Abstract
Background
Errors are commonplace in dentistry, it is therefore our imperative as dental professionals to intercept them before they lead to an adverse event, and/or mitigate their effects when an adverse event occurs. This requires a systematic approach at both the profession-level, encapsulated in the Agency for Healthcare Research and Quality’s Patient Safety Initiative structure, as well as at the practice-level, where Crew Resource Management is a tested paradigm. Supporting patient safety at both the dental practice and profession levels relies on understanding the types and causes of errors, an area in which little is known.
Methods
A retrospective review of dental adverse events reported in the literature was performed. Electronic bibliographic databases were searched and data were extracted on background characteristics, incident description, case characteristics, clinic setting where adverse event originated, phase of patient care that adverse event was detected, proximal cause, type of patient harm, degree of harm and recovery actions.
Results
182 publications (containing 270 cases) were identified through our search. Delayed and unnecessary treatment/disease progression after misdiagnosis was the largest type of harm reported. 24.4% of reviewed cases were reported to have experienced permanent harm. One of every ten case reports reviewed (11.1%) reported that the adverse event resulted in the death of the affected patient.
Conclusions
Published case reports provide a window into understanding the nature and extent of dental adverse events, but for as much as the findings revealed about adverse events, they also identified the need for more broad-based contributions to our collective body of knowledge about adverse events in the dental office and their causes.
Practical Implications
Siloed and incomplete contributions to our understanding of adverse events in the dental office are threats to dental patients’ safety.
Keywords: Dental care, patient safety, adverse events, case reports
Patient safety is fundamental to the delivery of high quality dental care1,2 and is one of the six aims for health care organizations described by the Institute of Medicine in their 2001 report, “Crossing the Quality Chasm: A New Healthcare System for the 21st Century.”3 Dental practitioners and dental institutions alike, are committed to delivering care that is safe, timely, efficient, effective, equitable and patient-centered, in keeping with these aims.4 At the same time, error is fundamental in health care, as our medical counterparts demonstrated over two decades ago,5–8 and indeed errors (lapses, slips, mistakes8,9) are commonplace in dentistry.10–12 Several theories have been formulated to explain the mechanism of errors and how unchecked latent systemic factors, threats or failures (e.g., provider fatigue or inexperience, understaffing, poor supervision, faulty equipment, teamwork, vague organizational policies/procedures and poor safety culture) can lead to the occurrence of an adverse event (unintended harm or injury to a patient due to medical/dental management rather than their underlying condition7, 9).13,14 Some of these theories include the Swiss Cheese Model by James Reason13 and the University of Texas Threat and Error Management Model by Robert Helmreich.14 It is our imperative as dental professionals to intercept errors and identify these latent systemic factors before they lead to the occurrence of adverse events, and/or mitigate their effects when they occur in our dental practices.2
Dentistry can learn from the successes of other industries including aviation, oil and gas, nuclear power plants and the military, which have developed sophisticated safety systems for minimizing errors and accidents.13,15 Crucial to their success is the emphasis on regular, good quality safety data collection, its prompt analysis and dissemination, which fosters learning across board.14 Non-punitive incident reporting systems such as the Aviation Safety Action Program,16 detailed incident analysis/accident investigations, routine reviews of deidentified aggregated flight data such as the Flight Operational Quality Assurance17 are some examples of safety systems that enable the understanding of the nature and extent of errors, contributing conditions and inform the development of countermeasures necessary for improving aviation safety.14 Countermeasures targeting human factors and human effectiveness through crew resource management (CRM) training have led to improved safety behaviors and attitudes amongst aviation workers.18 Our medical colleagues have pioneered efforts to translate these lessons into health care by establishing voluntary reporting systems19 (e.g. FDA Adverse Event Reporting System,20 USP MedMARx, JCAHO Sentinel Event Reporting System and National Nosocomial Reporting System)19 and adopting CRM training18 (e.g. Anesthesia Crisis Resource Management, in operating rooms, Medteams in emergency medicine and Neosim in pediatrics).18 While these safety systems are siloed, they are steps in the right direction and dentistry will benefit from adapting some of these systems21,22 as the profession moves towards developing a comprehensive patient safety initiative.23
With the exception of a few pioneer efforts,12,21,23,24 the dental profession has essentially watched from the sidelines, as medicine moved towards developing patient safety initiatives. The time has now come for dentistry to commit to patient safety by systematically addressing adverse events and errors in dentistry.23 As a first step of a dental patient safety initiative, we need to “identify the threats to dental patient safety by identifying errors and causes of patient injury associated with the delivery of dental care.”23,25
In the absence of a broad-based resource to capture errors, adverse events, and their causes, we turned to the biomedical literature, an untapped existing source of information regarding these events, which resulted in a database of events from multiple specialties across various clinical settings worldwide. Our primary objective was to characterize the types of patient safety events reported in the literature and raise awareness about identifying and tracking errors and their causes.
Methods
We conducted a retrospective review of published case reports/series on dental patient safety, from 1970 through June 2013. This study did not involve any direct interaction with human subjects.
Search methods
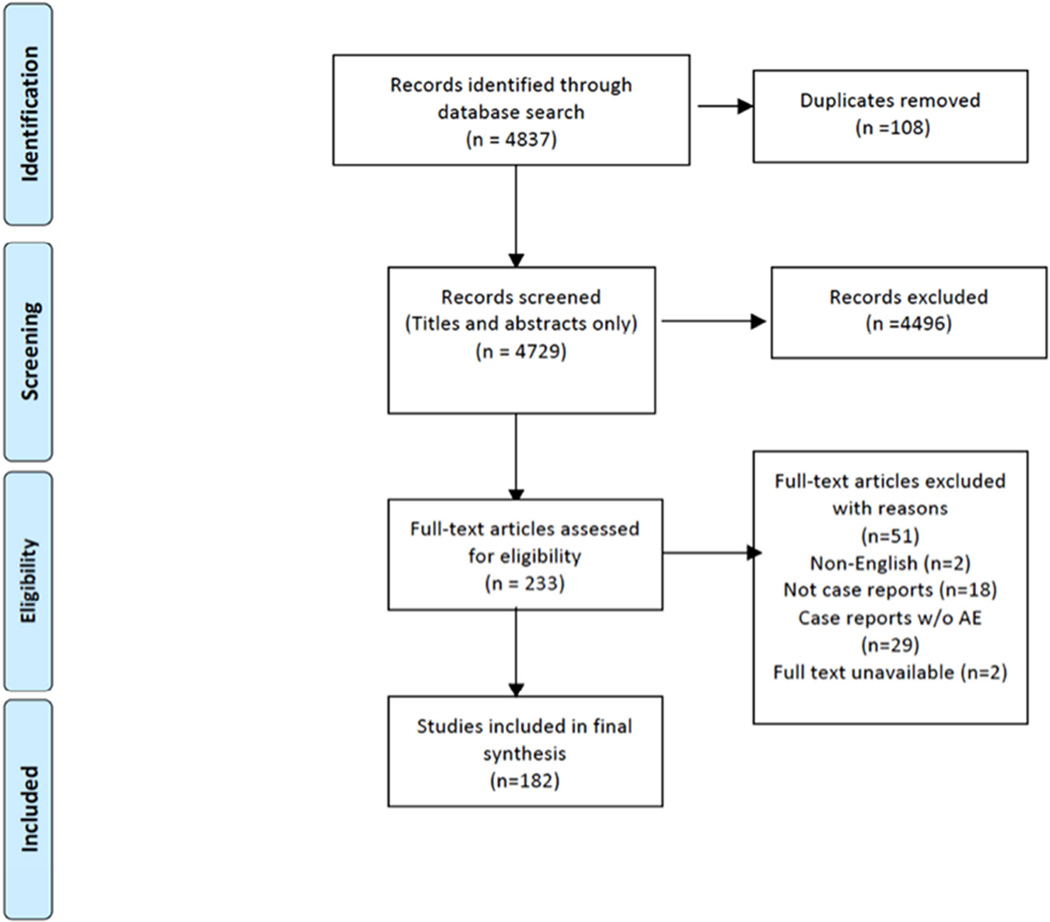
We searched electronic bibliographic databases (PubMed, EMBASE, Web of Science and CINAHL) using the following key words: patient safety, medical errors, adverse effects, dental care, dental procedures, dental treatment and facility. The final search date was June 30, 2013. The search yielded 4,837 publications. After the removal of duplicates, 4,729 unique articles were identified for screening.
Review process
A preliminary screening of the titles of these 4,729 articles resulted in the exclusion of 2449 articles that were not relevant to our objective. An example of an article that was captured by our search but not relevant was “Penetrability of dentinal tubules in adhesive-lined cavity walls.”26
Further exclusion of articles after abstract reviews was based on the following criteria: non-English publications (n = 124), nondental focus (n = 567), quality improvement focus w/o adverse events (n = 663), adverse events due to patients underlying condition (n = 29), guidelines, editorials, systematic reviews, clinical trials, observational studies, opinion pieces on dental adverse events/related patient safety issues (n = 664). The final phase of the review process involved assessing the full text of the remaining 233 articles, resulting in the exclusion of 51 studies (2 non-English, 29 noncase reports and 18 case reports without adverse events). Thus, final selection comprising 182 publications was included in the final synthesis (Figure 1).
Figure 1.
Dental adverse event case reports literature review process.
Data extraction
Two independent reviewers (EO, SS) extracted data from these case reports/series using an adverse event data collection form developed by the authors. Background characteristics were collected on authors, publication year, country, citation and PubMed ID/Accession number (if available). Each case was further characterized as follows: incident description, case characteristics (age, gender), clinic setting where adverse event originated, phase of patient care that adverse event was detected, proximal cause, type of patient harm, degree of harm and recovery actions. Through an iterative process among the authors (EO, RR, EK), preliminary categories (Appendix 1) were created to categorize the type of patient harm. We used a consensus process to assign each case to its associated harm category. The degree of harm was assessed using a newly developed Dental Adverse Event Severity Scale (Appendix 2), which is a modification of the Institute for Healthcare Improvement’s severity scale.27 Further publications on the development process for these tools are forthcoming.
Data analysis
Data were transferred to a spreadsheet using Microsoft Excel and analyzed. Descriptive statistics were obtained for each main category. The results are shown in the next section.
Results
270 cases from 182 published dental adverse event case reports were reviewed for this study. Background characteristics of these cases are described in Table 1.
Table 1.
Background Characteristics of Dental Patient Safety Case Reports.
| Frequency (n) | Percent (%) | |
|---|---|---|
| Description of Publications | n=182 | 100 |
| Publication Year | ||
| Before 1980 | 4 | 2.2 |
| 1981–1990 | 13 | 7.1 |
| 1991–2000 | 69 | 37.9 |
| 2001–2010 | 65 | 35.7 |
| 2010+ | 31 | 17.0 |
| WHO Region | ||
| Africa | 1 | 0.5 |
| Americas | 80 | 44.0 |
| Southeast Asia | 12 | 6.6 |
| Europe | 68 | 37.4 |
| Eastern Mediterranean | 2 | 1.1 |
| Western Pacific | 19 | 10.4 |
| Description of Cases | n=270 | 100 |
| Age (Years) | ||
| Younger than 15 | 35 | 13.0 |
| 15–24 | 42 | 15.6 |
| 25–44 | 77 | 28.5 |
| 45–64 | 76 | 28.1 |
| 65+ | 27 | 10.0 |
| Not specified | 13 | 4.8 |
| Gender | ||
| Female | 128 | 47.4 |
| Male | 141 | 52.2 |
| Not specified | 1 | 0.4 |
| Clinical Setting where AE Originated | ||
| Dental Office | 108 | 40.0 |
| Hospital | 94 | 34.8 |
| Not specified | 68 | 25.2 |
| Phase of Care When AE was Detected | ||
| During Visit | 96 | 35.6 |
| After Visit | 174 | 64.4 |
Background characteristics
There was a surge in the volume of publications between 1991 and 2000; 37.9% compared to 7.1% in the preceding decade. According to the World Health Organization (WHO) regional classification of countries,28 44% of the publications were from authors based in the region of the Americas (North and Latin America). The European region followed closely in frequency of publications with 37.4%. Over 50% of the reviewed cases were aged 25 to 64 years. Slightly more of the adverse events were reported to have occurred in males (52.2%) compared to females (47.4%). About two of every three (64.4%) adverse events reported were detected after the patient had concluded the dental encounter or left the dental facility. Although 25.2% of the authors did not specify the clinical setting where the error occurred, 40% of the adverse events originated at a dental office compared to 34.8% in hospitals or university-based dental clinics.
None of these percentages were standardized to any population size and/or number of available dentists/dental offices in the population because the aim of this paper was not to establish a prevalence of dental adverse events.
Degree of harm
Using the newly developed Dental Adverse Event Severity Scale, the cases were grouped according to the degree of harm that the patient experienced associated with the adverse event (Table 2). Interrater reliability with respect to this newly-developed scale was high between the two reviewers with a Cohen’s Kappa of 0.85. 24.1% of the adverse events required that the patient be transferred to an emergency department for further evaluation or hospitalization or had their hospital stay prolonged if they were already hospitalized (Category F). A similar number of cases were reported to have experienced permanent harm (24.4%; Category G1–G4) and in one of every ten case reports, the event resulted in the death of the affected patient (11.1%; Category I).
Table 2.
Degree of Harm*
| Degree of Harm | Frequency (n) |
Percent (%) |
|---|---|---|
| n=270 | 100 | |
| E1 (Temporary minimal harm w/ minimal intervention) | 18 | 6.7 |
| E2 (Temporary minimal harm w/ significant intervention) | 12 | 4.4 |
| E3 (Temporary significant harm w/ minimal intervention) | 23 | 8.5 |
| E4 (Temporary significant harm w/ significant intervention) | 38 | 14.1 |
| F (Temporary harm w/ emergency room transfer/hospitalization) | 65 | 24.1 |
| G1 (Permanent minimal harm w/ minimal intervention) | 3 | 1.1 |
| G2 (Permanent minimal harm w/ significant intervention) | 6 | 2.2 |
| G3 (Permanent significant harm w/ minimal intervention) | 16 | 5.9 |
| G4 (Permanent significant harm w/ significant intervention) | 41 | 15.2 |
| H (Intervention required to sustain life) | 18 | 6.7 |
| I (Patient death) | 30 | 11.1 |
See appendix 2 for details of the Dental Adverse Event Severity Scale
Type of harm
Using the type of harm categories we created (Appendix 1), the largest category was “delayed appropriate treatment/disease progression and/or unnecessary treatment associated with misdiagnosis,” comprising almost a quarter of all reported cases (23%). Systemic complications involving the cardiovascular, respiratory, neurologic/cerebral, renal and other body systems were the second largest harm category commonly reported (21.1%) (Table 3). 23.1% of the reported AEs were anesthesia-related, with general anesthesia accounting for 47.6% of these cases and local anesthesia accounting for 40.5%. Only a few reports were related to nitrous oxide and intravenous sedation (11.9%). Patients often required some form of intervention or a combination of several interventions to wholly or partially recover from an adverse event including: intraoral and extraoral radiographs, advanced imaging (CT, MRI, endoscopy, bronchoscopy), laboratory investigations, medication, re-treatment, changes to treatment plans, multiple dental visits, surgery, emergency room (ER) visits or prolonged hospital admissions.
Table 3.
Overview of Dental Adverse Events by Type of Harm.
| Type of Harm¶ | Example of Patient Harm | Frequency (n) | Percent (%)* |
|---|---|---|---|
| n=270 | 100 | ||
| Delayed appropriate treatment/ disease progression and/ or unnecessary treatment associated with misdiagnosis | Melkersson-Rosenthal syndrome misdiagnosed as angioedema and dental abscess resulting in multiple tooth extractions | 62 | 23.0 |
| Other systemic complications including adverse reactions to dental device/material/procedure | Intracerebral hematoma after tooth extraction | 57 | 21.1 |
| Allergy/ Hypersensitivity reactions | Latex allergy (bitewing radiograph pack, rubber dam, prophylaxis cup) | 29 | 10.7 |
| Systemic infection | Cerebral abscess after dental procedure | 28 | 10.4 |
| Soft tissue injury/ inflammation | Accidental injection of formalin into soft tissues instead of local anesthetic | 23 | 8.5 |
| Aspiration of foreign body | Aspiration of rubber mouth prop | 11 | 4.1 |
| Nerve damage or injury | Paresthesia of infraorbital region | 11 | 4.1 |
| Hard-tissue damage | Root perforation during endodontic treatment | 8 | 3.0 |
| Psychological distress/ disorder | Anorexia nervosa induced by painful orthodontic treatment | 7 | 2.6 |
| Toxicity/ drug overdose | Injection of 1:1000 adrenaline versus 1:100,000 | 7 | 2.6 |
| Orofacial infection | Necrotizing fasciitis of infraorbital region | 6 | 2.2 |
| Poor hemostasis/ prolonged bleeding | After traumatic tooth extraction in hemophiliac patient | 6 | 2.2 |
| Ingestion of foreign body | Ingestion of endodontic file | 5 | 1.9 |
| Other orofacial complications | Tear of suspensory ligaments in temporomandibular after excessive digital manipulation of chin by dentist | 5 | 1.9 |
| Retention of foreign object(s) with sequela(e) | Breakage of surgical bur and retention within bone | 3 | 1.1 |
| Poor aesthetic results postdental treatment | Malpositioned implants | 2 | 0.7 |
p-value: <0.001
Arranged in descending order of f requency.
Discussion
Our results reinforce that there is a level of risk associated with everyday dental practice. Dental patient safety events are a global phenomenon making it imperative that dental professionals worldwide acknowledge this reality to galvanize efforts to minimize patient harm. Based on the fact that most adverse events go unreported29 and an even fewer number are published in peer-reviewed journals, we suspect that many more opportunities will exist for learning about dental adverse events as more data sources become available. Our primary objective in this paper was to characterize dental adverse events from the biomedical literature using case reports/series. This article represents a call to action for the dental profession on patient safety. Our findings suggest that:
dentistry needs a standardized way of communicating about errors and adverse events;
dental professionals need a venue where they can efficiently report adverse events and nearmisses across a range of severities;
dental patient safety event case reports should be accompanied by a root cause analysis.
A dental patient safety classification system or taxonomy will enable us to communicate about errors and dental adverse events in a standardized manner. Categorizing the adverse events we identified in the case reports proved very challenging due to the absence of an established dental patient safety taxonomy as well as the tremendous variability in scope and content of the published case reports. Through a consensus process, we assigned each case to a type of harm category (Table 3). Delayed appropriate and unnecessary treatment/disease progression associated with misdiagnosis comprised almost a quarter of all cases reviewed (23%). This corresponds with observations in outpatient ambulatory practices where high rates of diagnostic errors have been detected.6
To understand the extent of harm experienced by the patients in the case reports, we categorized harm based on their degree of severity and the required intervention using the Dental Adverse Event Severity Scale (Table 2), which we developed. Our results illustrate that most patients experienced temporary harm significant enough to require a transfer to the emergency room or hospitalization (24.1%), permanent harm (24.4%), intervention required to sustain life (6.7%) or resulted in death (11.1%). While these aggregate numbers may be an overrepresentation of the true prevalence by virtue of reporting bias inherent to our data source, studies from Finland10 have estimated the prevalence of permanent harm due to dental adverse events as 13%. These estimates serve as a wake-up call for the profession to begin systematically addressing adverse events in dentistry. We need to develop safety systems and countermeasures using principles from other industries21,22 (e.g. CRM in aviation) to prevent errors, trap them before they lead to an adverse event and/or mitigate their effects when they occur.14
The path has been illuminated by safety science in other domains, as described in the introduction e.g., establishing nonpunitive incident reporting systems and conducting thorough root cause analyses when adverse events occur to foster better understanding of contributors to dental adverse events; developing checklists,21,30 protocols and computerized decision aids to reduce reliance on memory; promoting the use of electronic dental records31,32 to improve access to patient information or test results; the use of forcing functions to minimize the probability of making mistakes when such mistakes could cause unintended harm (e.g. a system that alerts the dentist when a drug to which the patient is allergic is prescribed or sensors to monitor the depth of endodontic files during root canal treatments); standardizing operating procedures to minimize variability based on dentists’ training or practice styles; and regular safety training for staff using a combination of didactic and simulation techniques which emphasize teamwork and working in emergency situations.8
In the absence of a broad-based dental patient safety reporting system, dental professionals can still contribute to the corpus of knowledge on dental patient safety events by writing and submitting manuscripts to peer-reviewed journals.33 Our results indicated that a good proportion (40%) of the adverse events originated at dental offices, although the reporting authors were typically based in a hospital or university-based dental clinic. Private practitioners, who represent the bulk of dental providers in the US, need to be actively engaged and incentivized to participate in the process of building this body of evidence. Journal editors are also encouraged to accept and publish more, and more detailed, case reports/series on dental patient safety events. It is our recommendation that these reports should, in addition to the standardized reporting guidelines for case reports,34 contain a root cause analysis and a follow-up to give a sense of the permanency of the harm.33 Admittedly, we recognize that the context of some case reports do not lend themselves to such detailed analysis, e.g. instances where an event caused at clinic A was identified and reported by clinic B. Under ideal circumstances, clinic B would seek additional information about the factors that contributed to the event, but this may not be practical in all cases. While it is not reasonable to propose that every lost temporary crown or perforated root should appear as a case report in a scientific or professional journal, a broad-based reporting system is a good forum for tracking the prevalence of these more common events.
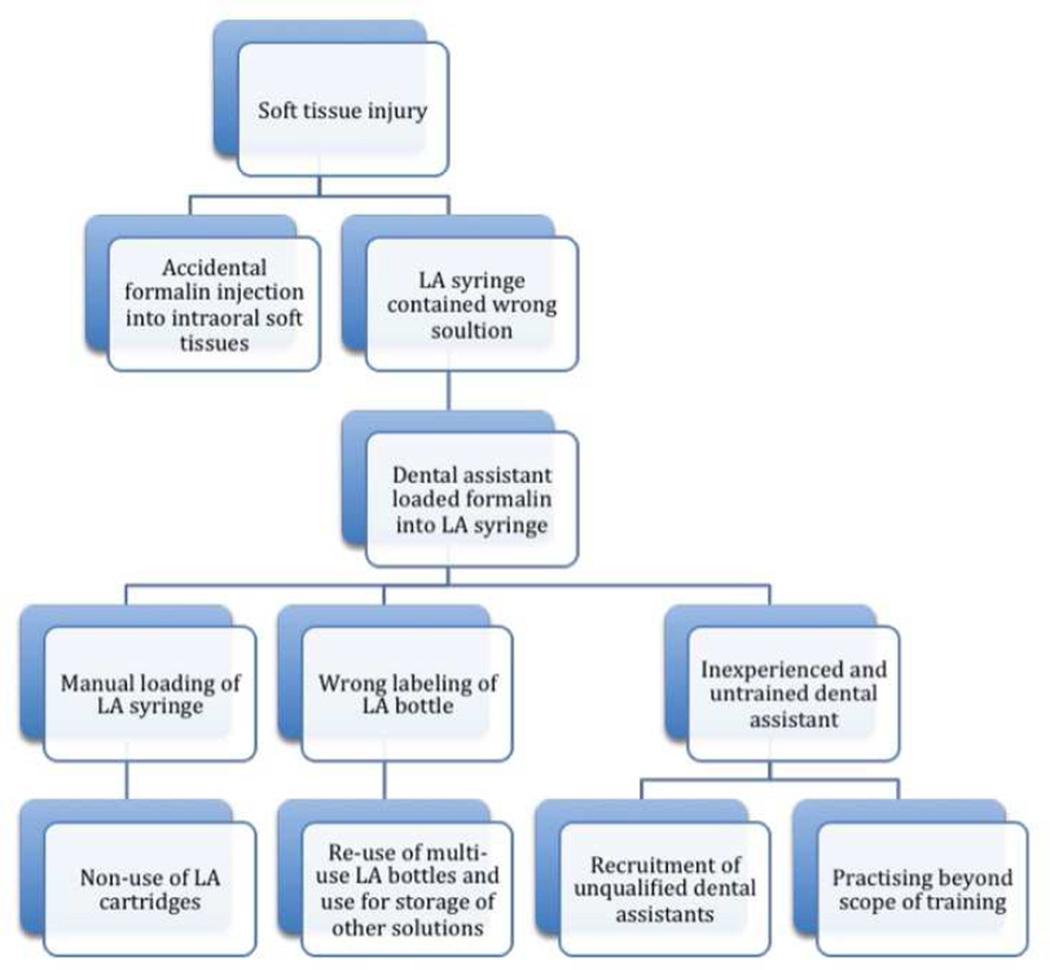
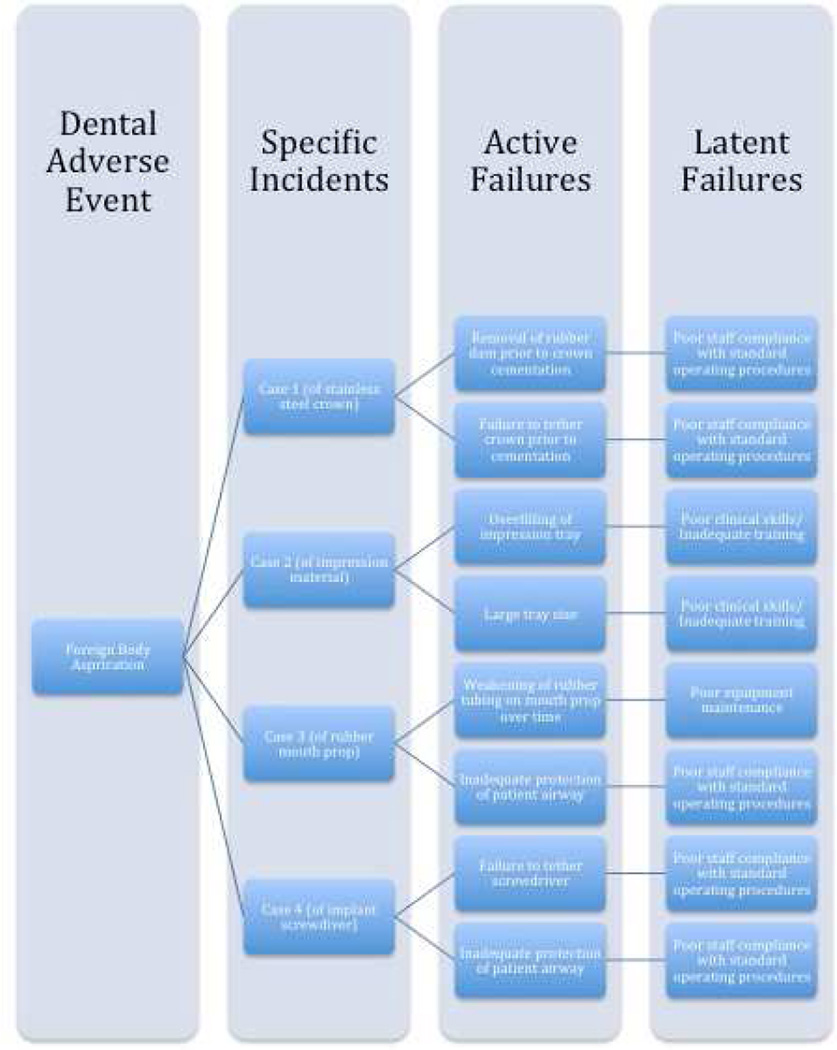
To illustrate the potential sense-making35 and learning opportunities present in a case report, a causal tree was constructed on the basis of information provided in one report where a root cause analysis was performed (Figure 2).36 Causal trees, also called fault or risk trees, are powerful visual tools for depicting a causal analysis of a patient safety event.35 They are useful for uncovering the underlying factors, circumstances, and decisions that contributed to the event. Figure 3 illustrates the benefits of examining case reports in the aggregate. This approach allows for the easy identification of common risk factors or latent failures and this is critical to understanding dental adverse events and preventing their future re-occurrence.37 Consider another example of a case report that did not provide sufficient information for a root cause analysis:
“A 78 year old black male presented to the oral and maxillofacial clinic at Columbia University. He had been referred on an emergency basis from the adjacent senior dental student clinic when his lower and upper lips suddenly swelled during the performance of complete denture impressions. The impressions were being made using permlastic, a polysulfide impression material… denied allergies… on exam, the patient appeared not to be in acute distress…. displayed significant lower lip edema with moderate upper lip edema… patient was given Benadryl 50mg intramuscularly and accompanied to the emergency room for observation… patient was discharged after five hours of observation with significantly decreased labial edema.”38
Figure 2.
Sample causal tree diagram for a dental adverse event case report. From top to bottom, this figure illustrates how the occurrence of an adverse event (soft-tissue injury, top row) can be traced to its root causes (bottom row) by continuously asking why when performing a root cause analysis.
Figure 3.
Hypothetical illustration of incident analyses from aggregated case reports. This figure shows, from left to right, that recurrent latent failures in a dental care delivery system (column 4) become apparent following the review of aggregated case reports (column 2). In this case, the adverse event (foreign body aspiration, column 1) occurred due to active failures (column 3) by frontline providers but can be traced to hidden latent failures in the care delivery system.
There was no documentation of any follow up with the patient after this encounter; information about whether a patch test was done to confirm the implied cause of the edema and information about the continued clinical course of the patient would have added value to the case report. The authors also did not report on the factors that might have contributed to or mitigated against the occurrence and severity of this adverse event. This is not intended to serve as an indictment of the authors of the case report, as it merely highlights the variability of content that has characterized case reports. However, it represents a missed learning opportunity for other dental professionals.
In light of the various issues discussed above, the authors conclude that:
Errors are commonplace in dentistry, it is our imperative as dental professionals to prevent them from occurring, trap them before they lead to an adverse event and/or mitigate their effects when an adverse events occur.
Identifying errors and the causes of dental adverse events is the first step towards a dental patient safety initiative aimed at reducing adverse events professionwide.
Dentistry can learn from the successes of other industries and adopt their safety systems including: establishing a broad-based nonpunitive dental patient safety reporting system, performing root cause analyses, and translating CRM techniques into dentistry.
Case reports provide a window into learning about the nature and extent of dental adverse events in the absence of a broad-based reporting system.
Acknowledgments
Special thanks to Sawsan Salih, instructor, Global and Community Health, Department of Oral Health Policy and Epidemiology, Harvard School of Dental Medicine, for her contribution to the data extraction portion of the study.
APPENDIX 1
Dental Adverse Event Type of Harm Classification
Allergy/Hypersensitivity reactions
Aspiration of foreign body
Delayed appropriate treatment/Disease progression and/or unnecessary treatment associated with misdiagnosis
Foreign body response/rejection
Hard-tissue damage
Harm, not otherwise specified
Ingestion of foreign body
Nerve damage or injury
Ocular damage
Orofacial infection
Other orofacial complications
Other systemic complications including adverse reactions to device/materials/procedure
Other Wrong/unnecessary treatment
Poor aesthetic results postdental treatment
Poor hemostasis/prolonged bleeding
Procedure on wrong patient
Procedure on wrong site
Psychological distress/disorder (including suicide)
Retention of foreign object(s) in patient with sequela (e)
Soft tissue injury/inflammation
Systemic infection
Toxicity-drug overdose
Transmission of infectious disease(s) 2
APPENDIX 2
Dental Adverse Event Severity Scale
Category A: Circumstances or events that have the capacity to cause error
Category B: An error that did not reach the patient
Category C: An error that reached the patient but did not cause harm
Category D: An error that reached the patient and required monitoring or intervention to confirm that it resulted in no harm to the patient (FDA medical device type 1: I.e. Patient treated with contaminated water in operatory–after f/u no evidence of harm; expired material or drug.
Category E1: Temporary (reversible or transient) minimal harm to the patient and required minimal intervention (FDA medical device type2: required intervention, healed or resolved with no permanent defect or disability. Stable and stationary).
Category E2: Temporary (reversible or transient) minimal harm to the patient and required significant intervention (FDA medical device type2: required intervention, healed or resolved with no permanent defect or disability. Stable and stationary).
Category E3: Temporary (reversible or transient) significant harm to the patient and required minimal intervention (FDA medical device type2: required intervention, healed or resolved with no permanent defect or disability. Stable and stationary).
Category E4: Temporary significant harm to the patient and required significant intervention (FDA medical device type2: required intervention, healed or resolved with no permanent defect or disability. Stable and stationary).
Category F: Temporary harm to the patient and required transfer to emergency room and/or hospitalization
Category G1: Permanent minimal patient harm requiring minimal intervention (FDA medical device type 3: required intervention, healed with permanent defect or disability, stable and stationary).
Category G2: Permanent minimal patient harm requiring significant intervention (FDA medical device type 3: required intervention, healed with permanent defect or disability, stable and stationary). E.g: lost tooth due to wrong extraction, iatrogenic pulpal damage.
Category G3: Permanent significant patient harm requiring minimal intervention (FDA medical device type 3: required intervention, healed with permanent defect or disability, stable and stationary).
Category G4: Permanent significant patient harm requiring significant intervention (FDA medical device type 3: required intervention, healed with permanent defect or disability, stable and stationary). E.g: lost tooth due to wrong extraction, needing implant or pros replacement; iatrogenic pulpal damage needing endodontic treatment.
Category H: Intervention required to sustain life
Category I: Patient death (FDA medical device type 4)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure. None of the authors reported any disclosures.
Contributor Information
Enihomo M. Obadan, Email: Enihomo_obadan@hsdm.harvard.edu, Department of Oral Health Policy and Epidemiology, Harvard School of Dental Medicine, 188 Longwood Avenue, Boston, MA 02115.
Rachel B. Ramoni, Oral Health Policy and Epidemiology, Harvard School of Dental Medicine, Boston, MA, and the executive director, Undiagnosed Diseases Coordinator Center, Center for Biomedical Informatics, Harvard Medical School, Boston, MA.
Elsbeth Kalenderian, Department of Oral Health Policy and Epidemiology, Harvard School of Dental Medicine, Boston, MA, and the chief of quality, Harvard Dental Center, Boston, MA.
References
- 1.Yamalik N, Perea Pérez B. Patient safety and dentistry: what do we need to know? Fundamentals of patient safety, the safety culture and implementation of patient safety measures in dental practice. International dental journal. 2012;62(4):189–196. doi: 10.1111/j.1875-595X.2012.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamalik N. Patient safety and quality assurance and improvement. Indian J Dent Res. 2014;25(2):139–141. doi: 10.4103/0970-9290.135898. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. The National Academies Press; 2001. [PubMed] [Google Scholar]
- 4.Dental Quality Alliance. Quality measurement in dentistry: a guidebook. American Dental Association; 2012. Advisory Committee on Education and Communication. [Google Scholar]
- 5.Kohn LT, Corrigan JM, Donaldson MS. To err is human: building a safer health system. National Academies Press; 2000. [PubMed] [Google Scholar]
- 6.Rockville, MD: 2014. [Accessed August 2014]. Agency for Healthcare Research and Quality Outpatient Diagnostic Errors Affect 1 in 20 U.S. Adults, AHRQ Study Finds. " http://www.webcitation.org/query?url=http%3A%2F%2Fwww.ahrq.gov%2Fnews%2Fnewsroom%2Fpress-releases%2F2014%2Fdiagnostic_errors.html.&date=2014-09-28. [Google Scholar]
- 7.Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370–376. doi: 10.1056/NEJM199102073240604. [DOI] [PubMed] [Google Scholar]
- 8.Leape LL. Error in medicine. JAMA-Journal of the American Medical Association-US Edition. 1994;272(23):1851–1856. [PubMed] [Google Scholar]
- 9.World Health Organization. World Health Organization; 2005. World alliance for patient safety: WHO draft guidelines for adverse event reporting and learning systems: from information to action. [Google Scholar]
- 10.Hiivala N, Mussalo-Rauhamaa H, Murtomaa H. Patient safety incidents reported by Finnish dentists; results from an internet-based survey. Acta Odontol Scand. 2013;71(6):1370–1377. doi: 10.3109/00016357.2013.764005. [DOI] [PubMed] [Google Scholar]
- 11.Thusu S, Panesar S, Bedi R. Patient safety in dentistry - state of play as revealed by a national database of errors. Br Dent J. 2012;213(3):E3. doi: 10.1038/sj.bdj.2012.669. [DOI] [PubMed] [Google Scholar]
- 12.Kalenderian E, Walji MF, Tavares A, Ramoni RB. An adverse event trigger tool in dentistry: A new methodology for measuring harm in the dental office. J Am Dent Assoc. 2013;144(7):808–814. doi: 10.14219/jada.archive.2013.0191. [DOI] [PubMed] [Google Scholar]
- 13.Reason J. Human error: models and management. 2000 doi: 10.1136/bmj.320.7237.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helmreich RL. On error management: lessons from aviation. 2000 doi: 10.1136/bmj.320.7237.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson P. Applying the lessons of high risk industries to health care. Quality and Safety in Health Care. 2003;12(suppl 1):i7–i12. doi: 10.1136/qhc.12.suppl_1.i7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Department of Transportation. Federal Aviation Administration. Aviation Safety Action Program (ASAP): AC 120-66B. 2002 Nov
- 17.US Department of Transportation. Federal Aviation Administration. Flight Operations Quality Assurance (FOQA). AC 120-82. 2004 Apr
- 18.Pizzi L, Goldfarb NI, Nash DB. Crew resource management and its applications in medicine. Making health care safer: A critical analysis of patient safety practices. 2001;44:511–519. [Google Scholar]
- 19.Leape LL. Reporting of Adverse Events. New England Journal of Medicine. 2002;347(20):1633–1638. doi: 10.1056/NEJMNEJMhpr011493. [DOI] [PubMed] [Google Scholar]
- 20.Silver Spring, MD: US Food and Drug Administration; [Accessed August 2014]. US Department of Health and Human Services Medwatch: The FDA Safety Information and Adverse Event Reporting System. " http://www.webcitation.org/query?url=https%3A%2F%2Fwww.accessdata.fda.g ov%2Fscripts%2Fmedwatch%2F&date=2014-09-28. [Google Scholar]
- 21.Pinsky HM, Taichman RS, Sarment DP. Adaptation of airline crew resource management principles to dentistry. J Am Dent Assoc. 2010;141(8):1010–1018. doi: 10.14219/jada.archive.2010.0316. [DOI] [PubMed] [Google Scholar]
- 22.Seager L, Smith DW, Patel A, Brunt H, Brennan PA. Applying aviation factors to oral and maxillofacial surgery--the human element. Br J Oral Maxillofac Surg. 2013;51(1):8–13. doi: 10.1016/j.bjoms.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Ramoni RB, Walji MF, White J, et al. From good to better: toward a patient safety initiative in dentistry. J Am Dent Assoc. 2012;143(9):956–960. doi: 10.14219/jada.archive.2012.0303. [DOI] [PubMed] [Google Scholar]
- 24.Ramoni R, Walji MF, Tavares A, et al. Open Wide: Looking into the Safety Culture of Dental School Clinics. Journal of Dental Education. 2014;78(5):745–756. [PubMed] [Google Scholar]
- 25.Agency for Healthcare Research and Quality. AHRQ's Patient Safety Initiative: Building Foundations, Reducing Risk: Interim Report to the Senate Committee on Appropriations. Rockville, MD: 2003. [Google Scholar]
- 26.Al-Turki M, Akpata ES. Penetrability of dentinal tubules in adhesive-lined cavity walls. Oper Dent. 2002;27(2):124–131. [PubMed] [Google Scholar]
- 27.Griffin F, Resar R. IHI Global Trigger Tool for measuring adverse events. Institute for Healthcare Improvement Innovation Series White Paper. 2009 [Google Scholar]
- 28.World Health Organization Annex Regional Classifications. Geneva, Switzerland: [Accessed August 2014]. " http://www.webcitation.org/query?url=http%3A%2F%2Fwww.who.int%2Fnutgrowthdb%2Fannex_regional_classifications.pdf&date=2014-09-28. [Google Scholar]
- 29.Wolf ZR, Hughes RG. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. Error Reporting and Disclosure. [PubMed] [Google Scholar]
- 30.Tokede O, Ramoni R, Kalenderian E. The value of checklists. J Am Dent Assoc. 2014;145(7):696. doi: 10.1016/s0002-8177(14)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tokede O, White J, Stark PC, et al. Assessing use of a standardized dental diagnostic terminology in an electronic health record. J Dent Educ. 2013;77(1):24–36. [PMC free article] [PubMed] [Google Scholar]
- 32.Kalenderian E, Ramoni RL, White JM, et al. The Development of a Dental Diagnostic Terminology. Journal of Dental Education. 2011;75(1):68–76. [PMC free article] [PubMed] [Google Scholar]
- 33.Obadan E, Kalenderian E, Ramoni RB. CASE REPORTS HAILED. The Journal of the American Dental Association. 2014;145(9):912–914. doi: 10.1016/s0002-8177(14)60134-3. [DOI] [PubMed] [Google Scholar]
- 34.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case report guideline development. Journal of clinical epidemiology. 2014;67(1):46–51. doi: 10.1016/j.jclinepi.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Battles JB, Dixon NM, Borotkanics RJ, Rabin-Fastmen B, Kaplan HS. Sensemaking of patient safety risks and hazards. Health Serv Res. 2006;41(4 Pt 2):1555–1575. doi: 10.1111/j.1475-6773.2006.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arakeri G, Brennan PA. Inadvertent injection of formalin mistaken for local anesthetic agent: report of a case. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2012;113(5):581–582. doi: 10.1016/j.tripleo.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Uberoi R, Swati E, Gupta U, Sibal A. Root Cause Analysis in Healthcare. Apollo Medicine. 2007;4(1):72–75. [Google Scholar]
- 38.Aziz SR, Tin P. Spontaneous angioedema of oral cavity after dental impressions. N Y State Dent J. 2002;68(2):42–45. [PubMed] [Google Scholar]