonese outbreak of 2007, Wauquier et al4 have shown the involvement of innate immunity with the production of numerous pro-inflammatory mediators in the plasma of acute patients. Similarly, in a study involving patients with acute chikungunya infection, Chow et al10 have reported specific patterns of plasma pro-inflammatory cytokines to be associated with disease severity. Muthumani et al11 have reported the utility of recombinant E1 and E2 proteins in mice as candidate vaccine against chikungunya virus infection. Subsequently, the same group reported the immunogenicity of the envelope protein in non-human primates12. CHIKV envelope-specific T cell responses as well as neutralization antibody responses have been observed in the immunized non-human primates12. These reports have suggested the possibility of an envelope based vaccine against chikungunya disease. Single linear epitope located at the N-terminus of the E2 glycoprotein was reported to be neutralizing and subsequent mice data provided a pre-clinical basis for the design of an effective vaccine against chikungunya13. This study was carried out to assess the cytokine profile and lymphocyte-proliferative responses directed against recombinant E1 and E2 proteins in individuals with different clinical presentations and unusually severe clinical course of chikungunya infection.
Material & Methods
This study was conducted in Ahmedabad, the capital of Gujarat State, India, between September and October 2006. The clinicians observed severe complications of chikungunya infection involving one/more organs in patients admitted to various hospitals in Ahmedabad. Following this information, a team from the National Institute of Virology, Pune, was stationed in Ahmedabad for one month. During this period, 46 individuals admitted in eight tertiary care hospitals (Sterling hospital, Sardar Patel hospital, VS General hospital, Civil General hospital, SCL hospital, Jivraj Mehta hospital, Medisurge hospital, Sushmsha hospital) were investigated. An acute case of chikungunya infection was defined clinically as the hospitalized patient presenting with acute onset of fever and arthralgia recently within 30 days of investigation and found negative for the known causes like malaria, leptospirosis and dengue. Further, a confirmed case of chikungunya was defined as the patient who was found positive for anti-CHIK IgM or RNA in body fluids either in serum or cerebrospinal fluid (CSF). The patients presenting with neurological manifestations indicating encephalopathy manifestations, with or without other systemic manifestations and with the serum or CSF positivity for either anti-CHIK IgM/chikungunya RNA/virus isolation were considered to have suffered from chikungunya encephalitis. The patients with other (non-neurological) systemic involvements (OSI) presented with hepatic, renal, cardiac, respiratory, haemorrhagic or metabolic/electrolytic dysfunction syndromes or any combinations. Classical chikungunya infection was diagnosed in absence of any of the systemic involvements. The current patient population is a small subset of the chikungunya epidemic investigation7. This exploratory study to assess the involvement of host factors was done in parallel in the Civil General hospital itself to identify factors of severity of chikungunya infection.
Based on the clinical manifestations, patients were grouped into three categories: neurological (mostly encephalitis) cases, patients with other (non-neurological) systemic involvements, OSI and classical fever cases with arthralgia. Repeat blood samples were collected from five encephalitis cases, five other systemic involvement cases and one classical chikungunya case. The patient care and/or diagnostics were similar in the eight hospitals. Due to logistical problems the blood samples drawn in most of the patients were less. Hence, the subjects assessed for lymphocyte proliferation assay and cytokine assay were not necessarily the same. The control group consisted of age and sex matched six apparently healthy individuals negative for IgM/IgG anti-chikungunya antibodies and were from the same hospital. Around 5-6 ml of blood sample was drawn from each patient. This study protocol was approved by the Ethics Committee of the National Institute of Virology (NIV), Pune, and informed written consents were obtained from both patients and controls.
Serology & molecular testing: Blood samples were collected in K3-EDTA and plasma was separated within four hours of collection. All the samples were screened for the presence of IgM anti-chikungunya antibodies by the in-house monoclonal antibody based IgM capture ELISA14. Presence of chikungunya virus RNA was carried out by PCR using the primers from the non-structural region according to the previously described protocol2.
Recombinant E1 & E2 antigens15: Structural proteins of chikungunya virus (E1and E2) were cloned and expressed in a bacterial system15. Complete E2 and E1 regions from chikungunya virus isolate (AP 061573) were amplified with primers containing restriction sites and were cloned in pET15b vector (Invitrogen, Life Technologies, Carlsbad, USA). The E1 and E2 clones were sequence confirmed using gene specific and T7 promoter specific primers. The start and stop codons were noted at correct positions and no mutations were observed. Recombinant pET15b plasmids were transformed in Escherichia coli BL21 RIL cells (Promega, WI, USA). The cells were induced at 37°C for two hours with 1mM isopropyl β-D-1-thiogalactopyranoside (IPTG). The expressed E2 and E1 proteins were purified using probond purification resin (Invitrogen, Life Technologies, Carlsbad, USA) with the hybrid purification protocol performed according to the manufacturer's instructions. The purity of the proteins was more than 90 per cent as assessed by SDS-PAGE (sodium dodecyl sulphate polyacrylamide gel electrophoresis).
Preparation of peripheral blood mononuclear cells (PBMCs): Peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood collected in K3-EDTA by Ficoll-Hypaque density gradient centrifugation. Cells were re-suspended in RPMI 1640 medium (Invitrogen, Carlsbad, USA) supplemented with 2 mmol/l L-glutamine, 1 mmol/l sodium pyruvate and 20 μg/ml of gentamycin.
Lymphocyte proliferation assay (LPA): LPA in the PBMCs of 22 encephalitis cases, 12 OSI cases, 12 classical chikungunya cases and six apparently healthy controls was carried out as previously described16. Briefly, PBMCs were cultured in quadruplicate (1x105 cells/well) in 96-well flat-bottom microtiter plates (Nunc, New York, USA) at 37°C with 5 per cent CO2. Cells cultured with media/phytohemmaglutinin (PHA) (5 μg/ml) (Sigma, USA) alone served as controls. Optimum dose of rE1p and rE2p was titrated to be 18 μg/ml. The cells were pulsed with tritiated thymidine (BARC, Mumbai, India). After 24 h, the DNA incorporated radioactivity was measured by using a liquid scintillation counter (LKB/Pharmacia, Uppasala, Sweden). Activity for each sample was expressed as stimulation index (SI), calculated as the ratio between mean counts per minute (cpm) obtained in the presence and absence of rE1p and rE2p. Samples showing SI values > 3 were considered positive16. For representative number of samples, assays were carried out with CD4 depletion using magnetic beads (BD Biosciences, San Diego, CA, USA).
Cytokine assay and estimation: Cytokine assay was carried out in 11 encephalitis cases, nine OSI cases, four classical chikungunya cases and in six healthy controls as previously described17. Briefly, PBMCs of the patients and the control individuals were washed and re-suspended in RPMI 1640 supplemented with 10 per cent heat-inactivated foetal calf serum (Invitrogen Carlsbad, USA). PBMCs at a concentration of 1x106 cells/ml were set up in 6-well plate (Nunc, Denmark). Cells were stimulated with rE2p protein. Optimum dose and optimum time for rE2p were standardized to be 18 μg/ml and 72 h, respectively. The cells were incubated at 37°C with 5 per cent CO2. The cytokine levels were measured in duplicate from the culture supernatants. Human Th1/Th2 CBA Cytokine Kit (Cytometric Bead Array, Becton-Dickinson, San Jose, CA, USA) having six analytes, i.e. interferon (IFN)-γ, tumour necrosis factor (TNF)-α, interleukin (IL)-10, IL-5, IL-2 and IL-4 was used for the estimation of cytokines from culture supernatants using flow cytometer (BD Facs Calibur system, Becton, Dickinson, Singapore). The individual standard curve range for a given cytokine defines the minimum and maximum quantifiable level using the BD CBA Human Th1/Th2 Cytokine Kit (i.e. 20 and 5000 pg/ml). For assessment of specificity, the antibody pairs used in the assay were screened with their specific cytokines. Analysis of samples containing only a single recombinant cytokine protein found no cross-reactivity or background detection of cytokine in other Capture Bead populations using this assay. Data were analyzed using Cell Quest Pro software (Becton Dickinson, Singapore). Assays with rE1 protein were not done in most samples due to insufficient number of PBMCs.
Statistical analysis: As distribution of cytokine levels was not normal, the inter- and intra-group comparisons were done by Mann-Whitney test using SPSS11.0 software (SPSS Inc., Chicago, IL, USA). For all analyses, P<0.05 derived from a two-tailed test was considered to be significant.
Results
Table I depicts demographic features, medical details and serological/molecular diagnostic marker status of the three categories of the patients investigated. Blood samples were collected from the patients at the time of admission, i.e. 1-30 days post onset of illness (POD). It was 3-30 PODs for encephalitis cases, 4-30 PODs for OSI cases and 1-30 PODs for the classical CHIK cases. Among the underlying medical conditions, type II diabetes and hypertension were the most prevalent co-morbid conditions (15.2% in both). Male to female ratio was 31:15. Mean age was 56±18.3 yr. Mean age of the encephalitis cases was 61 yr (range: 8 to 85 yr), of other systemic involvement cases was 68 yr (range: 44 to 87 yr), whereas that of classical fever cases was 46.6 yr (range: 36 to 75 yr). Seventeen of 46 cases had underlying medical conditions; seven of them had more than two underlying medical conditions. Ten of the 22 encephalitis cases, five of 12 other systemic involvement cases and two of 12 classical fever cases had underlying medical conditions. Twenty one out of 22 encephalitis cases were positive for IgM anti-chikungunya antibodies, the remaining patient was positive for chikungunya RNA alone. One patient positive for anti-CHIK IgM was also positive for chikungunya RNA. Of the 12 OSI cases, 11 were positive for anti-CHIK IgM while the remaining one was positive for CHIK RNA and negative for anti-CHIK IgM. Seven of 12 classical chikungunya cases were positive for anti-CHIK IgM while the remaining five chikungunya RNA positive alone. Five of 22 encephalitis cases and one of 12 OSI cases died. No mortality was recorded in classical chikungunya cases. Among the fatal cases, three had underlying medical conditions (Table I).
Table I.
Demographic features, clinical status, serological and molecular profiles of patients (n=46) with chikungunya infection
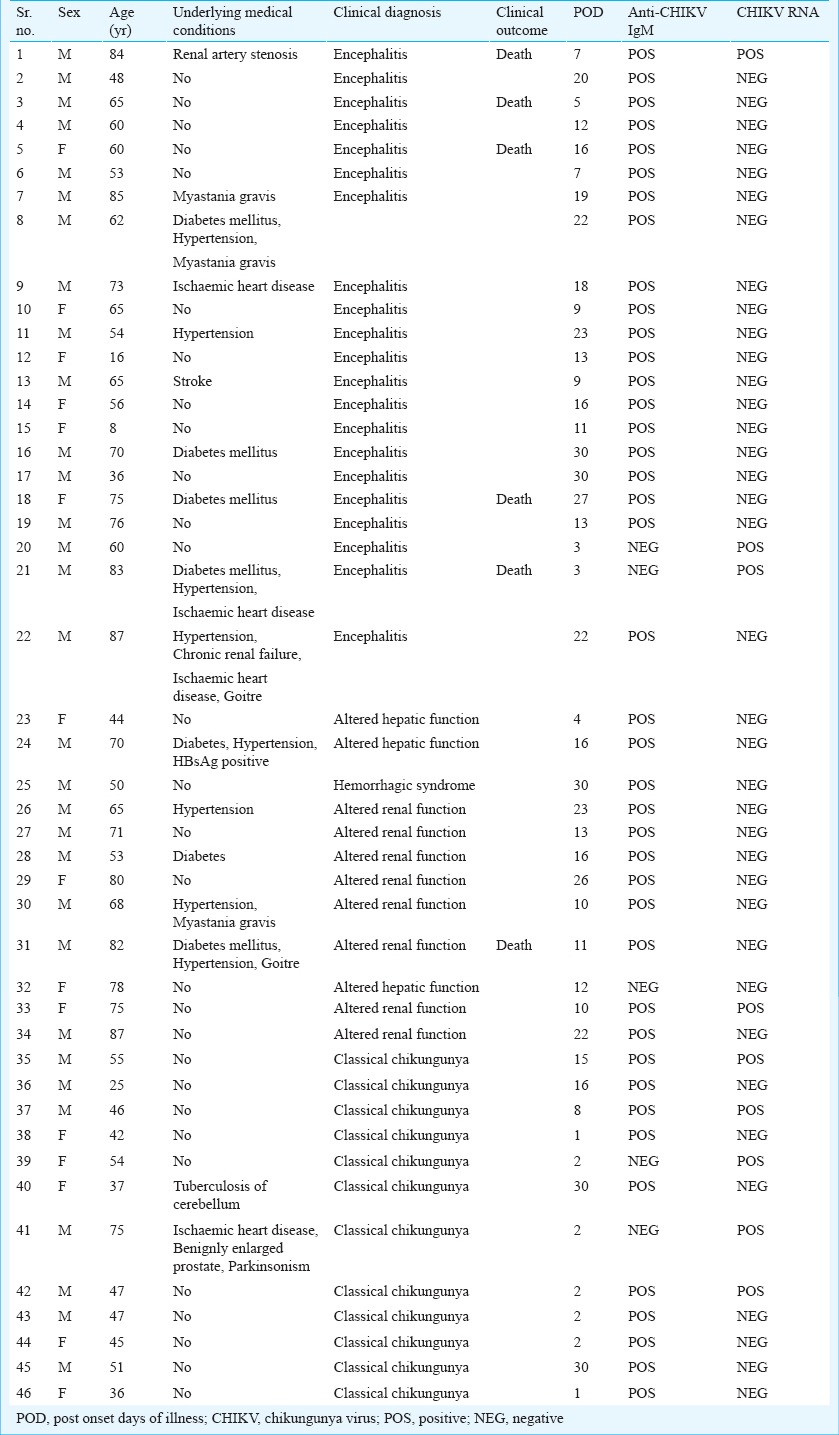
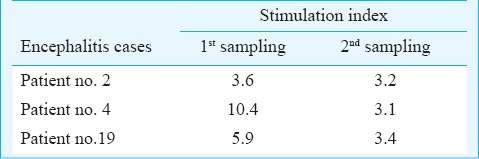
Lymphocyte proliferative responses to rE1 and rE2 proteins: The lymphocytes of 0/11 and 6/21 (0 and 28.5%) encephalitis cases, 1/6 and 4/12 (6.6 and 33.3%) OSI cases and 3/10 and 6/12 (30 and 50%) classical chikungunya cases could recognize rE1p and rE2p, respectively. Five of the six classical fever cases responding to rE2p were not having any underlying medical condition at the time of chikungunya infection. Among the responders, repeat samples were available for three encephalitis cases and all of them responded to the antigen, rE2p (Table II). None and one of four fatal encephalitis cases had proliferative response against rE1p and rE2p, respectively. The single death case from OSI group was a non-responder to both the antigens. None of the six control individuals responded to rE1p and rE2p.
Table II.
Lymphoproliferative response to rE2 protein in sequential chikungunya encephalitis patients
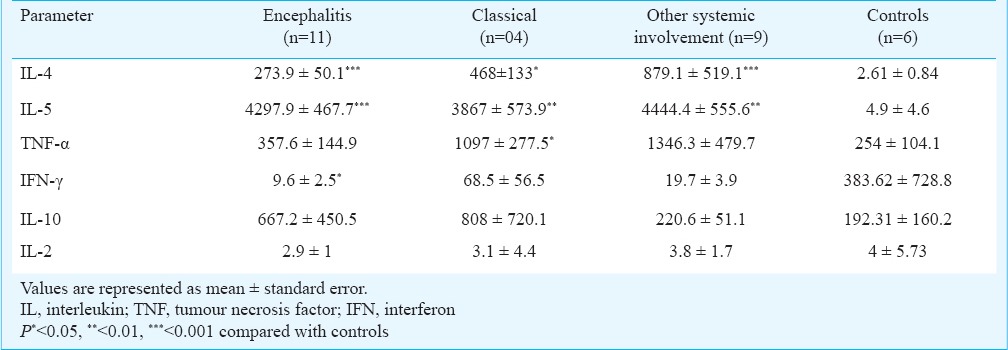
Due to insufficient number of PBMCs, rE1p induced cytokine assays were carried in very few samples. Hence the results of only rE2p induced cytokines were considered. The levels of IL-4 and IL-5 in all the patient categories were significantly elevated compared to the controls but comparable among patient categories. The levels of TNF-α were comparable in all the patient categories compared with the controls except in classical infection (P=0.02; Table III). The differences in the levels of Th1 cytokine IL-2 and anti-inflammatory cytokine IL-10 in the encephalitis patients, OSI, classical cases were insignificant in comparison to the control group as also among all the three patient categories. The level of IFN-γ was significantly (P<0.05) lower in encephalitis group compared to the control group and was significantly lower among the patient categories (P<0.05).
Table III.
Cytokine levels in culture supernatants of peripheral blood (PBMCs) stimulated with chikungunya virus rE2p in different categories of chikungunya patients and controls
Based on the post onset days of illness, all the patients were categorized into two groups: 1-14 days, acute infection, and ≥ 15 days convalescence/recovering and their cytokine levels were compared using Mann-Whitney test. There was no difference in cytokine levels in these two groups.
Discussion
On receiving information about the hospitalization of patients with primary chikungunya virus infection with a variety of complications, a team from NIV, Pune visited Ahmedabad to assess the involvement of chikungunya virus envelope specific T cell response/ cytokine profiles with the differential clinical presentations in patients. Similar to a report by Simon et al18 fever with painful arthralgia was present in all our patients. Except for one patient, the five fatal cases were elderly (60-84 yr) and three of them had underlying illness supporting by the findings of Borgherini et al19. Chikungunya probably played an indirect triggering role in these fatalities19.
Since the PODs and age were not significantly different among the three categories of patients, the differences in cytokine levels were not related to collection of samples at different time points/ age related factors. Hence, it was assumed that the variations in cytokine profiles could largely be attributed to acute chikungunya infection. In the absence of lymphoprolifarative response against rE1p in the encephalitis and other systemic involvement patients, it is difficult to comment on the association of the same with disease severity. Uniform lymphoprolifarative responses against rE2p in a uniform manner suggested the recognition of T cell epitopes on the envelope region of chikungunya virus by all the three categories of patients.
In a study by Ng et al20 the levels IL-4 were reported to be marginally increased in chikungunya fever group compared to those in the uninfected group. The upregulation of IL-4 in the first few days after symptom onset has been shown to be associated with the production of CHIKV-specific IgG3. This supports our observation of elevation of IL-4 levels in all categories of patients suggesting the skewing of host immunity towards a Th2 profile. Chaaitanya et al21 showed an elevation of IL-5 levels in patients who suffered and recovered from chikungunya infection and suggested that IL-5 might have a function in bone homeostasis, although it is not clear whether this cytokine has a direct effect or if it is mediated through eosinophils or B cells21. Ng et al20 also reported that the levels of IFN-α, IL-5, IL-6, IL-7, IL-10 and IL-15 were elevated in chikungunya infection. In a study on hospitalized patients from Reunion Island, Hoarau et al22 have reported a rapid innate immune antiviral response demonstrated by robust dendritic/NK/CD4/CD8 cell activation accompanied by a weak Th1/Th2 cytokine response. Low levels of TNF-α and IL-4 were reported in a few patients. This is surprising because these cytokines have been implicated in the development of joint pathologies, a common feature of chikungunya infection. Wauquier et al3 reported comparable levels of IL-5 and TNF-α among the patients and controls. The same group showed a significant increase in IFN-γ and IL-4 levels up to days 4 and 7, respectively suggesting the involvement of cellular responses in chikungunya infection3. Low levels of TNF-α, IFN- γ and IL-5 were reported in the initial acute phase chikungunya patients of the Italian outbreak that significantly increased at 6 months and 12 months follow up23. This suggests that production of these cytokines is a late event, and explains persistence of arthralgia after the virus disappearance from the circulation. Protective involvement of TNF-α and IFN- γ have been reported in neurotropic West Nile virus (WNV)24. Shrestha et al24 have reported that TNF-α likely enhances the clearance of WNV infection by facilitating migration or accumulation of protective leukocytes in the CNS. Experiments carried out in mice model by the same group have suggested a protective role of TNF-α during WNV infection23. TNF-α appears to function by regulating steps that lead to a decrease in viral burden and improvement in survival. Comparable levels of TNF-α in all categories of patients of the current study need further exploration. Alack of IFN-γ production has been shown to result in increased vulnerability to lethal neurotropic WNV infection in mice, with a rise in mortality and a decrease in the average survival time, suggesting that the dominant protective role of IFN-γ against WNV is antiviral in nature25. Significantly lower levels of IFN-γ in the severe encephalitis patients of the current study go hand in hand with the above observation. Insignificant difference in the levels of IL-2 in patients with different PODs and different clinical manifestations was similar to a study by Kelvin et al23 who reported a lack of association of the same cytokine with acute or convalescence phase of the disease. No difference in the cytokine levels between the two groups of patients based on the PODs of illness in the current study suggests the need of an in depth study in more number of patients from each category. However, lower levels of IFN-γ in encephalitis group vs controls suggest lack of association of this pro-inflammatory cytokine with the severity in chikungunya infection.
Knowing the sudden explosive nature of chikungunya outbreaks in the absence of any vaccine and unpreparedness of the healthcare system to tackle the same, it will be of interest to confirm the findings of the current study in a larger and longitudinal study group. Since timely induction of these pro-inflammatory molecules might be crucial for preventing severe form of chikungunya infection; this study may have important implications towards treatment of chikungunya virus infection.
Acknowledgment
The authors thank the Director, National Institute of Virology, Pune for the facilities provided, Dr S.N. Ranadhive for the recombinant chikungunya antigens, and all the staff members of B.J. Medical College and Hospital, Ahmedabad, Gujarat for co-operation. Statistical support received from Shri Walimbe is acknowledged. This study was funded by the Indian Council of Medical Research (ICMR), New Delhi.
References
- 1.Yergolkar PN, Tandale BV, Arankalle VA, Sathe PS, Sudeep AB, Gandhe SS, et al. Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis. 2006;12:1580–3. doi: 10.3201/eid1210.060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arankalle VA, Shrivastava S, Cherian S, Gunjikar RS, Walimbe AM, Jadhav SM, et al. Genetic divergence of Chikungunya viruses in India (1963-2006) with special reference to the 2005-2006 explosive epidemic. J Gen Virol. 2007;88:1967–76. doi: 10.1099/vir.0.82714-0. [DOI] [PubMed] [Google Scholar]
- 3.Powers AM, Brault AC, Tesh RB, Weaver SC. Reemergence of chikungunya and o’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81:471–9. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 4.Wauquier N, Becquart P, Nkoghe D, Padilla C, Ndjoyi- Mbiguino A, Leroy EM. The acute phase of Chikungunya virus infection in humans is associated with strong innate immunity and T CD8 cell activation. J Infect Dis. 2011;204:115–23. doi: 10.1093/infdis/jiq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porterfield JH. Antigenic characteristics and classification of the Togaviridae. In: Schlesinger R, editor. The togaviruses. New York: Academic Press; 1980. pp. 13–46. [Google Scholar]
- 6.Economopoulou A, Dominguez M, Helynck B, Sissoko D, Wichmann O, Quenel P, et al. Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005-2006 outbreak on Reunion. Epidemiol Infect. 2009;137:534–41. doi: 10.1017/S0950268808001167. [DOI] [PubMed] [Google Scholar]
- 7.Tandale BV, Sathe PS, Arankalle VA, Wadia RS, Kulkarni R, Shah SV, et al. Systemic involvements and fetalities during chikungunya epidemic in India, 2006. J Clin Virol. 2009;46:145–9. doi: 10.1016/j.jcv.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Mavalankar D, Shastri P, Bandyopadhyay T, Parmar J, Ramani KV. Increased mortality rate associated with Chikungunya epidemic, Ahmedabad, India. Emerg Infect Dis. 2008;14:412–5. doi: 10.3201/eid1403.070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavalankar D, Shastri P, Raman P. Chikungunya epidemic in India: a major public-health disaster. Lancet Infect Dis. 2007;7:306–7. doi: 10.1016/S1473-3099(07)70091-9. [DOI] [PubMed] [Google Scholar]
- 10.Chow A, Her Z, Ong EK, Chen JM, Dimatatac F, Kwek DJ, et al. Persistent arthralgia induced by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J Infect Dis. 2011;203:149–57. doi: 10.1093/infdis/jiq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muthumani K, Lankaraman KM, Laddy DJ, Sundaram SG, Chung CW, Sako E, et al. Immunogenicity of novel consensus based DNA vaccine against Chikungunya virus. Vaccine. 2008;26:5128–34. doi: 10.1016/j.vaccine.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallilankaraman K, Shedlock DJ, Bao H, Kawalekar OU, Fagone P, Ramanathan AA, et al. A DNA vaccine against Chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl Trop Dis. 2011;5:e928. doi: 10.1371/journal.pntd.0000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kam YW, Lum FM, Teo TH, Lee WW, Simarmata D, Harjanto S, et al. Early neutralizing IgG response to Chikungunya virus in infected patients targets a dominant linear epitope on the E2 glycoprotein. EMBO Mol Med. 2012;4:330–43. doi: 10.1002/emmm.201200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sitz KV, Birx DL. Lymphocyte proliferation assay. Methods Mol Med. 1999;17:343–53. doi: 10.1385/0-89603-369-4:343. [DOI] [PubMed] [Google Scholar]
- 15.Kumar M, Sudeep AB, Arankalle VA. Evaluation of recombinant E2 protein-based and whole-virus inactivated candidate vaccines against chikungunya virus. Vaccine. 2012;30:6142–9. doi: 10.1016/j.vaccine.2012.07.072. [DOI] [PubMed] [Google Scholar]
- 16.Saravanabalaji S, Tripathy AS, Dhoot RR, Chadha MS, Kakrani AL, Arankalle VA. Viral load antibody titers and recombinant open reading frame 2 protein-induced Th1/Th2 cytokines and cellular immune responses in self-limiting and fulminant hepatitis E. Intervirology. 2009;52:78–85. doi: 10.1159/000214862. [DOI] [PubMed] [Google Scholar]
- 17.Tripathy AS, Das R, Chadha MS, Arankalle VA. Epidemic of hepatitis B with high mortality in India: association of fulminant disease with lack of CCL4 and natural killer T cells. J Viral Hepat. 2011;18:e415–22. doi: 10.1111/j.1365-2893.2011.01457.x. [DOI] [PubMed] [Google Scholar]
- 18.Simon F, Parola P, Grandadam M, Fourcade S, Oliver M, Brouqui P, et al. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean Islands. Report of 47 cases. Medicine (Baltimore) 2007;86:123–37. doi: 10.1097/MD/0b013e31806010a5. [DOI] [PubMed] [Google Scholar]
- 19.Borgherini G, Poubeau P, Staikowsky F, Lory M, Le Moullec N, Becquart JP, et al. Outbreak of Chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clin Infect Dis. 2007;44:1401–7. doi: 10.1086/517537. [DOI] [PubMed] [Google Scholar]
- 20.Ng LF, Chow A, Sun YJ, Kwek DJC, Lim PL, Dimatatac F, et al. IL-1b, IL-6, and RANTES as biomarkers of Chikungunya severity. PLoS One. 2009;4:e4261. doi: 10.1371/journal.pone.0004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaaitanya IK, Muruganandam N, Sundaram SG, Kawalekar O, Sugunan AP, Manimunda SP, et al. Role of proinflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral Immunol. 2011;24:265–71. doi: 10.1089/vim.2010.0123. [DOI] [PubMed] [Google Scholar]
- 22.Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184:5914–27. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 23.Kelvin AA, Banner D, Silvi G, Moro ML, Spataro N, Gaibani P, et al. Inflammatory cytokine expression is associated with Chikungunya virus resolution and symptom severity. PLoS Negl Trop Dis. 2011;5:e1279. doi: 10.1371/journal.pntd.0001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrestha B, Zhang B, Purtha WE, Klein RS, Diamond MS. Tumor necrosis factor alpha protects against lethal West Nile virus infection by promoting trafficking of mononuclear leukocytes into the central nervous system. J Virol. 2008;82:8956–64. doi: 10.1128/JVI.01118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shrestha B, Wang T, Samuel MA, Whitby K, Craft J, Fikrig E, et al. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J Virol. 2006;80:5338–48. doi: 10.1128/JVI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


