Abstract
In a search for nonpeptide agonists for the neurotensin receptor (NTR1), we replaced the adamantyl amino acid moiety found in the antagonist SR48692 (1a) with leucine and related α-alkylamino acids found in peptide agonists. When tested in a calcium mobilization assay, we found that both d- and l-leucine confer partial agonist activity to the pyrazole scaffold with the l-enantiomer (3a) providing a significantly greater response. A brief SAR survey demonstrated that the observed agonist activity was resilient to changes made to the dimethoxyaryl ring in 3a. The resulting compounds were less potent relative to 3a but showed greater agonist responses. The partial agonist activity was extinguished when the chloroquinoline ring was replaced with naphthalene. Thus, while l-leucine appears to possess a powerful agonist directing affect for the NTR1 receptor, its presence alone in the molecular architecture is not sufficient to insure agonist behavior.
Keywords: Neurotensin, Partial agonist, Antagonist, Nonpeptide
Abuse of methamphetamine represents a major and increasing threat to public health.1 Yet despite many years of research, no pharmacotherapies have been identified for psychostimulant abuse. Neurotensin (NT, pGlu-Leu-Tyr-Glu-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu), a tridecapeptide identified over 30 years ago2 is widely distributed in the central and peripheral nervous system and functions as both a neurotransmitter and neuromodulator.3–6 It is a key player with regard to dopamine control in an area of the brain that is central to the mediation of reward behavior. It is co-localized with mesolimbic dopamine and modulates its transmission,7–9 functionally antagonizing dopamine in the mesolimbic system while increasing dopaminergic transmission in the nigrastriatal system.10,11 It modulates both dopaminergic and glutamatergic inputs to the nucleus accumbens, a region critical to the brains response to psychostimulants.12–14 Such an ability to modulate dopamine has drawn the attention of researchers evaluating the role that NT plays in a number of maladies including schizophrenia and abuse of psychostimulants.15,16 NT receptor peptide agonists and nonpeptide antagonists have both received attention in these efforts. Along similar lines, our interest in identifying pharmacotherapies for methamphetamine abuse directed us to the discovery of nonpeptide small-molecule NT receptor agonists, an area of research that has received little attention over the years.
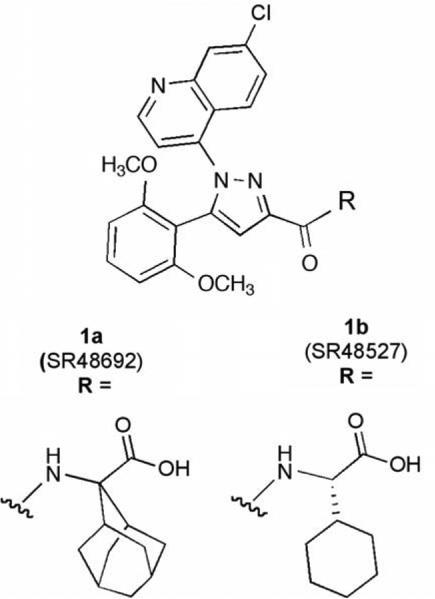
Neurotensin achieves its effects via three receptor proteins, NTR1, NTR2, and NTR3.17–22 The first two are seven-transmembrane domain G-protein-coupled receptors (GPCRs) while the third is a single-transmembrane domain sorting protein. Despite the fact that this receptor system was identified many years ago, very few nonpeptide ligands have been described for any NT receptor.23–25 For NTR1, the most widely studied small-molecule ligands are those related to the antagonist SR48692 (1a, Chart 1) that shows potent antagonist activity at NTR1 and good selectivity versus NTR2.20,26 Despite the paucity of information regarding nonpeptide NT receptor compounds, much information supporting their design was available. Two research groups using point mutation studies have reported ligand-binding site models for the NTR1 receptor.27–30 The model put forth by Barroso et al. and Labbe-Jullie et al. was of particular importance for the development of NTR1 nonpeptide small-molecule agonists as they proposed overlapping binding sites for the peptide agonist neurotensin-(8–13) and the nonpeptide antagonist 1a. This suggested that it might be possible to obtain nonpeptide agonists through modification of the nonpep-tide small-molecule antagonists.27–29
Chart 1.
Standard antagonists for the NTR1 receptor.
All of the receptor modeling studies to date have demonstrated that the terminal amino acid in both the agonist and antagonist ligands is of primary importance to the ligand/receptor interaction. Both ligand types were proposed to anchor on three key residues Arg327 Met,208 and Phe.331 The Arg327 residue was suggested to bind the Leu13 terminal residue in the peptide agonist and the carboxyl group in the nonpeptide antagonist 1a. The side chains of the amino acids were proposed to interact with Met208 and Phe.331 Taken together, these findings strongly suggested that replacement of the amino acids in the small-molecule antagonists (1a and 1b) with those found in peptide agonists, l-leucine and similar l-alkyl-amino acids, was a reasonable strategy for discovery of small-molecule nonpeptide agonists.
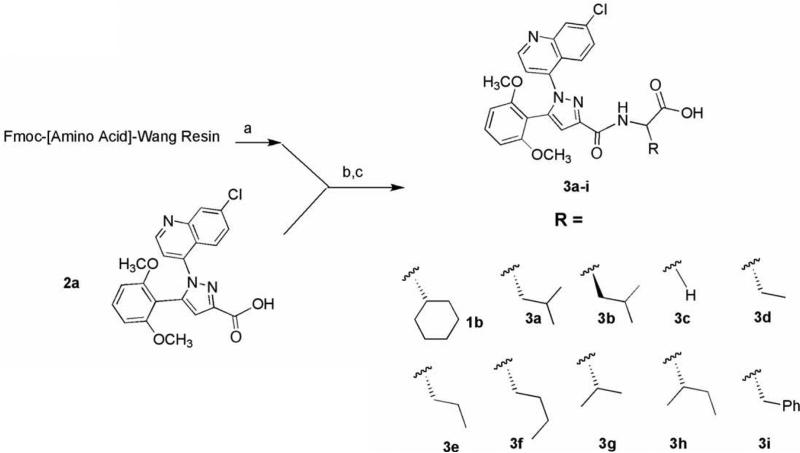
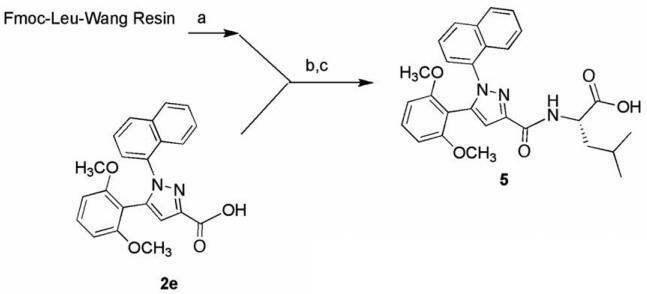
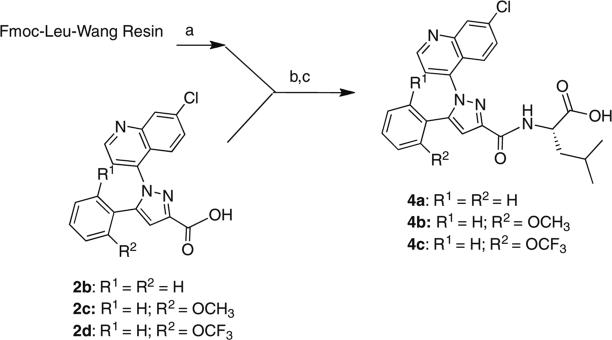
The synthesis of the target compounds described in this study was accomplished by coupling pyrazole carboxylic acids 2a–e with Fmoc-protected amino acids pre-loaded onto Wang resin (Schemes 1–3). The names of the amino acids used to prepare specific target compounds are listed in Table 1. The pyrazole acids 2a–e were prepared according to the method previously described by Labeeuw et al.31 In each synthesis, the resin was first Fmoc-deprotected with piperidine and then coupled to 2a–e using benzotriazole-1-yl-oxytris-(dimethylamino)-phosphonium hexafluorophosphate (BOP) and triethylamine. Cleavage of the target compounds from the resin was accomplished using 95% trifluoroacetic acid in water to give the desired products 3a–i (Scheme 1), 4a–c (Scheme 2), and 5 (Scheme 3) in 60–80% yield.32
Scheme 1.
Reagents: (a) 20% piperidine, NMP; (b) BOP, Et3N, NMP, 95:5, TFA:H2O.
Scheme 3.
Reagents: (a) 20% piperdine, NMP; (b) BOP, Et3N, NMP; (c) 95:5, TFA:H2O.
Table 1.
Fmoc-amino acid Wang resins used in target compound synthesis
| Target compound | Fmoc-[amino acid]-Wang |
|---|---|
| 1b | [l-Cyclohexylglycine] |
| 3a, 4a-c, 5 | [l-Leucine] |
| 3b | [d-Leucine] |
| 3c | [Glycine] |
| 3d | [l-α-aminobutyric] |
| 3e | [l-Norvaline] |
| 3f | [l-Norleucine] |
| 3g | [l-Valine] |
| 3h | [l-Isoleucine] |
| 3i | [l-Phenylalanine] |
Scheme 2.
Reagents: (a) 20% piperdine, NMP; (b) BOP, Et3N, NMP; (c) 95:5, TFA:H2O.
Instead of using a binding assay to test our compounds 3a–i, 4a–c, and 5, we chose to use a high-throughput functional assay. To this end, we generated a CHO-K1 cell line stably expressing NTR1.33 The receptor specificity of agonist activity was determined by the ability of the NTR1 antagonist 1a to block test compound NTR1 activation. For these experiments, the cells were pre-incubated with 1 or 10 mM 1a for 15 min at 37 °C prior to the addition of the test compound at its EC50. The apparent affinity (Ke) of 1a and b and 5 were determined by measuring the EC50 of neurotensin in their presence and absence.34
The screening data obtained from test compounds 3a–i (Scheme 1) is shown in Table 2. As depicted, many of the compounds demonstrated agonist behavior while leucine, both l and d isomers, showed the greatest effect on calcium release. In contrast, the previously described antagonist, compound 1b bearing the amino acid l-cyclohexylglycine, did not stimulate calcium release in our assay. Interestingly, the amount of activity observed varied with the structure of the side chain and the isobutyl group appeared to be preferred. The EC50 data was obtained for compounds 3a and 3b. Compound 3a bearing the l-leucine amino acid, was found to be a partial agonist with an EC50 of 67 nM and an Emax of 51% of NT. Its enantiomer, 3b, also showed partial agonist activity but was significantly less potent (EC50 = 986 nM). Both of these are considerably less potent than NT. Overall, this data set clearly demonstrates that the amino acid in the test compounds 1a, 1b, and 3a–i has a powerful directing effect on the compounds intrinsic behavior and can confer either agonist or antagonist activity depending on its substructure. The NTR1 specificity of the observed calcium release for 3a and 3b was demonstrated by blocking with the NTR1 antagonist (1a) as described earlier (data not shown).
Table 2.
Data obtained for target and standard compounds in an NTR1 agonist-mediated calcium mobilization assay
| Compound | Screena (10 μM) | EC50b (nM ± SEM) | Emax (%NT ± SEM) | Ke (nM ± SEM) |
|---|---|---|---|---|
| NT | 0.23 ± 0.04 | 100±6 | ||
| 1a | 36 ± 10 | |||
| 1b | NAc | 35 ± 9 | ||
| 3a | 55 ±12 | 67 ± 26 | 54 ± 18 | |
| 3b | 57 ±10 | 986 ± 496 | 37 ± 2 | |
| 3c | NA | |||
| 3d | 11 ±1 | |||
| 3e | 18± 4 | |||
| 3f | 20 ± 3 | |||
| 3g | 5±1 | |||
| 3h | NA | |||
| 3i | NA | |||
| 4a | 62 ± 6 | 2043 ± 86 | 56 ± 7 | |
| 4b | 86 ±7 | 220 ± 42 | 81 ± 7 | |
| 4c | 84 ± 7 | 1214 ± 197 | 91 ± 16 | |
| 5 | NA | 93 ± 32 |
Test compounds were screened in triplicate at 10 μM and data is presented as percent stimulation relative to NT.
Data represent means ± SE from at least two independent experiments.
Not active.
Compounds 4a–c were prepared to determine if the agonist directing effect of the l-leucine group in 3a would be resilient to changes made to the substituents in its dimethoxyaryl ring (Scheme 2). Quéré had previously shown that these groups were essential to high affinity binding in 1a.35 As depicted in Table 2, analogs 4a–c retained their partial agonist behavior and, 4b and 4c showed a higher agonist response relative to NT though they all had lower EC50 values. Compound 4a with no methoxy groups was significantly less potent than 3a and also less potent than 4b with a single methoxy group. The lower potency of 4c relative to 4b suggests that the aryl ring prefers a greater degree of electron density. The NTR1 specificity of the observed calcium release for 4a–c was demonstrated by blocking with the NTR1 antagonist (1a) as described earlier (data not shown).
Compound 5 was prepared to determine if the agonist directing effect of the l-leucine group in 3a would be affected with changes made to its chloroquinoline ring (Scheme 3). We replaced the chloroquinoline group in 3a with a naphthalene ring system since it is of similar size and had been shown to produce compounds with high binding affinity.35 Interestingly, this compound (5) showed no agonist activity when screened at 10 mM but instead was found to be an antagonist with an apparent affinity (Ke) of 93 nM for the NTR1 receptor (Table 2). This finding illustrates that the presence of l-leucine in target molecules derived from 1a is insufficient to guarantee that the resulting ligand will display agonist behavior for the NTR1 receptor. When considered together with the data from 4a–c, the results from 5 suggest that each of the groups pendant to the pyrazole core can impact ligand activity which in turn suggests that each of these groups are interacting with the receptor protein, a notion consistent with the receptor modeling studies of Labbe-Jullie et al.29 Taken together, this compound survey demonstrated that changing the amino acid residue in the antagonist 1a to l-leucine (3a) was sufficient to convert from antagonist to partial agonist activity. Beyond this we have shown that this agonist activity was resilient to some (4a–c) but not all (5) changes to the scaffold. We have also shown that the presence of l-leucine alone (5) is insufficient to guarantee partial agonist activity.
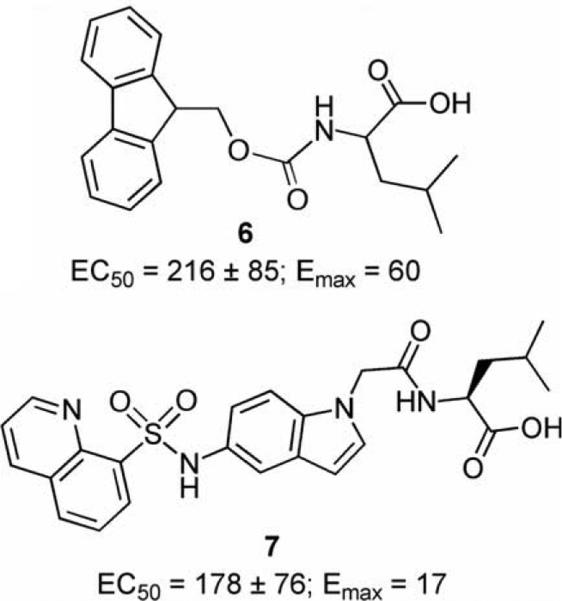
During the course of this investigation, Fan and coworkers disclosed the structures for two NTR1 partial agonists (6 and 7, Fig. 1).36 These compounds arose from a campaign to identify NT small-molecule agonists using virtual screening techniques. The investigators confirmed the NTR1 receptor partial agonist activity of 6 and 7 in a calcium release (FLIPR) assay. We found this of interest, and based on our observations, do not believe that it is a coincidence that both of these compounds incorporate the amino acid leucine. Though there is much more to learn regarding small-molecule NTR1 compounds, it will be of interest to discover why leucine appears to be a privileged group for promoting agonist activity in NTR1 ligands.
Figure 1.
NTR1 receptor partial agonists disclosed by Wyeth Laboratories.
In conclusion, we have demonstrated that replacement of the amino acid group in NTR1 antagonists such as 1a or 1b with leucine afforded small-molecule partial agonists (3a and 3b) with the l-leucine enantiomer providing significantly higher potency. The propensity of this amino acid to confer agonist behavior was also observed in analogs of 3a (4a–c). In addition to this, we have also shown that the presence of leucine as part of a molecular structure (5) is not enough to guarantee an NTR1 partial agonist. Finally, the results from this study also provide support for the receptor-binding models that proposed overlapping active sites for NT agonists and antagonists. Additional studies to identify potent NTR1 small-molecule agonists are currently in progress and will be reported in due course.
Acknowledgments
This research was supported by the National Institute on Drug Abuse, Grant DA024702-01. We gratefully acknowledge the NIMH Chemical Synthesis and Drug Supply Program for providing us with the standard NTR1 antagonist SR48692 (1a).
References and notes
- 1.Roehr B. BMJ. 2005;331:476. doi: 10.1136/bmj.331.7515.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caraway R, Leeman SE. J. Biol. Chem. 1973;248:6854. [PubMed] [Google Scholar]
- 3.Quirion R, Rowe WB, Lapchak PA, Araujo DM, Beaudet A. Ann. N.Y. Acad. Sci. 1992;668:109. doi: 10.1111/j.1749-6632.1992.tb27343.x. [DOI] [PubMed] [Google Scholar]
- 4.Sarret P, Perron A, Stroh T, Beaudet AJ. Comp. Neurol. 2003;461:520. doi: 10.1002/cne.10718. [DOI] [PubMed] [Google Scholar]
- 5.Uhl GR. Ann. N.Y. Acad. Sci. 1982;400:132. doi: 10.1111/j.1749-6632.1982.tb31565.x. [DOI] [PubMed] [Google Scholar]
- 6.Walker N, Lepee-Lorgeoux I, Fournier J, Betancur C, Rostene W, Ferrara P, Caput D. Brain Res. Mol. Brain Res. 1998;57:193. doi: 10.1016/s0169-328x(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 7.Hokfelt T, Everitt BJ, Theodorsson-Norheim E, Goldstein MJ. Comp. Neurol. 1984;222:543. doi: 10.1002/cne.902220407. [DOI] [PubMed] [Google Scholar]
- 8.Kalivas PW, Miller JS. Brain Res. 1984;300:157. doi: 10.1016/0006-8993(84)91351-9. [DOI] [PubMed] [Google Scholar]
- 9.Kalivas PW, Nemeroff CB, Prange AJ., Jr. Eur. J. Pharmacol. 1982;78:471. doi: 10.1016/0014-2999(82)90491-5. [DOI] [PubMed] [Google Scholar]
- 10.Ford AP, Marsden CA. Brain Res. 1990;534:243. doi: 10.1016/0006-8993(90)90135-x. [DOI] [PubMed] [Google Scholar]
- 11.Nemeroff CB, Hernandez DE, Luttinger D, Kalivas PW, Prange AJ., Jr. Ann. N.Y. Acad. Sci. 1982;400:330. doi: 10.1111/j.1749-6632.1982.tb31579.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee K, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Proc. Natl. Acad. Sci. U.S.A. 2006;103:3399. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuyama S, Fukui R, Higashi H, Nishi A. Eur. J. Neurosci. 2003;18:1247. doi: 10.1046/j.1460-9568.2003.02859.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsuyama S, Higashi H, Maeda H, Greengard P, Nishi AJ. Neurochem. 2002;81:325. doi: 10.1046/j.1471-4159.2002.00822.x. [DOI] [PubMed] [Google Scholar]
- 15.Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Biol. Psychiatry. 2001;50:856. doi: 10.1016/s0006-3223(01)01211-2. [DOI] [PubMed] [Google Scholar]
- 16.Kitabgi P. Curr. Opin. Drug Discov. Dev. 2002;5:764. [PubMed] [Google Scholar]
- 17.Tanaka K, Masu M, Nakanishi S. Neuron. 1990;4:847. doi: 10.1016/0896-6273(90)90137-5. [DOI] [PubMed] [Google Scholar]
- 18.Vita N, Laurent P, Lefort S, Chalon P, Dumont X, Kaghad M, Gully D, Le Fur G, Ferrara P, Caput D. FEBS Lett. 1993;317:139. doi: 10.1016/0014-5793(93)81509-x. [DOI] [PubMed] [Google Scholar]
- 19.Chalon P, Vita N, Kaghad M, Guillemot M, Bonnin J, Delpech B, Le Fur G, Ferrara P, Caput D. FEBS Lett. 1996;386:91. doi: 10.1016/0014-5793(96)00397-3. [DOI] [PubMed] [Google Scholar]
- 20.Mazella J, Botto J, Guillemare E, Coppola T, Sarret P, Vincent JP. J. Neurosci. 1996;16:5613. doi: 10.1523/JNEUROSCI.16-18-05613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, Roigaard H, Gliemann J, Madsen P, Moestrup SK. J. Biol. Chem. 1997;272:3599. doi: 10.1074/jbc.272.6.3599. [DOI] [PubMed] [Google Scholar]
- 22.Mazella J, Zsurger N, Navarro V, Chabry J, Kaghad M, Caput D, Ferrara P, Vita N, Gully D, Maffrand JP, Vincent JP. J. Biol. Chem. 1998;273:26273. doi: 10.1074/jbc.273.41.26273. [DOI] [PubMed] [Google Scholar]
- 23.Gully D, Canton M, Boigegrain R, Jeanjean F, Molimard J, Poncelet M, Gueudet C, Heaulme M, Leyris R, Brouard A, Pelaprat D, Labbe-Jullie C, Mazella J, Soubrie P, Maffrand JP, Rostene W, Kitabgi P, Le Fur G. Proc. Natl. Acad. Sci. U.S.A. 1993;90:65. doi: 10.1073/pnas.90.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gully D, Labeeuw B, Boigegrain R, Oury-Donat F, Bachy A, Poncelet M, Steinberg R, Suaud-Chagny MF, Santucci V, Vita N, Pecceu F, Labbe-Jullie C, Kitabgi P, Soubrie P, Le Fur G, Maffrand JP. J. Pharmacol. Exp. Ther. 1997;280:802. [PubMed] [Google Scholar]
- 25.Hong F, Zaidi J, Cusack B, Richelson E. Bioorg. Med. Chem. 2002;10:3849. doi: 10.1016/s0968-0896(02)00342-5. [DOI] [PubMed] [Google Scholar]
- 26.Richard F, Barroso S, Martinez J, Labbe-Jullie C, Kitabgi P. Mol. Pharmacol. 2001;60:1392. doi: 10.1124/mol.60.6.1392. [DOI] [PubMed] [Google Scholar]
- 27.Barroso S, Richard F, Nicolas-Etheve D, Reversat JL, Bernassau JM, Kitabgi P, Labbe-Jullie CJ. Biol. Chem. 2000;275:328. doi: 10.1074/jbc.275.1.328. [DOI] [PubMed] [Google Scholar]
- 28.Labbe-Jullie C, Barroso S, Delphine N, Reversat J, Botto J, Mazella J, Bernassau J, Kitabgi PJ. Biol. Chem. 1998;273:16351. doi: 10.1074/jbc.273.26.16351. [DOI] [PubMed] [Google Scholar]
- 29.Labbe-Jullie C, Barroso S, Nicolas-Eteve D, Reversat JL, Botto JM, Mazella J, Bernassau JM, Kitabgi PJ. Biol. Chem. 1998;273:16351. doi: 10.1074/jbc.273.26.16351. [DOI] [PubMed] [Google Scholar]
- 30.Pang Y, Cusack B, Groshan K, Richelson EJ. Biol. Chem. 1996;271:15060. doi: 10.1074/jbc.271.25.15060. [DOI] [PubMed] [Google Scholar]
- 31.Labeeuw B, Gully D, Jeanjean F, Molimard J-C, Boigegrain R. WO. 1996;032382:1996. [Google Scholar]
- 32.All compounds` showed satisfactory NMR and combustion data. The NMR spectrum of 3a was recorded using a 300 MHz (Bruker AVANCE 300) spectrometer using tetramethylsilane as the internal standard. 1H NMR (CDCl3) δ 8.8 (d, 1H, J = 4.72 Hz), 8.14 (d, 1H, J = 1.81 Hz), 7.95 (d, 1H, J = 9.1), 7.5 (dd, 1H, J = 1.89 and 9.1 Hz), 7.4 (d, 1H, J = 7.95 Hz), 7.20 (t, 1H, J = 8.42 Hz), 7.10 (s, 1H), 7.08 (d, 1H, J = 4.72 Hz), 6.38 (d, 2H, J = 8.42 Hz), 4.81 (br s, 1H), 3.39 (s, 6H), 1.74 (m, 3H), 0.96 (dd, 6H, J = 5.49 and 10.21 Hz).
- 33.Agonist activity was measured in these cells by monitoring increases in internal calcium concentrations using the calcium-sensitive dye, calcium-4, and a FlexStation II384 fluorometric imaging plate reader (MDS Analytical Technologies, Sunnyvale, CA). Cells were plated in 96-well plates at 20,000 cells per well 24 h before assay. Prior to assay, the media was removed and the cells were washed 1 × with 100 μl dye loading buffer (HBSS plus 20 mM HEPES, 2 mM probenecid) followed by addition of 100 ll dye loading buffer. Calcium-4 dye was reconstituted in dye loading buffer as per manufacturer’s instructions and 100 ll dye was added to wells. Dye loading was performed for 1 h at 37 °C in 5% CO2. Fluorescent intensities were measured for 17 s (baseline intensity) before and for 40 s after compound addition. Compound-induced fluorescence increases were measured as the difference between peak and baseline intensity for each well. Test compounds were initially screened at 10 lM final concentration and an EC50 was determined for compounds exhibiting at least 50% neurotensin Emax. EC50 values were determined from 10-point halflog concentration–response curves.
- 34.The assays were run as above except that during the last 15 min of the incubation the cells were pretreated (15 min, in duplicate) with 1a (1 or 4 μM) or 1b (1 or 10 μM) or 5 (1 or 10 μM) followed by the addition of one of eight different concentrations of neurotensin. Test compounds and neurotensin were dissolved in DMSO (1% final assay concentration). The Ke was determined using the equation: Ke = [antagonist]/((EC50 Ant/EC50control) − 1). The EC50 values were determined using Prism (v 5.0, Graph Pad, San Diego, CA).
- 35.Quéré L, Boigegrain R, Jeanjean F, Gully D, Evrard G, Durant F. J. Chem. Soc., Perkin Trans. 1996;2:2639. [Google Scholar]
- 36.Fan Y, Lai MH, Sullivan K, Popiolek M, Andree TH, Dollings P, Pausch MH. Bioorg. Med. Chem. Lett. 2008;18:5789. doi: 10.1016/j.bmcl.2008.09.075. [DOI] [PubMed] [Google Scholar]