Abstract
Purpose of Review
Malaria is caused by the infection and proliferation of parasites from the genus Plasmodium in red blood cells (RBCs). A free Plasmodium parasite, or merozoite, released from an infected RBC must invade another RBC host cell to sustain a blood-stage infection. Here, we review recent advances on RBC invasion by Plasmodium merozoites, focusing on specific molecular interactions between host and parasite.
Recent findings
Recent work highlights the central role of host-parasite interactions at virtually every stage of RBC invasion by merozoites. Biophysical experiments have for the first time measured the strength of merozoite-RBC attachment during invasion. For P. falciparum, there have been many key insights regarding the invasion ligand PfRh5 in particular, including its influence on host species tropism, a co-crystal structure with its RBC receptor basigin, and its suitability as a vaccine target. For P. vivax, researchers identified the origin and emergence of the parasite from Africa, demonstrating a natural link to the Duffy-negative RBC variant in African populations. For the simian parasite P. knowlesi, zoonotic invasion into human cells is linked to RBC age, which has implications for parasitemia during an infection and thus malaria.
Summary
New studies of the molecular and cellular mechanisms governing RBC invasion by Plasmodium parasites have shed light on various aspects of parasite biology and host cell tropism; and indicate opportunities for malaria control.
Keywords: Plasmodium, malaria, parasite, red blood cell, invasion
INTRODUCTION
To ensure their survival, malaria parasites use sophisticated mechanisms to engage and infiltrate host cells. In a human host, following a bite from an infected mosquito and an initial phase of infection and replication in liver cells, parasites enter the bloodstream where they may proliferate indefinitely in red blood cells (RBCs). For RBC infection, a parasite must invade the host cell, a process requiring attachment to the plasma membrane surface followed by entry into the cytoplasmic space. The clinical symptoms and pathogenicity of malaria are caused entirely by this blood-stage of infection, wherein parasites proliferate and persist through alternating cycles of RBC invasion and asexual replication within the RBC.
Plasmodium falciparum is the most virulent malaria species responsible for the greatest number of fatalities (~0.5 million per year globally), primarily among children in sub-Saharan Africa. Plasmodium vivax continues to be responsible for a substantial burden of disease, and an increasing incidence of malaria caused by Plasmodium knowlesi in Southeast Asia is an emerging matter of public health. Plasmodium ovale and Plasmodium malariae, though less well studied, also cause human malaria.
RBC invasion by malaria parasites is ordered and complex
The fully differentiated forms of Plasmodium parasites at the end of each asexual, blood-stage cell cycle are merozoites – small, polarized, pear-shaped cells that are committed to invade uninfected RBCs upon their release into the bloodstream. Perhaps the most distinctive cell biological features of merozoites are their micronemes and rhoptries, organelles located near their smaller apical ends. These organelles secrete factors for invasion either onto the parasite surface or into the red blood cell. Our modern understanding of RBC invasion by merozoites (Fig. 1) is based primarily on microscopic studies to define the kinetic and morphological features [1,2,3,4,5,6]; and molecular studies to establish the functions of specific protein factors [7,8,9,10**,11]. We refer the reader to excellent reviews that provide a detailed account of RBC invasion by Plasmodium merozoites [7,12].
Figure 1. An overview of RBC invasion by Plasmodium merozoites.
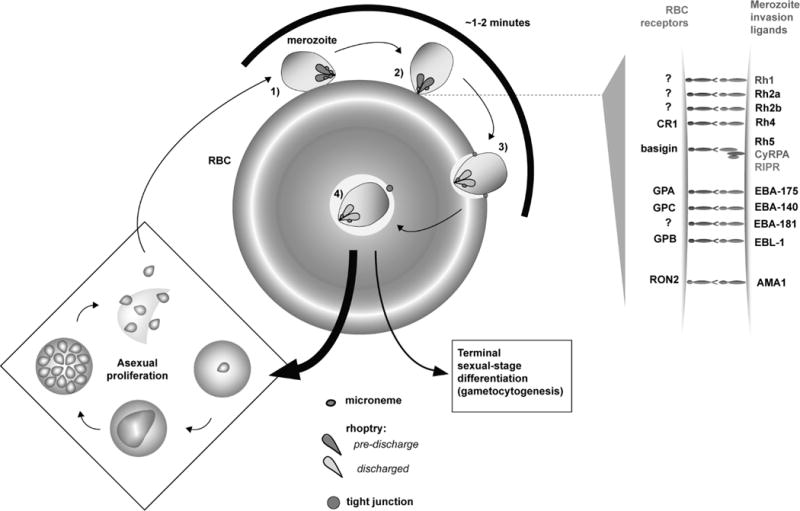
Following release into the bloodstream with rupture of an infected RBC, a merozoite encounters a new RBC and requires only ~1-2 minutes to complete re-invasion to establish a new round of the cell cycle. During encounter, the merozoite and the RBC undergo an ordered series of adhesive interactions, most of which are mediated by interaction of RBC receptors with parasite proteins, or invasion ligands, anchored to the merozoite plasma membrane surface. 1) A free merozoite can attach at any point of its cell body to the RBC. During this primary phase of encounter, adhesion is very dynamic, but interaction culminates in 2) direct, oriented attachment of merozoite apical end to the RBC membrane that is more stable. RBC receptors engage parasite invasion ligands on the parasite apical surface. These invasion ligands are secreted onto the merozoite apical end from micronemes and anterior necks of the rhoptry organelles. To the right, we schematically depict known P. falciparum invasion ligands. Where the RBC receptor for a given ligand is known, this molecule is also identified. PfRh5 forms a functional complex with P. falciparum Rh5-interacting protein (PfRIPR) and the glycophosphatidylinositol-conjugated protein CyRPA. For AMA1, the receptor RON2 is secreted by the parasite into the RBC membrane. 3) Oriented RBC attachment is clearly separated from a subsequent phase, where the parasite uses its own actin-based cytoskeletal machinery to burrow and enter the RBC cytoplasmic space. After apical attachment but before intracellular entry, the merozoite creates a “junction” with the RBC that seals the plasma membranes of the two cells. This tight junction (also called moving junction) acts as a foothold for the parasite as it enters the RBC, forming a circumferential ring around the parasite that also demarcates the nascent parasitophorous vacuole (PV) from the RBC membrane. Formation of the PV itself is stimulated by discharge of the rhoptry, which results in secretion of protein and lipid into the RBC following apical attachment. 4) Invasion is completed when the PV, which will house the parasite through its ensuing development and replication, pinches off the from the RBC membrane into the host cytoplasm, with the vestige of the tight junction still apparent at the posterior. While most invaded parasites re-enter the asexual cell cycle for further parasite proliferation, a small fraction commit to gametocytogenesis, a developmental program for terminal sexual-stage differentiation that ensures parasite transmission. Circulating gametocytes, when taken up by a feeding mosquito, undergo sexual recombination and further differentiation for re-infection of another individual.
The primary purpose of this review is to highlight significant, recent cellular and molecular advances in research of RBC invasion by Plasmodium merozoites. We also discuss progress in vaccine efforts targeting RBC invasion.
PRIMARY PARASITE-RBC ENCOUNTERS: FAST BUT NOT THAT LOOSE?
Live video microscopy has shown that free merozoites of both P. knowlesi and P. falciparum can attach to the RBC membrane at any point along the parasite plasma membrane surface. Upon RBC encounter, merozoites appear to “roll” on the RBC surface and dissociate with some frequency [1,2]; these primary interactions have been thought to be weak. The use of optical tweezers to study P. falciparum merozoite-RBC interactions for the first time directly addresses the strength of cellular attachment, providing evidence that non-oriented adhesion is in fact similarly strong (~35–40 pN) to oriented, stable attachment [13*]. These results may suggest that thermodynamic parameters of the aggregate cellular interaction remain constant over time, even perhaps as kinetic details of the molecular interactions vary to allow dynamic adhesion upon first encounter and stable adhesion later with reorientation.
Video microscopy of invasion shows that the RBC membrane undergoes substantial deformation during the primary, dynamic phase of adhesion [1,2]. The optical tweezer-based study discussed above shows that perturbation of a stable merozoite-RBC interaction, which must involve rearrangement of individual molecular interactions between host and parasite, stimulates transient RBC membrane deformation [13*]. When considered with fact that merozoites in these experiments were attached but could not invade RBCs, these observations are consistent with an intimate, likely causal link between merozoite-RBC adhesion and RBC membrane deformation. Based on theoretical constraints, “wrapping” of the merozoite by a deformed RBC membrane, combined with the energy of adhesive interactions was suggested to explain the tendency of a merozoite to reorient and attain stable attachment from first encounter [14]. Future studies might consider the use of drugs, or select parasite or RBC variants to address the roles of the RBC membrane dynamics and host-parasite interactions in merozoite attachment.
Merozoite surface proteins (MSPs), present along the entire merozoite surface, are strong candidates for invasion ligands that mediate primary encounter with an RBC, either directly or indirectly [15]. Lin and colleagues [16] identified a functional complex of MSP1 with MSPDBL1 and MSPDBL2, both of which were shown to bind RBCs. In P. falciparum infections in Kenya, the presence of serum antibodies to MSPDBL2 is associated with protective effects from malaria [17], supporting a study demonstrating strong selection on the gene [18].
RBC ATTACHMENT THROUGH PARASITE RBL AND EBL LIGANDS: FROM REDUNDANCY TO ESSENTIALITY
For Plasmodium species, the invasion ligands from the erythrocyte binding-like (EBL) and reticulocyte binding-like (RBL) families interact with specific RBC surface receptors to permit apical attachment following reorientation (Fig. 1). EBL proteins contain DBL domains for receptor binding, while the determinants of RBC binding by RBL proteins have only recently begun to be elucidated (see below). Unlike MSPs, which are present constitutively all along the parasite surface, EBL and RBL proteins are stored in parasite micronemes and the anterior “necks” of rhoptries prior to invasion. Near the time of invasion, their release at the apical end is related to increases in cellular calcium in the parasite [19,20]. For Plasmodium and the distantly related parasite Toxoplasma gondii, an active topic of research has been the identification of parasite signal transduction molecules that regulate and respond to cellular calcium levels near the time of host cell invasion, reviewed in [21].
Most RBL and EBL ligands have been shown to be individually dispensable for invasion by Plasmodium merozoites [8,9]. Indeed, as antigens, it is thought that variant and compensatory expression of distinct but functionally redundant paralogs of invasion ligands could be a mechanism for the merozoite to evade the host immune system, reviewed in [22]. Nevertheless, because of their functional importance, researchers consider merozoite invasion ligands as targets for potential blood-stage vaccines for P. falciparum. To block RBC invasion by diverse strains of P. falciparum utilizing variable complements of invasion ligands, recent studies have explored combinations of EBL and P. falciparum RBL homolog (PfRh) antigens that might produce a broadly neutralizing vaccine [23,24,25].
RBC-invasion ligand interactions mediate host cell tropism for P. falciparum
In Plasmodium species, the specificity of RBL and EBL proteins for RBC receptors is thought to be a primary mechanism for host cell restriction in blood-stage parasites. Indeed, P. falciparum merozoites use the EBL ligands EBA-175 and EBA-140 to engage the RBC-specific receptors glycophorin A (GPA) and glycophorin C (GPC), respectively, for RBC host invasion [8,9]. In addition to tropism for RBCs, it has long been suspected that RBL and EBL invasion ligands govern host species preference (e.g. [26]), a view for which various recent studies provide support. Otto and colleagues [27*] performed whole genome sequencing of the chimpanzee parasite P. reichenowi, and showed that the strongest regions of genetic divergence with human-specific P. falciparum include the loci for the EBL proteins and PfRh proteins. It is thus possible that diversification of these invasion ligands was a principal driving force for the varying host preference of these otherwise highly related parasite species.
In striking contrast to RBC interaction by many other RBL and EBL ligands, the interaction of PfRh5 with its receptor basigin is essential for P. falciparum invasion. The identification of the receptor-ligand pair was initially carried out by a biochemical approach designed to detect low-affinity affinity interactions (AVEXIS), and further validated with in vitro reverse genetic analysis of red blood cells [28]. The same study showed that numerous, diverse strains of P. falciparum require the PfRh5-basigin interaction. Genetic studies show that PfRh5 can regulate the host species tropism of P. falciparum parasites [29,30], and biochemical studies provide evidence that species-specific polymorphisms in basigin influence binding by PfRh5. In assays with purified proteins, PfRh5 from P. falciparum displays a markedly stronger affinity for human basigin than for basigin orthologs from non-hominid great apes [31*]. Intriguingly, the same study identified a single “humanizing” mutation in chimpanzee basigin sufficient to confer strong binding to PfRh5.
The determination of the co-crystal structure of PfRh5 with basigin provides for the first time structural insights into the function of any RBL protein [32**]. The flattened “kite”-like architecture of PfRh5 comprised of two alpha-helical bundles is novel. PfRh5 makes contacts with basigin primarily along elements of the peptide backbone of the receptor, suggesting that to a degree, the complex could tolerate sequence polymorphisms in basigin that chiefly influence the amino acid side chain character of the protein. It is interesting to consider if this feature of PfRh5-basigin might explain how P. falciparum has come to rely so heavily on the interaction for RBC invasion. As the authors show, the structure of the comparatively small PfRh5 protein facilitates prediction of plausible receptor-binding modules in considerably more complex and distantly related PfRh and RBL proteins from P. falciparum and other Plasmodium species, respectively. Presently, the receptor for only one other RBL protein is known; PfRh4 binds Complement Receptor (CR) 1 [33,34].
Invasion ligands as targets for vaccination against P. falciparum: all eyes on PfRh5
In recent years, PfRh5 has emerged as perhaps the most promising candidate antigen for a next generation blood-stage vaccine. PfRh5 evades many of the challenges that confound other potential vaccine targets [22]. An essential function for PfRh5 ensures that P. falciparum parasites cannot circumvent its use for invasion. Additionally, PfRh5 is comparatively low in its sequence polymorphism, allowing specific anti-PfRh5 antibodies to potently inhibit RBC invasion by diverse strains of P. falciparum [35,36,37,38,39].
Douglas and colleagues [40**] recently reported the results of the first anti-PfRh5 vaccine trial administered in Aotus nancymaae monkeys, which are animal models for P. falciparum infection. While all animals in a control group developed high parasitemia that required drug treatment, most animals treated with PfRh5 vaccines were protected from such severe infection. Several monkeys vaccinated with a human compatible formulation self-cured. Anti-PfRh5 IgG levels in immunized animals were correlated with protection from P. falciparum, and antibodies inhibited growth of parasites in vitro. Recently, a short peptide epitope of PfRh5 was reported to elicit strong invasion-inhibitory antibodies following immunization [39], a finding that may facilitate design of a future PfRh5-based vaccine.
Recently, it was reported that PfRh5 forms a functional complex with a glycophosphatidylinositol-linked extracellular protein, cysteine-rich protective antigen (CyRPA), thus providing an explanation for how the invasion ligand is anchored to the merozoite plasma membrane surface [10**]. The same study reported that like anti-PfRh5 antibodies, antibodies to CyRPA potently inhibit RBC invasion by diverse strains of P. falciparum, suggesting that CyRPA may also prove a promising vaccine target.
Duffy RBC receptor: a central (but not essential?) receptor for P. vivax
Experimental studies have shown that P. vivax relies strongly on the RBC receptor Duffy for invasion through an EBL family ligand, Duffy Binding Protein (DBP). The erythroid-specific Duffy negative mutation, also known as FyBES, is common in ethnic sub-Saharan Africans and is credited for the highly diminished incidence of P. vivax in the continent [41]. In spite of the near absence of P. vivax in African people, Liu and colleagues [42**] detected comparatively diverse P. vivax and P. vivax-like parasites in wild Central African apes using a mitochondrial DNA sequencing-based approach. This finding establishes the African origin of P. vivax present in modern human populations, suggesting that the Duffy-negative RBC variant that prevails today in ethnic Africans was historically important in limiting spread of the parasitic infection.
Biochemical work on the interaction of P. vivax DBP with Duffy has also yielded basic insights about interaction of invasion ligands with RBC receptors. It had previously been shown that binding to Duffy drives the dimerization of DBP [43]. More recent work shows that the effect is reciprocal: dimerized DBP bound to a single binding domain from Duffy recruits an additional Duffy molecule into the complex with even higher affinity [44*]. Overall, this work reveals a stepwise, cooperative mode of binding, showing at the resolution of a single interaction between a parasite ligand and RBC receptor how Plasmodium merozoites can develop strong binding to their target cells.
Nevertheless, studies focusing on the centrality of Duffy to P. vivax invasion must contend with observations of P. vivax infections in Duffy-negative in individuals in Madagascar [45]. Future work might consider alternative receptor-ligand interactions for P. vivax invasion, as has been described for P. falciparum [8,9].
NEW INSIGHTS ON A RBC RECEPTOR-INDEPENDENT MODE OF HOST CELL ADHESION
For the Plasmodium invasion ligand apical membrane antigen (AMA)1, the parasite supplies its own receptor to allow the merozoite to engage the RBC membrane. The merozoite secretes the rhoptry neck (RON) complex of proteins into the host cell membrane, so that the RON2 subunit faces the parasite plasma membrane from the RBC surface for engagement with AMA1 [3,46]. The AMA1-RON2 binding interaction is conserved in the distantly related parasite species Toxoplasma gondii, indicating that these molecules are part of an ancient molecular strategy for host cell invasion that predates specific tropism for RBCs, reviewed in [47].
The preponderance of evidence suggests that following apical reorientation, the AMA1-RON2 interaction is required for formation of the tight junction that allows the merozoite to efficiently enter the RBC [48,49]. Yap and colleagues [50*] investigated the function of P. falciparum AMA1 by disrupting the endogenous gene during merozoite development. These researchers observed defects in tight junction formation by some merozoites, but likely owing to residual AMA1 protein, also an intermediate phenotype in a fraction of mutant merozoites attempting RBC invasion. As observed by video microscopy, some merozoites penetrated the RBC cytoplasmic space but could not seal and pinch off the PV membrane to complete invasion. The AMA1-RON2 interaction may thus help overcome energetic barriers associated with reconfiguration of plasma membrane topology for PV resealing, as well as at tight junction formation.
The AMA1 antigen has previously failed to elicit protection from P. falciparum in human vaccine trials [51]. Srinivasan and colleagues [52**] revisited the potential of AMA1 as a vaccine target in a mouse model, and made the remarkable finding that immunization with the recombinant extracellular domain of AMA1 specifically in complex with a RON2 peptide fragment provides sterile protection against Plasmodium yoelii. Despite extensive sequence polymorphism, a recent study proposes that for P. falciparum AMA1, a minimal combination of antigenic AMA1 alleles might produce antibodies that react with most natural variants of the target protein [53].
RED BLOOD CELL AGE AND INVASION
P. falciparum merozoites invade and grow in RBCs of all ages, but blood-stage parasites of other species of Plasmodium are more restricted in their age preference. The selectivity of P. vivax for reticulocytes, which is a relatively transient stage in erythropoiesis, has made in vitro culture of the parasite difficult. Using an ex vivo assay with parasites drawn from patients, Malleret and colleagues [54*] showed that the selectivity of P. vivax for reticulocytes is at least partially explained by host cell invasion, demonstrating that invasion is restricted to very young reticulocytes still bearing the CD71 surface marker of erythroid precursors.
RBC age was also shown to be a factor for RBC invasion by Plasmodium knowlesi, which naturally infects rhesus monkeys but has become a significant source of human infection. In in vitro lab cultures, the parasite weakly invades human RBCs, displaying a distinct preference for young cells. Nevertheless, extended culture of P. knowlesi with human RBCs allows parasites to adapt [55,56,57], resulting in increased invasion of older RBCs and perhaps providing an explanation for unusually high parasitemia observed in some natural human infections by P. knowlesi [55]. From an experimental standpoint, P. knowlesi lines that proliferate in readily available human blood may expand the use of this comparatively genetically tractable parasite for in vitro studies of Plasmodium biology, reviewed in [57].
CONCLUSIONS AND FUTURE DIRECTIONS
How malaria merozoites selectively invade RBCs has been of long-standing interest in the malaria research community, and the recent work summarized here indicates substantial progress towards a cohesive, mechanistic explanation. Biophysical approaches like optical tweezers help clarify how molecular interactions between merozoites and RBCs give rise to the unique features of the cellular interaction. With continued development of tools for Plasmodium genetics and more recently RBC genetics [58], tools like video microscopy will be key to measuring how specific parasite and host factors influence transient processes and cellular behaviors on a timescale relevant to invasion. Basic research on the function of invasion ligands has been particularly relevant to efforts to develop a blood-stage P. falciparum vaccine, for which there has tangible progress, especially with regards to the PfRh5 and AMA1 antigens. Identification of previously unexplored, protective antigens indicates further avenues for development in this field [59]. In short, recent work on RBC invasion by Plasmodium merozoites has yielded many new insights regarding this unique parasitic behavior and is likely to advance efforts to treat malaria.
KEY POINTS.
The use of optical tweezers to interrogate Plasmodium merozoite-RBC attachment provides evidence that molecular interactions are optimized to provide uniformly strong adhesion through various phases of cellular interaction.
Whole genome sequencing and protein biochemistry provide evidence that RBC interactions with RBL and EBL invasion ligands on the merozoite surface regulate the host species preference of Plasmodium parasites.
Anti-PfRh5 antibodies strongly inhibit RBC invasion; in the first such vaccine trial for PfRh5, immunization with PfRh5 antigens protects monkeys against P. falciparum.
P. vivax requires the RBC receptor Duffy to invade RBCs, and new evidence that the parasite originated in Africa strongly suggests that the Duffy-negative RBC variant common today in ethnic Africans was historically relevant in human interactions with the parasite.
Acknowledgments
None
FINANCIAL SUPPORT AND SPONSORSHIP
This work was supported by NIH F32 AI093059 (A.S.P.), NIH 1K08AI103034-01A1 (E.S.E.), and NIH R01 AI091787 (M.T.D.).
Footnotes
FINANCIAL CONFLICTS OF INTEREST
The authors do not have any conflicts of interests.
REFERENCES AND RECOMMENDED READING
- 1.Dvorak JA, Miller LH, Whitehouse WC, Shiroishi T. Invasion of erythrocytes by malaria merozoites. Science. 1975;187:748–750. doi: 10.1126/science.803712. [DOI] [PubMed] [Google Scholar]
- 2.Gilson PR, Crabb BS. Morphology and kinetics of the three distinct phases of red blood cell invasion by Plasmodium falciparum merozoites. Int J Parasitol. 2009;39:91–96. doi: 10.1016/j.ijpara.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Riglar DT, Richard D, Wilson DW, et al. Super-Resolution Dissection of Coordinated Events during Malaria Parasite Invasion of the Human Erythrocyte. Cell Host Microbe. 2011;9:9–20. doi: 10.1016/j.chom.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Miller LH, Aikawa M, Johnson JG, Shiroishi T. Interaction between cytochalasin B-treated malarial parasites and erythrocytes. Attachment and junction formation. J Exp Med. 1979;149:172–184. doi: 10.1084/jem.149.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aikawa M, Miller LH, Johnson J, Rabbege J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol. 1978;77:72–82. doi: 10.1083/jcb.77.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sam-Yellowe TY, Shio H, Perkins ME. Secretion of Plasmodium falciparum rhoptry protein into the plasma membrane of host erythrocytes. J Cell Biol. 1988;106:1507–1513. doi: 10.1083/jcb.106.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowman AF, Berry D, Baum J. The cell biology of disease: The cellular and molecular basis for malaria parasite invasion of the human red blood cell. J Cell Biol. 2012;198:961–971. doi: 10.1083/jcb.201206112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duraisingh MT, DeSimone T, Jennings C, et al. Erythrocyte invasion by Plasmodium falciparum: multiple ligand-receptor interactions and phenotypic switching. Subcell Biochem. 2008;47:46–57. doi: 10.1007/978-0-387-78267-6_3. [DOI] [PubMed] [Google Scholar]
- 9.Tham W-H, Healer J, Cowman AF. Erythrocyte and reticulocyte binding-like proteins of Plasmodium falciparum. Trends Parasitol. 2012;28:23–30. doi: 10.1016/j.pt.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 10**.Reddy KS, Amlabu E, Pandey AK, et al. Multiprotein complex between the GPI-anchored CyRPA with PfRH5 and PfRipr is crucial for Plasmodium falciparum erythrocyte invasion. Proc Nat Acad Sci U S A. 2015 doi: 10.1073/pnas.1415466112. (in press). This study availed of biochemical approaches to identify the association in a physical complex of PfRh5 with the GPI-conjugated parasite protein CyRPA, providing an explanation for how the invasion ligand is linked to the parasite plasma membrane surface. The authors showed that like anti-PfRh5 antibodies, anti-CyRPA antibodies display strain-transcending inhibition of P. falciparum invasion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Lopaticki S, Riglar DT, et al. An EGF-like protein forms a complex with PfRh5 and is required for invasion of human erythrocytes by Plasmodium falciparum. PLoS Pathog. 2011;7:e1002199. doi: 10.1371/journal.ppat.1002199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey KL, Gilson PR, Crabb BS. A model for the progression of receptor-ligand interactions during erythrocyte invasion by Plasmodium falciparum. Int J Parasitol. 2012;42:567–573. doi: 10.1016/j.ijpara.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 13*.Crick AJ, Theron M, Tiffert T, et al. Quantitation of malaria parasite-erythrocyte cell-cell interactions using optical tweezers. Biophys J. 2014;107:846–853. doi: 10.1016/j.bpj.2014.07.010. This study measured the strength of attachment between free P. falciparum merozoites and RBCs with optical tweezers, the first such application of the technology to these biological interactions. The authors provide evidence that the interaction is similar strong (~35-40 pN) throughout qualitatively distinct phases of cellular attachment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dasgupta S, Auth T, Gov NS, et al. Membrane-wrapping contributions to malaria parasite invasion of the human erythrocyte. Biophys J. 2014;107:43–54. doi: 10.1016/j.bpj.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldwin M, Yamodo I, Ranjan R, et al. Human erythrocyte band 3 functions as a receptor for the sialic acid-independent invasion of Plasmodium falciparum. Role of the RhopH3-MSP1 complex. Biochim Biophys Acta. 2014;1843:2855–2870. doi: 10.1016/j.bbamcr.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin CS, Uboldi AD, Marapana D, et al. The Merozoite Surface Protein 1 Complex Is a Platform for Binding to Human Erythrocytes by Plasmodium falciparum. J Biol Chem. 2014;289:25655–25669. doi: 10.1074/jbc.M114.586495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tetteh KKA, Osier FHA, Salanti A, et al. Analysis of antibodies to newly described Plasmodium falciparum merozoite antigens supports MSPDBL2 as a predicted target of naturally acquired immunity. Infect Immun. 2013;81:3835–3842. doi: 10.1128/IAI.00301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amambua Ngwa A, Tetteh KKA, Manske M, et al. Population genomic scan for candidate signatures of balancing selection to guide antigen characterization in malaria parasites. PLoS Genet. 2012;8:e1002992. doi: 10.1371/journal.pgen.1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S, Alam MM, Pal-Bhowmick I, et al. Distinct External Signals Trigger Sequential Release of Apical Organelles during Erythrocyte Invasion by Malaria Parasites. PLoS Pathog. 2010;6:e1000746. doi: 10.1371/journal.ppat.1000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao X, Gunalan K, Yap SSL, Preiser PR. Triggers of key calcium signals during erythrocyte invasion by Plasmodium falciparum. Nat Commun. 2013;4:2862. doi: 10.1038/ncomms3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma P, Chitnis CE. Key molecular events during host cell invasion by Apicomplexan pathogens. Curr Opin Microbiol. 2013;16:432–437. doi: 10.1016/j.mib.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Wright GJ, Rayner JC. Plasmodium falciparum Erythrocyte Invasion: Combining Function with Immune Evasion. PLoS Pathog. 2014;10:e1003943. doi: 10.1371/journal.ppat.1003943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandey AK, Reddy KS, Sahar T, et al. Identification of a potent combination of key Plasmodium falciparum merozoite antigens that elicit strain-transcending parasite-neutralizing antibodies. Infect Immun. 2013;81:441–451. doi: 10.1128/IAI.01107-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Healer J, Thompson JK, Riglar DT, et al. Vaccination with Conserved Regions of Erythrocyte-Binding Antigens Induces Neutralizing Antibodies against Multiple Strains of Plasmodium falciparum. PLoS ONE. 2013;8:e72504. doi: 10.1371/journal.pone.0072504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams AR, Douglas AD, Miura K, et al. Enhancing Blockade of Plasmodium falciparum Erythrocyte Invasion: Assessing Combinations of Antibodies against PfRH5 and Other Merozoite Antigens. PLoS Pathog. 2012;8:e1002991. doi: 10.1371/journal.ppat.1002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rayner JC, Huber CS, Galinski MR, Barnwell JW. Rapid evolution of an erythrocyte invasion gene family: the Plasmodium reichenowi Reticulocyte Binding Like (RBL) genes. Mol Biochem Parasitol. 2004;133:287–296. doi: 10.1016/j.molbiopara.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 27*.Otto TD, Rayner JC, Böhme U, et al. Genome sequencing of chimpanzee malaria parasites reveals possible pathways of adaptation to human hosts. Nat Commun. 2014;5:4754. doi: 10.1038/ncomms5754. The authors performed whole-genome sequencing of the chimpanzee parasite P. reichenowi, which shares a relatively recent common ancestry and high degree of genomic orthology with P. falciparum. The loci for the Rh and EBL ligands were among the genes that differed most strongly in sequence between the two parasite species, showing that these ligands could be principal determinants of host species preference. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crosnier C, Bustamante LY, Bartholdson SJ, et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayton K, Gaur D, Liu A, et al. Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of Plasmodium falciparum invasion. Cell Host Microbe. 2008;4:40–51. doi: 10.1016/j.chom.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayton K, Dumoulin P, Henschen B, et al. Various PfRH5 polymorphisms can support Plasmodium falciparum invasion into the erythrocytes of owl monkeys and rats. Mol Biochem Parasitol. 2013;187:103–110. doi: 10.1016/j.molbiopara.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Wanaguru M, Liu W, Hahn BH, et al. RH5-Basigin interaction plays a major role in the host tropism of Plasmodium falciparum. Proc Nat Acad Sci U S A. 2013;110:20735–20740. doi: 10.1073/pnas.1320771110. Using purified proteins, this study shows that PfRh5 binds more strongly to human basigin than it binds to basigin from other great apes, leading the authors to propose that this receptor-ligand interaction is a strong determinant of host species tropism for P. falciparum. Site-directed mutagenesis in human and chimpanzee basigin reveal loss and gain-of-function mutations, respectively, with regard to PfRh5 binding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Wright KE, Hjerrild KA, Bartlett J, et al. Structure of malaria invasion protein RH5 with erythrocyte basigin and blocking antibodies. Nature. 2014;515:427–430. doi: 10.1038/nature13715. This study reports the first protein structure of a Plasmodium RBL-type invasion ligand, revealing a novel protein fold. The co-crystal structure of PfRh5 with its physiological RBC receptor basigin is also significant because PfRh5 is the only universally essential Rh or EBL ligand for P. falciparum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tham W-H, Wilson DW, Lopaticki S, et al. Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand. Proc Nat Acad Sci U S A. 2010;107:17327–17332. doi: 10.1073/pnas.1008151107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park HJ, Guariento M, Maciejewski M, et al. Using mutagenesis and structural biology to map the binding site for the Plasmodium falciparum merozoite protein PfRh4 on the human immune adherence receptor. J Biol Chem. 2014;289:450–463. doi: 10.1074/jbc.M113.520346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Douglas AD, Williams AR, Illingworth JJ, et al. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat Commun. 2011;2:601. doi: 10.1038/ncomms1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel SD, Ahouidi AD, Bei AK, et al. Plasmodium falciparum merozoite surface antigen, PfRH5, elicits detectable levels of invasion-inhibiting antibodies in humans. J Infect Dis. 2013;208:1679–1687. doi: 10.1093/infdis/jit385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy KS, Pandey AK, Singh H, et al. Bacterially Expressed Full-Length Recombinant Plasmodium falciparum RH5 Protein Binds Erythrocytes and Elicits Potent Strain-Transcending Parasite-Neutralizing Antibodies. Infect Immun. 2014;82:152–164. doi: 10.1128/IAI.00970-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bustamante LY, Bartholdson SJ, Crosnier C, et al. A full-length recombinant Plasmodium falciparum PfRH5 protein induces inhibitory antibodies that are effective across common PfRH5 genetic variants. Vaccine. 2013;31:373–379. doi: 10.1016/j.vaccine.2012.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ord RL, Caldeira JC, Rodriguez M, et al. A malaria vaccine candidate based on an epitope of the Plasmodium falciparum RH5 protein. Malar J. 2014;13:326. doi: 10.1186/1475-2875-13-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40**.Douglas AD, Baldeviano GC, Lucas CM, et al. A PfRH5-Based Vaccine Is Efficacious against Heterologous Strain Blood-Stage Plasmodium falciparum Infection in Aotus Monkeys. Cell Host Microbe. 2015;17:130–139. doi: 10.1016/j.chom.2014.11.017. The authors report findings from the first vaccine trial of PfRh5, showing that Aotus nancymaae monkeys immunized with Rh5 antigens are protected from high parasitemia following challenge with P. falciparum. Protection from high parasitemia in vivo and separately in vitro inhibition of parasite growth were each correlated with serum levels of anti-PfRh5 IgG antibody. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmerman PA, Ferreira MU, Howes RE, Mercereau-Puijalon O. Red blood cell polymorphism and susceptibility to Plasmodium vivax. Adv Parasitol. 2013;81:27–76. doi: 10.1016/B978-0-12-407826-0.00002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42**.Liu W, Li Y, Shaw KS, et al. African origin of the malaria parasite Plasmodium vivax. Nat Commun. 2014;5:3346. doi: 10.1038/ncomms4346. Applying a mitochondrial DNA sequencing-based approach to material from fecal samples left by wild, Central African apes, the authors reconstructed the phylogeny of P. vivax and P. vivax-like parasites endemically infecting these animals. The data show that human P. vivax forms a monophyletic lineage included within the range of genetic diversity of the ape parasites, arguing that the clinically relevant form of the parasite emerged from this region. A major implication of this finding is that the parasite must have naturally interacted with ancestors of modern human populations in Africa that express the Duffy-negative RBC variant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Batchelor JD, Zahm JA, Tolia NH. Dimerization of Plasmodium vivax DBP is induced upon receptor binding and drives recognition of DARC. Nat Struct Mol Biol. 2011;18:908–914. doi: 10.1038/nsmb.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Batchelor JD, Malpede BM, Omattage NS, et al. Red blood cell invasion by Plasmodium vivax: structural basis for DBP engagement of DARC. PLoS Pathog. 2014;10:e1003869. doi: 10.1371/journal.ppat.1003869. P. vivax DBP dimerization was previously shown to be induced by binding with the RBC receptor Duffy. Here, the authors show that this complex recruits an additional minimal binding domain from Duffy, arguing for a stepwise, cooperative process for assembly of an oligomeric RBC receptor-parasite ligand interaction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menard D, Barnadas C, Bouchier C, et al. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Nat Acad Sci U S A. 2010;107:5967–5971. doi: 10.1073/pnas.0912496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamarque M, Besteiro S, Papoin J, et al. The RON2-AMA1 Interaction is a Critical Step in Moving Junction-Dependent Invasion by Apicomplexan Parasites. PLoS Pathog. 2011;7:e1001276. doi: 10.1371/journal.ppat.1001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harvey KL, Yap A, Gilson PR, et al. Insights and controversies into the role of the key apicomplexan invasion ligand, Apical Membrane Antigen 1. Int J Parasitol. 2014;44:853–857. doi: 10.1016/j.ijpara.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Treeck M, Zacherl S, Herrmann S, et al. Functional Analysis of the Leading Malaria Vaccine Candidate AMA-1 Reveals an Essential Role for the Cytoplasmic Domain in the Invasion Process. PLoS Pathog. 2009;5:e1000322. doi: 10.1371/journal.ppat.1000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srinivasan P, Beatty WL, Diouf A, et al. Binding of Plasmodium merozoite proteins RON2 and AMA1 triggers commitment to invasion. Proc Nat Acad Sci U S A. 2011;108:13275–13280. doi: 10.1073/pnas.1110303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Yap A, Azevedo MF, Gilson PR, et al. Conditional expression of apical membrane antigen 1 in Plasmodium falciparum shows it is required for erythrocyte invasion by merozoites. Cell Microbiol. 2014;16:642–656. doi: 10.1111/cmi.12287. Using a genetic approach to conditionally disrupt the AMA1 genetic locus in P. falciparum and video microscopy to observe mutant merozoites, the authors provide support for the model that the invasion ligand is required for formation of the tight junction. The authors additionally observe defects in PV resealing following merozoite entry into the RBC, suggesting the AMA1-RON2 interaction is important even subsequent to junction formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spring MD, Cummings JF, Ockenhouse CF, et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS ONE. 2009;4:e5254. doi: 10.1371/journal.pone.0005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Srinivasan P, Ekanem E, Diouf A, et al. Immunization with a functional protein complex required for erythrocyte invasion protects against lethal malaria. Proc Nat Acad Sci U S A. 2014;111:10311–10316. doi: 10.1073/pnas.1409928111. The authors found that immunization of mice with AMA1 antigen in complex with a peptide fragment of RON2 provides sterile protection against P. yoelii. This finding underscores the importance of this molecular interaction to Plasmodium parasites and suggests the viability of AMA1 as a vaccine target. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terheggen U, Drew DR, Hodder AN, et al. Limited antigenic diversity of Plasmodium falciparum apical membrane antigen 1 supports the development of effective multi-allele vaccines. BMC Med. 2014;12:183. doi: 10.1186/s12916-014-0183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Malleret B, Li A, Zhang R, et al. Plasmodium vivax: restricted tropism and rapid remodelling of CD71 positive reticulocytes. Blood. 2015 doi: 10.1182/blood-2014-08-596015. (in press). The researchers investigated if the well-known restriction of P. vivax to reticulocytes is related to host cell invasion. In ex vivo assays, P. vivax displays a strong preference for invasion into very young reticulocytes still bearing the CD71+ surface marker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim C, Hansen E, Desimone TM, et al. Expansion of host cellular niche can drive adaptation of a zoonotic malaria parasite to humans. Nat Commun. 2013;4:1638. doi: 10.1038/ncomms2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moon RW, Hall J, Rangkuti F, et al. Adaptation of the genetically tractable malaria pathogen Plasmodium knowlesi to continuous culture in human erythrocytes. Proc Nat Acad Sci U S A. 2013;110:531–536. doi: 10.1073/pnas.1216457110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grüring C, Moon RW, Lim C, et al. Human red blood cell-adapted Plasmodium knowlesi parasites: a new model system for malaria research. Cell Microbiol. 2014;16:612–620. doi: 10.1111/cmi.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bei AK, Brugnara C, Duraisingh MT. In Vitro Genetic Analysis of an Erythrocyte Determinant of Malaria Infection. J Infect Dis. 2010;202:1722–1727. doi: 10.1086/657157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osier FH, Mackinnon MJ, Crosnier C, et al. New antigens for a multicomponent blood-stage malaria vaccine. Sci Transl Med. 2014;6:247ra102. doi: 10.1126/scitranslmed.3008705. [DOI] [PMC free article] [PubMed] [Google Scholar]


