Abstract
Tendon disorders are common and lead to significant disability, pain, healthcare cost, and lost productivity. A wide range of injury mechanisms exist leading to tendinopathy or tendon rupture. Tears can occur in healthy tendons that are acutely overloaded (e.g., during a high speed or high impact event) or lacerated (e.g., a knife injury). Tendinitis or tendinosis can occur in tendons exposed to overuse conditions (e.g., an elite swimmer’s training regimen) or intrinsic tissue degeneration (e.g., age-related degeneration). The healing potential of a torn or pathologic tendon varies depending on anatomic location (e.g., Achilles vs. rotator cuff) and local environment (e.g., intrasynovial vs. extrasynovial). Although healing occurs to varying degrees, in general healing of repaired tendons follows the typical wound healing course, including an early inflammatory phase, followed by proliferative and remodeling phases. Numerous treatment approaches have been attempted to improve tendon healing, including growth factor- and cell-based therapies and rehabilitation protocols. This review will describe the current state of knowledge of injury and repair of the three most common tendinopathies-- flexor tendon lacerations, Achilles tendon rupture, and rotator cuff disorders-- with a particular focus on the use of animal models for understanding tendon healing.
Keywords: animal models, inflammation, healing, tendinopathy
Epidemiology and Etiology of Tendon Injury
Flexor tendon injury occurs most commonly by laceration, with the highest incidence in persons aged 20–29 years, with a higher incidence in males than females.1 Work-related injuries account for ~25% of acute traumatic flexor tendon injuries, most commonly in construction and extraction (44%), food preparation and serving (14%), and transportation and material moving (12%) occupations.1 The Achilles tendon, the largest and strongest tendon in the human body, is involved in as much as half of all sports-related injuries. The vast majority (~75%) of Achilles tendon ruptures occur in men aged 30–49, and participating in a sports activity is the most common etiologic factor for injury.2,3 Biopsies retrieved at surgery have demonstrated degenerative changes in most ruptured Achilles tendons4, suggesting that Achilles tendon ruptures could be characterized as acute trauma of chronically degenerated tendons. Rotator cuff disorders are the most common causes of shoulder disability and are very common in the aging population5. Full-thickness rotator cuff tears are present in approximately 13% of individuals in their 50s6, 25% of individuals in their 60s and 50% of individuals in their 80s5. The etiology of rotator cuff tearing is multifactorial and likely a combination of age-related degenerative changes7 and micro/macrotrauma. Besides age, smoking, hypercholesterolemia, and family history have been shown to predispose individuals to rotator cuff tearing5. It should be appreciated that injuries to the flexor and rotator cuff tendons are intra-synovial and do not undergo spontaneous healing, whereas injury to the Achilles tendon is extra-synovial where fibrous tissue formation can and does occur after injury. Since the local environment and mechanisms of tendon injury are quite different among these three tendinopathic conditions, research questions and models must be framed in the context of these distinctions to produce clinically relevant studies that can eventually be translated to clinical care.
Animal Models of Tendon Injury and Repair
Animal models are the primary means by which fundamental and translational questions related to the complex processes of tendon injury, healing, and repair are investigated. In general, the specific research question should drive the choice of animal model (Table 1). Below, we provide some considerations for choosing appropriate animal models in tendon injury and repair research. The citations provided are intended to be representative of the various animal models and are by no means exhaustive.
Table 1.
Animal models for studying tendon healing.
| Research Question | Animal Models |
|---|---|
| Basic Mechanisms of Chronic Tendon Injury |
Models of Inducing Chronic Tendon Injury (Mouse, Rat, Rabbit, Sheep)
|
| Basic Mechanisms of Tendon Healing |
Models of Tendon Healing (Mouse, Rat, Rabbit) A) Intra-tendinous healing following injury induced by:
B) Tendon-bone healing following sharp transection andsuture repair to bone |
| Translation to Clinical Care |
Translational Models of Tendon Injury and Repair
|
Animal models for examining basic mechanisms of chronic tendon injury
Understanding the basic mechanisms of chronic tendon degeneration and subsequent injury would allow for the prevention and/or early treatment of ruptures. This is particularly relevant to the rotator cuff or Achilles tendon, as they typically advance through chronic, degenerative conditions over extended time prior to injury. Chronic tendon injuries are a common musculoskeletal problem in horses8, however, naturally occurring equine flexor tendon injury is impractical for broad use as a research model because the severity of equine disease is highly variable and there are practical issues related to animal size, housing and cost. Hence, investigators have used various methods to artificially induce chronic tendon injuries in animal models. For overuse injuries, uphill or downhill treadmill running in rats or mice has been used to induce injury to the rotator cuff9 or Achilles tendons10, respectively. Other investigators have induced overuse injury by applying controlled fatigue loading directly to the patellar tendons of anesthesized rats11 and mice12. Still others have used full-thickness, partial-width, laceration of the infraspinatus tendon in sheep to induce overstressed and stress-deprived portions of the tendon13. Finally, collagenase injection has been used to induce chronic tendon injury in the rat14 rabbit15 and sheep16 models. While all of these models capture important aspects of tendon degeneration and injury, it is important to keep in mind that none captures the complete etiology of the chronic tendon injuries seen in human patients. Therefore, care must be taken in the choice and interpretation of the animal model used.
Animal models for examining basic mechanisms of tendon healing
Understanding the basic mechanisms of tendon healing would inform the development of new treatments strategies for tendon repair. Animal models to investigate intra-tendinous healing in the absence of repair have largely been performed in rat and mouse models of Achilles, patellar, and flexor tendon injury, where injuries have been induced by a variety of methods, including full-width sharp17 or blunt transection18, punch biopsy or window defect19, collagenase injection14, partial-width incision19, or needle stick20. The healing of intra-tendinous injury with repair has been investigated using mouse and rat models of flexor or Achilles tendon mid-substance tenotomy21 or tenectomy22. The healing of tendon-to-bone repair has largely been studied in mouse and rat models of rotator cuff23, Achilles24 and flexor tendon25 injury by sharp transection and then suture repair to bone. Tendon-to-bone healing has also been studied in the rabbit rotator cuff model26. The mouse is a particularly attractive animal model to study tendon healing due to the availability of a wide range of genetically manipulated targets thought to be involved in tendon healing and regeneration. Critical pathways of healing can therefore be studied in a mechanistic manner. A recent paper also describes tendon development in the zebrafish27, introducing an additional animal model for studying tendon biology to the community that is even easier to manipulate genetically than the mouse. Although it is implicitly assumed that the mechanisms observed in animal models are generalizable to the biology of human tendon healing, the extent to which the mechanisms of inducing injury, the particular tendon that is injured, or the age or species of the animal influence findings is currently unknown. Hence further work is needed to validate the generalizability and translatability of basic science studies of tendon healing in these animal models.
Animal models for translation to clinical care
A number of patient, surgical and post-operative influences should be considered in the choice and development of translational animal models for tendon repair and healing. The research variables of interest may include surgical technique, co-morbidities (e.g., obesity, smoking), repair augmentation strategies (e.g., grafts, cells, growth factors), and post-operative loading (rehabilitation). Because the goal of these research questions is translation to patient care, the animal model used should reflect the specific tendon of interest and incorporate clinically relevant features to the extent possible. Although the use of translational animal models may not allow for unraveling mechanistic links between functional outcomes and underlying biological events, results are intended to inform and improve clinical practice.
Flexor Tendon Repair
Translational studies of flexor tendon repair have largely been performed in the canine model. Dog flexor tendon anatomy is similar to humans28, and they are large enough to perform an operative repair that is identical to that used in clinical practice. The canine flexor tendon repair model has been used over the past thirty years to investigate strategies for optimal suture repair29, autogenic and allogenic graft repair30, enhancing tendon-tendon or tendon-bone repair using growth factors, cells and other therapeutics31, and reducing tendon adhesions using biologic lubricants32,33. Furthermore, post-operative rehabilitation can be controlled in the canine model using a specially designed removable cast system which allows for replication of the controlled physical therapy that patients receive after tendon repair. The canine model has been used extensively to investigate the optimal rehabilitation parameters following flexor tendon repair34.
Achilles Tendon Repair
Translational studies of Achilles tendon repair include those which investigate intra-tendinous repair as well as tendon-bone repair. The canine35 and rabbit36 have been used for translational studies of Achilles tendon repair using a variety of techniques and therapeutics for several decades. Their size allows for clinically relevant operative technique, and each model can be manipulated to control post-operative rehabilitation through casting and/or treadmill activity37,38. In more recent years, the rat model of Achilles tendon repair has been used extensively as well39. Although its size limits the use of some standard-of-care surgical techniques, post-operative loading of the repair can also be controlled through a variety of means such as casting, treadmill running or swimming in the rat model40–42.
Rotator Cuff Repair
The rat model has been used most extensively to study the factors and strategies that influence rotator cuff repair43. The rat’s bony and muscle anatomy greatly resembles that of humans44. Re-tear of rotator cuff repairs has not been observed post-operatively in the rat model45. Hence, the rat model lends itself particularly well to studying regenerative (biologic) strategies for rotator cuff repair, but is a less suitable model for translational studies of mechanically motivated standard-of-care repair techniques and strategies. The rat has been used to study the effect of post-operative activity levels46 (see section below), chronic tears47, and chronic tears followed by surgical repair48. Because chronic tendon tears in the rat are reparable through at least 16 weeks 48, the rat allows for studies of tendon-to-bone repair in the context of a clinically relevant chronic tendon injury, although in the absence of persistent degenerative muscle changes49.
Large animals, such as the rabbit, dog, sheep and goat, have also been used to study surgical techniques50 and regenerative strategies for rotator cuff repair, including using growth factors51, scaffold interposition52 and scaffold augmentation53. Because of their size, many standard-of-care surgical techniques can be reproduced in large animals. However, rotator cuff repairs in large animals uniformly undergo re-tear post-operatively54–56, which confounds interpretation of the mechanical effectiveness of various repair strategies for the human condition. Further, the high incidence of tendon re-tear makes large animal models less suited to study biologic treatments aimed at tendon-to-bone healing because of the difficulty keeping the tendon and bone in close proximity after repair. The sheep and canine do not lend themselves to the study of chronic rotator cuff repair because chronically released rotator cuff tendons become irreparable after approximately 6 weeks57,58. Rabbit rotator cuff tendons, however, are reparable out to 12 weeks59. As a consequence of chronic tendon release, significant muscle atrophy and fatty infiltration develop and persist in large animal models58,60,61 making them well-suited to study the mechanism and treatment of associated rotator cuff muscle pathology. A more exhaustive review of animal models for rotator cuff repair can be found elsewhere62.
Tendon healing
Inflammation, proliferation, and remodeling in tendon healing
Tendon healing after surgical repair generally progresses through a short inflammatory phase, which lasts about a week, followed by a proliferative phase, which lasts a few weeks, followed by a remodeling phase, which lasts many months.63 During the inflammatory phase, vascular permeability increases and an influx of inflammatory cells enter the healing site. These cells produce a number of cytokines and growth factors that lead to recruitment and proliferation of macrophages and resident tendon fibroblasts. During the proliferative and remodeling phases of healing, fibroblasts proliferate and begin to produce, deposit, orient, and crosslink fibrillar collagens.
Tendon healing generally involves the contributions of cells from multiple sources, including infiltrating inflammatory cells, resident fibroblasts from the tendon surface or midsubstance, and tendon or marrow-derived mesenchymal stem cells. Yet the specific cellular events in healing depend on the anatomy and physiology of a given tendon injury and repair. For example, healing of flexor tendon injuries begins with angiogenesis and epitenon fibroblast migration to the wound site.64,65 Cells from the intrasynovial sheath infiltrate to the repair site, leading to adhesions between the sheath and the tendon surface, which impairs tendon gliding (and hence decreases digital range of motion).65 In the rotator cuff, on the other hand, injuries typically require repair of tendon to bone. In this case, abundant fibroblasts from the tendon and surrounding tissues produce a disorganized collagen scar tissue at the attachment site of the two tissues.46 Osteoclasts are also attracted to the repair site, and resorption of bone at the repair site can impair healing.66 Understanding how different tendons heal is an important consideration for post-operative treatment and rehabilitation.
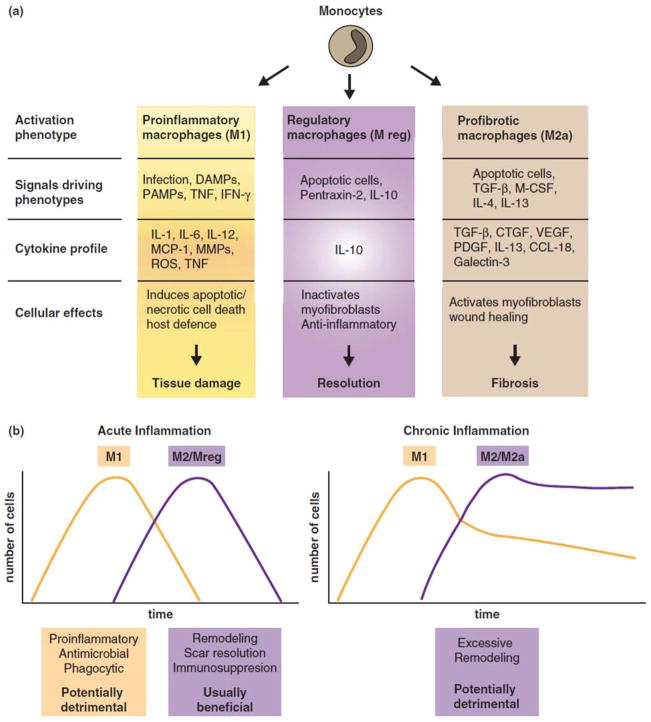
Recent evidence suggests that modulation of inflammation in the early stages following tendon repair may lead to improved healing.67 It is important to recognize that regulated inflammation is largely beneficial to tissue repair, whereas excessive or persistence inflammation can be damaging. Indeed, whereas inflammatory cytokines attract fibroblasts to the repair site, excessive inflammation may lead to poor clinical outcomes.68,69 Macrophages play essential roles in both promoting and resolving inflammation and in both facilitating and moderating tissue repair (Figure 1). That a single cell type can serve opposing functions may seem counterintuitive, but dramatic phenotypic changes occur when macrophages respond to local stimuli.69,70 Macrophages are broadly classified into two groups, classically activated (M1) or alternatively activated (M2) cells, although it is important to note that many more phenotypes exist, each driven by specific activation conditions (Figure 1).70 M1 macrophages, which are stimulated by bacterial products or Th1 cytokines, are pro-inflammatory (via release of IL1β, IL12, TNFα, others) and stimulate scarring and fibrosis. M2 macrophages, which are induced by Th2 cytokines, are anti-inflammatory (via release of IL10, TGFβ1, others) and are effective at clearing excess extracellular matrix (ECM) in scars. In an injury setting, M1 cells predominate early, whereas M2 macrophages accumulate later.69 Ablation studies in liver, skin, and tendon show that during the early stages post-injury, macrophages (presumably M1) promote repair processes (i.e., re-epithelialization, myofibroblast activation, scarring, etc.) and inflammation, whereas at later stages, these cells (presumably M2) suppress inflammation and resolve scarring. Hence, in tendon injury, it would be reasonable to hypothesize that M1 macrophages promote repair by stimulating ECM production and that later on M2 macrophages repress inflammation and clear excess ECM, a concept that is consistent with experimental evidence.68 Disturbing the balance between these macrophage subtypes may result in defective repair and impaired tissue function. For example, over-activation or an abundance of M1 macrophages could lead to deleterious inflammation and excess ECM production, whereas sustained or an excess of M2 cells could cause excess tissue remodeling resulting in tissue damage. Thus, understanding the signals that control macrophage activation will provide fundamental insights to how tissue repair processes are orchestrated and balanced.
Figure 1.
(a) Macrophages can differentiate into specific subpopulations with distinct phenotypes and functions. (b) In acute inflammation, macrophage phenotypes such as M2 and Mreg are usually beneficial for immunosuppression, scar resolution, and remodeling. In chronic inflammation, macrophage phenotypes such as M2 and M2a can stimulate excessive tissue remodeling resulting in fibrosis. [Reproduced, with permission, from 69]
A number of growth factors, powerful regulators of biological function, play important roles during the remodeling phase of tendon healing.71 The patterns of natural expression of platelet derived growth factor (PDGF-BB), basic fibroblast growth factor (bFGF), transforming growth factor β (TGF-β), and vascular endothelial growth factor (VEGF) vary dramatically over time during tendon healing. Manipulation of the growth factor environment has therefore been an important strategy for improving the outcomes of repaired tendon and ligament.32,72 PDGF and bFGF have been effective in promoting fibroblast proliferation and collagen remodeling; however, bFGF has also been shown to promote adhesions in a flexor tendon animal model.32,73 TGF-β has received particular attention in the tendon literature due to its critical role in tendon development and its potent affect in promoting matrix remodeling.74 Furthermore, fetal tendon wounds have been shown to heal in a regenerative manner (i.e., the repaired tissue is identical to the original tissue) and this process may be regulated by TGF-β isoforms.75,76 Specifically, regenerative fetal wound healing was characterized by low expression of TGF-β1 and TGF-β2 and high expression of TGF-β3. In contrast, adult scar-mediated wound healing was characterized by high levels of TGF-β1 and TGF-β2 and low levels of TGF-β3. However, therapeutic application of this concept has not to date been successful, as control of TGF-β isoforms during tendon-to-bone healing in a rat rotator cuff model did not lead to regenerative healing.77 These initial therapeutic studies using growth factors to improve tendon healing demonstrate that dosage, time of administration, residence time and synergistic effects significantly complicate the use of growth factors as a treatment strategy.
Rehabilitation strategies for enhanced tendon healing
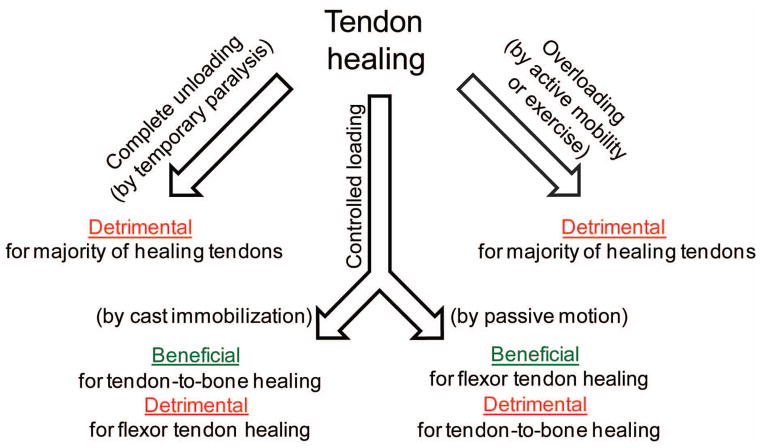
Tendon development, homeostasis, and healing are influenced by their loading environments.78 Muscle loading is necessary for tendon development and maintenance of adult tendon mass and mechanical properties. The effects of loading on healing tendons, however, are complex. Optimal post-repair rehabilitation strategies for tendon depend on the particular tendon’s environment and functional requirements. For example, successful repair of flexor tendons requires both gliding and strength for digital function. Immobilization after repair of flexor tendons leads to adhesions between the tendon and its synovial sheath, limiting tendon excursion and hence decreasing finger range of motion and tendon strength.79 Passive motion rehabilitation, on the other hand, has been shown in animal models and clinical practice to greatly improve post-repair function, leading to improved tendon gliding and increased repair strength compared to both immobilization and active force rehabilitation.80
In contrast, studies in the rat rotator cuff model have suggested a beneficial effect of immobilization to prevent post-repair gapping and aid in healing.46 Protective immobilization was shown to improve healing compared to other post-repair loading protocols such exercise or complete tendon unloading.46 The mechanisms behind the benefits of immobilization are unclear, but likely include mechanical (i.e., prevention of gap formation) and biologic effects (e.g., reduced phagocytic macrophage accumulation81). Recent evidence that rotator cuff re-tears in human patients occur within the first 3–6 months post-operation82,83 supports a conservative approach to rehabilitation after repair, though to date no apparent advantage or disadvantage of shoulder immobilization compared with early passive range of motion has been shown.84
In summary, rehabilitation strategies must balance the negative outcomes that can arise from immobilization (e.g., increased adhesions, retarded repair tissue maturation, joint stiffness) with the negative outcomes that can arise from too much load (e.g., repair tissue rupture) (Figure 2). Furthermore, the particular anatomy and functional requirement of a given tendon repair must be considered when determining the optimal rehabilitation scenario.
Figure 2.
To achieve effective healing, a balance must be reached between loads that are too low (leading to increased adhesions, retarded repair tissue maturation, and/or joint stiffness) and loads that are too high (leading to repair site gapping or rupture).78
Conclusions and open questions
Treatment of tendon injuries is a significant clinical challenge. The basic science of intra-tendinous and tendon-bone healing remains only partially understood. Over the past three decades, advances have been made in the treatment of certain tendinopathies by first understanding injury and healing in animal models and then translating that understanding to clinical care. Despite these advances, a number of open questions remain, including:
Does it matter which injury mechanism and/or tendon we use to explore the basic science of tendon healing?
What is the “Goldilocks balance” for macrophage response (e.g., M1 vs. M2), rehabilitation (e.g., immobilization vs. loading), and other mechanisms that influence repair?
What are the appropriate animal models for basic and translational questions related to various tendon injury and repair scenarios?
How can we harness the power of growth factors to improve tendon repair?
Are there translational questions that cannot be answered in animal models?
How does physical therapy translate to cell-level responses?
Acknowledgments
Support from the National Institutes of Health (AR057836, AR060820).
References
- 1.de Jong JP, Nguyen JT, Sonnema AJ, et al. The incidence of acute traumatic tendon injuries in the hand and wrist: a 10-year population-based study. Clinics in orthopedic surgery. 2014;6:196–202. doi: 10.4055/cios.2014.6.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarvinen TA, Kannus P, Maffulli N, et al. Achilles tendon disorders: etiology and epidemiology. Foot and ankle clinics. 2005;10:255–266. doi: 10.1016/j.fcl.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a United States population. Foot & ankle international/American Orthopaedic Foot and Ankle Society [and] Swiss Foot and Ankle Society. 2013;34:475–480. doi: 10.1177/1071100713477621. [DOI] [PubMed] [Google Scholar]
- 4.Astrom M, Rausing A. Chronic Achilles tendinopathy. A survey of surgical and histopathologic findings. Clin Orthop Relat Res. 1995;(316):151–164. [PubMed] [Google Scholar]
- 5.Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31:589–604. doi: 10.1016/j.csm.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Tempelhof S. Age-related prevalance of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg. 1999;8:296–299. doi: 10.1016/s1058-2746(99)90148-9. [DOI] [PubMed] [Google Scholar]
- 7.Longo UG, Franceschi F, Ruzzini L, et al. Histopathology of the supraspinatus tendon in rotator cuff tears. Am J Sports Med. 2008;36:533–538. doi: 10.1177/0363546507308549. [DOI] [PubMed] [Google Scholar]
- 8.Goodship AE, Birch HL, Wilson AM. The pathobiology and repair of tendon and ligament injury. The Veterinary clinics of North America Equine practice. 1994;10:323–349. doi: 10.1016/s0749-0739(17)30359-0. [DOI] [PubMed] [Google Scholar]
- 9.Soslowsky LJ, Thomopoulos S, Tun S, et al. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- 10.Pingel J, Wienecke J, Kongsgaard M, et al. Increased mast cell numbers in a calcaneal tendon overuse model. Scand J Med Sci Sports. 2013;23:e353–360. doi: 10.1111/sms.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andarawis-Puri N, Sereysky JB, Sun HB, et al. Molecular response of the patellar tendon to fatigue loading explained in the context of the initial induced damage and number of fatigue loading cycles. J Orthop Res. 2012;30:1327–1334. doi: 10.1002/jor.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sereysky JB, Andarawis-Puri N, Jepsen KJ, et al. Structural and mechanical effects of in vivo fatigue damage induction on murine tendon. J Orthop Res. 2012;30:965–972. doi: 10.1002/jor.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith MM, Sakurai G, Smith SM, et al. Modulation of aggrecan and ADAMTS expression in ovine tendinopathy induced by altered strain. Arthritis Rheum. 2008;58:1055–1066. doi: 10.1002/art.23388. [DOI] [PubMed] [Google Scholar]
- 14.Solchaga LA, Bendele A, Shah V, et al. Comparison of the effect of intra-tendon applications of recombinant human platelet-derived growth factor-BB, platelet-rich plasma, steroids in a rat achilles tendon collagenase model. J Orthop Res. 2014;32:145–150. doi: 10.1002/jor.22483. [DOI] [PubMed] [Google Scholar]
- 15.Chang KV, Wu CH, Ding YH, et al. Application of contrast-enhanced sonography with time-intensity curve analysis to explore hypervascularity in Achilles tendinopathy by using a rabbit model. Journal of ultrasound in medicine: official journal of the American Institute of Ultrasound in Medicine. 2012;31:737–746. doi: 10.7863/jum.2012.31.5.737. [DOI] [PubMed] [Google Scholar]
- 16.Lacitignola L, Staffieri F, Rossi G, et al. Survival of bone marrow mesenchymal stem cells labelled with red fluorescent protein in an ovine model of collagenase-induced tendinitis. Veterinary and comparative orthopaedics and traumatology: VCOT. 2014;27:204–209. doi: 10.3415/VCOT-13-09-0113. [DOI] [PubMed] [Google Scholar]
- 17.Guerquin MJ, Charvet B, Nourissat G, et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J Clin Invest. 2013;123:3564–3576. doi: 10.1172/JCI67521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schizas N, Li J, Andersson T, et al. Compression therapy promotes proliferative repair during rat Achilles tendon immobilization. J Orthop Res. 2010;28:852–858. doi: 10.1002/jor.21066. [DOI] [PubMed] [Google Scholar]
- 19.Beason DP, Kuntz AF, Hsu JE, et al. Development and evaluation of multiple tendon injury models in the mouse. J Biomech. 2012;45:1550–1553. doi: 10.1016/j.jbiomech.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien EJ, Frank CB, Shrive NG, et al. Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int J Exp Pathol. 2012;93:319–331. doi: 10.1111/j.1365-2613.2012.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman BR, Sarver JJ, Buckley MR, et al. Biomechanical and structural response of healing Achilles tendon to fatigue loading following acute injury. J Biomech. 2014;47:2028–2034. doi: 10.1016/j.jbiomech.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams SB, Jr, Thorpe MA, Parks BG, et al. Stem cell-bearing suture improves Achilles tendon healing in a rat model. Foot & ankle international/American Orthopaedic Foot and Ankle Society [and] Swiss Foot and Ankle Society. 2014;35:293–299. doi: 10.1177/1071100713519078. [DOI] [PubMed] [Google Scholar]
- 23.Galatz LM, Sandell LJ, Rothermich SY, et al. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006;24:541–550. doi: 10.1002/jor.20067. [DOI] [PubMed] [Google Scholar]
- 24.Fujioka H, Thakur R, Wang GJ, et al. Comparison of surgically attached and non-attached repair of the rat Achilles tendon-bone interface. Cellular organization and type X collagen expression. Connect Tissue Res. 1998;37:205–218. doi: 10.3109/03008209809002440. [DOI] [PubMed] [Google Scholar]
- 25.Loiselle AE, Bragdon GA, Jacobson JA, et al. Remodeling of murine intrasynovial tendon adhesions following injury: MMP and neotendon gene expression. J Orthop Res. 2009;27:833–840. doi: 10.1002/jor.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi M, Itoi E, Minagawa H, et al. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg. 2006;15:371–377. doi: 10.1016/j.jse.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Chen JW, Galloway JL. The development of zebrafish tendon and ligament progenitors. Development. 2014;141:2035–2045. doi: 10.1242/dev.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potenza AD. Tendon healing within the flexor digital sheath in the dog. J Bone Joint Surg Am. 1962;44-A:49–64. [PubMed] [Google Scholar]
- 29.Winters SC, Gelberman RH, Woo SL, et al. The effects of multiple-strand suture methods on the strength and excursion of repaired intrasynovial flexor tendons: a biomechanical study in dogs. J Hand Surg Am. 1998;23:97–104. doi: 10.1016/s0363-5023(98)80096-8. [DOI] [PubMed] [Google Scholar]
- 30.Gelberman RH, Chu CR, Williams CS, et al. Angiogenesis in healing autogenous flexor-tendon grafts. J Bone Joint Surg Am. 1992;74:1207–1216. [PubMed] [Google Scholar]
- 31.Zhao C, Ozasa Y, Reisdorf RL, et al. CORR(R) ORS Richard A. Brand Award for Outstanding Orthopaedic Research: Engineering flexor tendon repair with lubricant, cells, and cytokines in a canine model. Clin Orthop Relat Res. 2014;472:2569–2578. doi: 10.1007/s11999-014-3690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomopoulos S, Das R, Silva MJ, et al. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J Orthop Res. 2009;27:1209–1215. doi: 10.1002/jor.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao C, Hashimoto T, Kirk RL, et al. Resurfacing with chemically modified hyaluronic acid and lubricin for flexor tendon reconstruction. J Orthop Res. 2013;31:969–975. doi: 10.1002/jor.22305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva MJ, Brodt MD, Boyer MI, et al. Effects of increased in vivo excursion on digital range of motion and tendon strength following flexor tendon repair. J Orthop Res. 1999;17:777–783. doi: 10.1002/jor.1100170524. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, et al. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89:621–630. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 36.Deng D, Wang W, Wang B, et al. Repair of Achilles tendon defect with autologous ASCs engineered tendon in a rabbit model. Biomaterials. 2014;35:8801–8809. doi: 10.1016/j.biomaterials.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen C, Pluhar GE. Outcome following surgical repair of achilles tendon rupture and comparison between postoperative tibiotarsal immobilization methods in dogs: 28 cases (1997–2004) Veterinary and comparative orthopaedics and traumatology: VCOT. 2006;19:246–249. [PubMed] [Google Scholar]
- 38.West JR, Juncosa N, Galloway MT, et al. Characterization of in vivo Achilles tendon forces in rabbits during treadmill locomotion at varying speeds and inclinations. J Biomech. 2004;37:1647–1653. doi: 10.1016/j.jbiomech.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 39.Kraus TM, Imhoff FB, Wexel G, et al. Stem cells and basic fibroblast growth factor failed to improve tendon healing: an in vivo study using lentiviral gene transfer in a rat model. J Bone Joint Surg Am. 2014;96:761–769. doi: 10.2106/JBJS.L.01794. [DOI] [PubMed] [Google Scholar]
- 40.Murrell GA, Jang D, Deng XH, et al. Effects of exercise on Achilles tendon healing in a rat model. Foot & ankle international/American Orthopaedic Foot and Ankle Society [and] Swiss Foot and Ankle Society. 1998;19:598–603. doi: 10.1177/107110079801900906. [DOI] [PubMed] [Google Scholar]
- 41.Bring D, Reno C, Renstrom P, et al. Prolonged immobilization compromises up-regulation of repair genes after tendon rupture in a rat model. Scand J Med Sci Sports. 2010;20:411–417. doi: 10.1111/j.1600-0838.2009.00954.x. [DOI] [PubMed] [Google Scholar]
- 42.Karpakka J, Vaananen K, Virtanen P, et al. The effects of remobilization and exercise on collagen biosynthesis in rat tendon. Acta Physiol Scand. 1990;139:139–145. doi: 10.1111/j.1748-1716.1990.tb08906.x. [DOI] [PubMed] [Google Scholar]
- 43.Thomopoulos S, Soslowsky LJ, Flanagan CL, et al. The effect of fibrin clot on healing rat supraspinatus tendon defects. J Shoulder Elbow Surg. 2002;11:239–247. doi: 10.1067/mse.2002.122228. [DOI] [PubMed] [Google Scholar]
- 44.Soslowsky LJ, Carpenter JE, DeBano CM, et al. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 45.Galatz LM, Charlton N, Das R, et al. Complete removal of load is detrimental to rotator cuff healing. Journal of Shoulder and Elbow Surgery. 2009;18:669–675. doi: 10.1016/j.jse.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 46.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. Journal of Biomechanical Engineering. 2003;125:106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 47.Gimbel JA, Van Kleunen JP, Mehta S, et al. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 2004;37:739–749. doi: 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Gimbel JA, Van Kleunen JP, Lake SP, et al. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 2007;40:561–568. doi: 10.1016/j.jbiomech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Barton ER, Gimbel JA, Williams GR, et al. Rat supraspinatus muscle atrophy after tendon detachment. J Orthop Res. 2005;23:259–265. doi: 10.1016/j.orthres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 50.Klinger HM, Buchhorn GH, Heidrich G, et al. Biomechanical evaluation of rotator cuff repairs in a sheep model: suture anchors using arthroscopic Mason-Allen stitches compared with transosseous sutures using traditional modified Mason-Allen stitches. Clin Biomech (Bristol, Avon) 2008;23:291–298. doi: 10.1016/j.clinbiomech.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Seeherman HJ, Archambault JM, Rodeo SA, et al. rhBMP-12 accelerates healing of rotator cuff repairs in a sheep model. J Bone Joint Surg Am. 2008;90:2206–2219. doi: 10.2106/JBJS.G.00742. [DOI] [PubMed] [Google Scholar]
- 52.Adams JE, Zobitz ME, Reach JS, Jr, et al. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22:700–709. doi: 10.1016/j.arthro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 53.Derwin KA, Codsi MJ, Milks RA, et al. Rotator cuff repair augmentation in a canine model with use of a woven poly-L-lactide device. J Bone Joint Surg Am. 2009;91:1159–1171. doi: 10.2106/JBJS.H.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Derwin KA, Baker AR, Codsi MJ, et al. Assessment of the canine model of rotator cuff injury and repair. J Shoulder Elbow Surg. 2007;16:S140–148. doi: 10.1016/j.jse.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodeo SA, Potter HG, Kawamura S, et al. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007;89:2485–2497. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- 56.Schlegel TF, Hawkins RJ, Lewis CW, et al. An in vivo comparison of the modified Mason-Allen suture technique versus an inclined horizontal mattress suture technique with regard to tendon-to-bone healing: a biomechanical and histologic study in sheep. J Shoulder Elbow Surg. 2007;16:115–121. doi: 10.1016/j.jse.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Coleman SH, Fealy S, Ehteshami JR, et al. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am. 2003;85-A:2391–2402. doi: 10.2106/00004623-200312000-00018. [DOI] [PubMed] [Google Scholar]
- 58.Safran O, Derwin KA, Powell K, et al. Changes in rotator cuff muscle volume, fat content, and passive mechanics after chronic detachment in a canine model. J Bone Joint Surg Am. 2005;87:2662–2670. doi: 10.2106/JBJS.D.02421. [DOI] [PubMed] [Google Scholar]
- 59.Koike Y, Trudel G, Curran D, et al. Delay of supraspinatus repair by up to 12 weeks does not impair enthesis formation: A quantitative histologic study in rabbits. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2006;24:202–210. doi: 10.1002/jor.20031. [DOI] [PubMed] [Google Scholar]
- 60.Gerber C, Meyer DC, Schneeberger AG, et al. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86-A:1973–1982. doi: 10.2106/00004623-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 61.Uhthoff HK, Matsumoto F, Trudel G, et al. Early reattachment does not reverse atrophy and fat accumulation of the supraspinatus--an experimental study in rabbits. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2003;21:386–392. doi: 10.1016/S0736-0266(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 62.Derwin KA, Baker AR, Iannotti JP, et al. Preclinical models for translating regenerative medicine therapies for rotator cuff repair. Tissue Eng Part B Rev. 2010;16:21–30. doi: 10.1089/ten.teb.2009.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47–71. doi: 10.1146/annurev-bioeng-071811-150122. [DOI] [PubMed] [Google Scholar]
- 64.Manning CN, Havlioglu N, Knutsen E, et al. The early inflammatory response after flexor tendon healing: a gene expression and histological analysis. J Orthop Res. 2014;32:645–652. doi: 10.1002/jor.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gelberman RH, Vandeberg JS, Manske PR, et al. The early stages of flexor tendon healing: a morphologic study of the first fourteen days. J Hand Surg Am. 1985;10:776–784. doi: 10.1016/s0363-5023(85)80151-9. [DOI] [PubMed] [Google Scholar]
- 66.Ditsios K, Boyer MI, Kusano N, et al. Bone loss following tendon laceration, repair and passive mobilization. J Orthop Res. 2003;21:990–996. doi: 10.1016/S0736-0266(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 67.Hays PL, Kawamura S, Deng XH, et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90:565–579. doi: 10.2106/JBJS.F.00531. [DOI] [PubMed] [Google Scholar]
- 68.Sugg KB, Lubardic J, Gumucio JP, et al. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J Orthop Res. 2014;32:944–951. doi: 10.1002/jor.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lichtnekert J, Kawakami T, Parks WC, et al. Changes in macrophage phenotype as the immune response evolves. Curr Opin Pharmacol. 2013;13:555–564. doi: 10.1016/j.coph.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Medicine. 2003;33:381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 72.Spindler KP, Dawson JM, Stahlman GC, et al. Collagen expression and biomechanical response to human recombinant transforming growth factor beta (rhTGF-beta2) in the healing rabbit MCL. Journal of Orthopaedic Research. 2002;20:318–324. doi: 10.1016/S0736-0266(01)00107-3. [DOI] [PubMed] [Google Scholar]
- 73.Thomopoulos S, Kim HM, Das R, et al. The effects of exogenous basic fibroblast growth factor on intrasynovial flexor tendon healing in a canine model. J Bone Joint Surg Am. 2010;92:2285–2293. doi: 10.2106/JBJS.I.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glass ZA, Schiele NR, Kuo CK. Informing tendon tissue engineering with embryonic development. J Biomech. 2014;47:1964–1968. doi: 10.1016/j.jbiomech.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. Journal of cell science. 1995;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 76.Beredjiklian PK, Favata M, Cartmell JS, et al. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31:1143–1152. doi: 10.1114/1.1616931. [DOI] [PubMed] [Google Scholar]
- 77.Kim HM, Galatz LM, Das R, et al. The role of transforming growth factor beta isoforms in tendon-to-bone healing. Connect Tissue Res Epub. 2011 doi: 10.3109/03008207.2010.483026. [DOI] [PubMed] [Google Scholar]
- 78.Killian ML, Cavinatto L, Galatz LM, et al. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21:228–237. doi: 10.1016/j.jse.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woo SL, Gelberman RH, Cobb NG, et al. The importance of controlled passive mobilization on flexor tendon healing. A biomechanical study. Acta Orthop Scand. 1981;52:615–622. doi: 10.3109/17453678108992156. [DOI] [PubMed] [Google Scholar]
- 80.Boyer MI, Goldfarb CA, Gelberman RH. Recent progress in flexor tendon healing. The modulation of tendon healing with rehabilitation variables. J Hand Ther. 2005;18:80–85. doi: 10.1197/j.jht.2005.02.009. quiz 86. [DOI] [PubMed] [Google Scholar]
- 81.Dagher E, Hays PL, Kawamura S, et al. Immobilization modulates macrophage accumulation in tendon-bone healing. Clin Orthop Relat Res. 2009;467:281–287. doi: 10.1007/s11999-008-0512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller BS, Downie BK, Kohen RB, et al. When do rotator cuff repairs fail? Serial ultrasound examination after arthroscopic repair of large and massive rotator cuff tears. Am J Sports Med. 2011;39:2064–2070. doi: 10.1177/0363546511413372. [DOI] [PubMed] [Google Scholar]
- 83.Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95:965–971. doi: 10.2106/JBJS.L.00708. [DOI] [PubMed] [Google Scholar]
- 84.Keener JD, Galatz LM, Stobbs-Cucchi G, et al. Rehabilitation following arthroscopic rotator cuff repair: a prospective randomized trial of immobilization compared with early motion. J Bone Joint Surg Am. 2014;96:11–19. doi: 10.2106/JBJS.M.00034. [DOI] [PubMed] [Google Scholar]