Sir,
Foodborne gastroenteritis outbreaks due to non-typhoidal Salmonella represent important public health problem globally1. In the last two decades, Salmonella enterica serovar Weltevreden (S. Weltevreden) has emerged as a dominant foodborne pathogen globally especially in South-East Asian countries, being increasingly isolated from vegetables, meat and seafoods1,2,3,4,5,6. S. Weltevreden associated foodborne outbreaks of gastroenteritis have been reported from Scandinavian countries, Réunion Island (France) and India in the past7,8,9,10. Although, the complete genome sequence of S. Weltevreden outbreak strain 2007-60-3289-1 isolated in Scandinavia was available in EMBL nucleotide sequence database7, the implicating agent of S. Weltevreden associated outbreaks in India have not been characterized earlier.
Here we report occurrence of S. Weltevreden associated foodborne gastroenteritis outbreak among student staying in a hostel at Pune, Maharashtra, India, and characterization of the isolates by antimicrobial susceptibility pattern, plasmid profiling and typing, pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST).
On January 19, 2010, 150 students aged between 20-30 yr had consumed food comprising milk, rice, sprouted pulses and curd from their hostel canteen for lunch in Pune, Maharashtra. Almost all of them developed acute watery diarrhoea within 12 h of having food, associated with fever, abdominal cramps, nausea and vomiting. Most of them were immediately admitted to the local private hospitals (Aditya Birla Group Hospital) for treatment. Stool samples (n=25) were collected from the admitted patients, processed by standard microbiological method and the isolated organism (n=14) were sent to B. J. Medical College, Pune, for identification. The patients were discharged following treatment with either ciprofloxacin metronidazole combination or ceftriaxone alone. Food samples were not available to identify the source of infection due to delay (>12 h) in carrying out the investigation. A total of nine isolates, provisionally identified as Salmonella spp. were sent to National Institute of Cholera and Enteric Diseases (NICED), Kolkata, for confirmation of identification and characterization.
At NICED, the Salmonella isolates were identified by standard biochemical tests, followed by serotyping using Salmonella specific poly- and monovalent O and H antisera (Denka Seiken Pvt. Ltd, Tokyo, Japan) and nomenclature was done following Kauffmann White Scheme11. The isolates were tested for their antimicrobial susceptibility using 17 antibiotic discs (BD BBL™, Marryland, USA); ampicillin, chloramphenicol, tetracycline, co-trimoxazole, nalidixic acid, ciprofloxacin, norfloxacin, ofloxacin, gentamicin, amikacin, streptomycin, cefotaxime, ceftazidime, ceftriaxone, aztreonam, azithromycin and erythromycin, by disc diffusion method and results were interpreted following CLSI (Clinical and Laboratory Standards Institute) guidelines12. Escherichia coli ATCC 25922 was used as control. Plasmid DNA was extracted by Kado and Liu method13, electrophoresed on 0.8 per cent agarose gel and stained with ethidium bromide to determine the plasmid profiles. E. coli V517 was used as plasmid molecular weight marker. The plasmid incompatibility type was determined by PCR using published primers followed by sequencing14.
DNA fingerprinting was performed by PFGE in CHEF-DRIII system (BioRad Laboratories, Hercules, CA, USA), following standard PulseNet one-day protocol (http://www.cdc.gov/pulsenet/PDF/ecoli-shigella-salmonella-pfge-protocol-508c.pdf) and using Salmonella Braenderup H9812 as control. The PFGE profiles were analyzed using FP Quest™ software version 4.5 (Bio-Rad, CA, USA). The extent of homology was determined by Dice coefficient, and clustering was based on unweighted pair group method with arithmetic mean (UPGMA)15. The isolates with ≥ 90 per cent similarity threshold were grouped under one cluster.
Phylogenetic relatedness of the isolates was studied by MLST. The amplification of the seven housekeeping genes followed by sequencing was done as previously described16. Sequences obtained were submitted to the MLST database (http://mlst.ucc.ie/mlst/dbs/Senterica) to assign sequence type (ST) of each isolate based on the set of allele types derived from all the seven loci.
Two local S. Weltevreden isolates from sporadic diarrhoea cases in Kolkata were also included in the study and characterized for comparison.
Out of nine outbreak isolates, eight were identified as S. Weltevreden. All the study isolates (Pune outbreak and Kolkata sporadic) were uniformly susceptible to the 17 antimicrobials tested. Pan susceptible S. Weltevreden isolates have earlier been reported from outbreaks in France (2007) and Mangalore (2008)8,9. However, isolation of multi-drug resistant S. Weltevreden (resistant to ampicillin, streptomycin, co-trimoxazole, erythromycin and tetracycline) has also been reported from environmental samples in different countries2,3,4,5,6.
Single plasmid of 63kb size belonging to IncFIIS type was observed in all the study isolates. Earlier studies have documented presence of single or more plasmids ranging from <2 to 89.1 kb sizes5. The plasmids of type IncFIIS are considered as virulence plasmids in Salmonella14, but its role in disease pathogenesis is yet to be established.
The Pune outbreak (A) and Kolkata sporadic (B) isolates formed distinct clusters in PFGE (Figure). All the outbreak isolates were found to be clonal (100%) and had 87 per cent similarity with the sporadic isolates. MLST database characterized all the study isolates as ST1500 [Clonal complex (CC) type 205] based on their sequence data. The allelic types of the seven genes were aroC: 130, dnaN: 97, hemD: 25, hisD: 125, purE: 422, sucA: 9, and thrA: 101. The current data have been entered in the MLST database and are available at the website (http://mlst.ucc.ie/mlst/dbs/Senterica/GetTableInfo_html). S. Weltevreden human isolates from Sri Lanka, Australia, Indonesia, Zimbabwe, Philippines and Germany belonging to ST365 and that of an environmental isolate from UK belonging to ST1500 were available in the aforesaid MLST database. This report showed that the foodborne outbreak caused by Indian S. Weltevreden isolates belonged to ST1500 (CC205).
Fig.
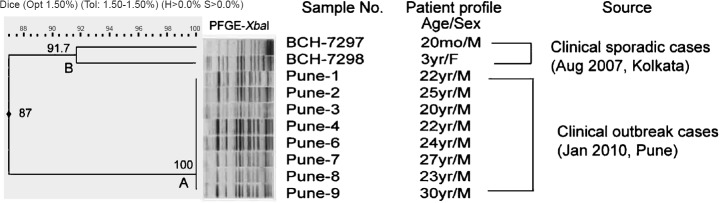
Pulsed field gel electrophoresis (PFGE) profiles of XbaI digest of DNA of S. Weltevreden Pune outbreak isolates, 2010 by cluster analysis and comparison with S. Weltevreden Kolkata sporadic isolates. Band comparison was performed using Dice coefficient with 1.50 per cent optimization (Opt) and 1.50 per cent position tolerance (Tol). H, minimum height; S, minimum surface; mo, months; M, male; F, female.
The use of molecular methods and antibiotic susceptibility profiles for characterization of Salmonella outbreak isolates in various geographical regions is useful for studying global epidemiology of the organism which might result in controlling further spread of the organism.
Acknowledgment
The study was supported by the Indian Council of Medical Research (ICMR) intramural fund. ICMR-SRF to the first author (PJ) and technical assistance by NICED staff are acknowledged. Authors also thank the Curator of the MLST database (http://mlst.ucc.ie), which is currently supported by a grant from the Science Foundation of Ireland (05/FE1/B882) for enabling us to submit our data.
References
- 1.World Health Organization Global Salm-Surv. Progress Report (2000-2005): Building capacity for laboratory-based foodborne disease surveillance and outbreak detection and response. WHO. 2006. [accessed on July 7, 2012]. Available from http://www.who.int/salmsurv/en/
- 2.Aarestrup FM, Lertworapreecha M, Evans MC, Bangtrakulnonth A, Chalermchaikit T, Hendriken RS, et al. Antimicrobial susceptibility and occurrence of resistance genes among Salmonella enterica serovar Weltevreden from different countries. J Antimicrob Chemother. 2003;52:715–8. doi: 10.1093/jac/dkg426. [DOI] [PubMed] [Google Scholar]
- 3.Sirichote P, Bangtrakulnonth A, Tianmanee K, Unahalekhaka A, Oulai A, Chittaphithakchai P, et al. Serotypes and antimicrobial resistance of Salmonella enterica ssp. in central Thailand, 2001-2006. Southeast Asian J Trop Med Public Health. 2010;41:1405–15. [PubMed] [Google Scholar]
- 4.Thong KL, Goh YL, Radu S, Noorzaleha S, Yasin R, Koh YT, et al. Genetic diversity of clinical and environmental strains of Salmonella enterica serotype Weltevreden isolated in Malaysia. J Clin Microbiol. 2002;40:2498–503. doi: 10.1128/JCM.40.7.2498-2503.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponce E, Khan AA, Cheng C, Summage-West C, Cerniglia CE. Prevalence and characterization of Salmonella enterica serovar Weltevreden from imported seafood. Food Microbiol. 2008;25:29–35. doi: 10.1016/j.fm.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Deekshit VK, Kumar BK, Rai P, Srikumar S, Karunasagar I, Karunasagar I. Detection of class 1 integrons in Salmonella Weltevreden and silent antibiotic resistance genes in some seafood-associated nontyphoidal isolates of Salmonella in south-west coast of India. J Appl Microbiol. 2012;112:1113–22. doi: 10.1111/j.1365-2672.2012.05290.x. [DOI] [PubMed] [Google Scholar]
- 7.Brankatschk K, Blom J, Goesmann A, Smits THM, Duffy B. Genome of an European fresh-vegetable food safety outbreak strain of Salmonella enterica subsp. enterica serovar Weltevreden. J Bacteriol. 2011;193:2066. doi: 10.1128/JB.00123-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Ortenzio E, Weill FX, Ragonneau S, Lebon JA, Renault P, Pierre Vl. First report of a Salmonella enterica serovar Weltevreden outbreak on Réunion Island, France, August 2007. Euro Surveill. 2008;13:7–9. [PubMed] [Google Scholar]
- 9.Antony B, Dias M, Shetty AK, Rekha B. Food poisoning due to Salmonella enterica serotype Weltevreden in Mangalore. Indian J Med Microbiol. 2009;27:257–8. doi: 10.4103/0255-0857.53211. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury G, Sarkar A, Pazhani GP, Mukhopadhyay AK, Bhattacharya MK, Ramamurthy T. An outbreak of foodborne gastroenteritis caused by dual pathogens, Salmonella enterica Serovar Weltevreden and Vibrio fluvialis in Kolkata, India. Foodborne Pathog Dis. 2013;10:904–6. doi: 10.1089/fpd.2013.1491. [DOI] [PubMed] [Google Scholar]
- 11.Grimont PAD, Weill FX. 9th ed. Paris: World Health Organization Collaborating Centre for Reference and Research on Salmonella, Institut Pasteur; 2007. Antigenic formulae of the Salmonella serovars. [Google Scholar]
- 12.Wayne, PA: CLSI; 2012. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 22nd Informational Supplement. CLSI document M100-S22. [Google Scholar]
- 13.Kado CI, Liu ST. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–73. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villa L, García-Fernández A, Fortini D, Carattoli A. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J Antimicrob Chemother. 2010;65:2518–29. doi: 10.1093/jac/dkq347. [DOI] [PubMed] [Google Scholar]
- 15.Tatavarthy A, Sanderson R, Peak K, Scilabro G, Davenhill P, Cannons A, et al. Molecular typing and resistance analysis of travel-associated Salmonella enterica serotype Typhi. J Clin Microbiol. 2012;50:2631–8. doi: 10.1128/JCM.00593-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noda T, Murakami K, Asai T, Etoh Y, Ishihara T, Kuroki T, et al. Multi-locus sequence typing of Salmonella enterica subsp. enterica serovar Enteritidis strains in Japan between 1973 and 2004. Acta Vet Scand. 2011;53:38. doi: 10.1186/1751-0147-53-38. [DOI] [PMC free article] [PubMed] [Google Scholar]


