Abstract
The intestine is segmented into functionally discrete compartments (duodenum, jejunum, ileum, and colon). The present study examined whether alcohol combined with burn injury differently influences cytokine levels in different parts of the intestine. Male mice were gavaged with alcohol (~2.9 g/kg) 4 h prior to receiving a ~12.5% total body surface area full thickness burn. Mice were sacrificed 1, 3, and 7 days after injury. The intestine segments (duodenum, jejunum, ileum, and colon) were harvested, homogenized, and analyzed for inflammatory mediators (IL-6, IL-18, and KC) using their respective ELISAs. KC levels were significantly increased in the jejunum, ileum, and colon following alcohol and burn injury as compared to shams. The increase in KC was ~28-fold higher in the colon as compared to the levels observed in duodenum following alcohol and burn injury. Both IL-6 and IL-18 levels were significantly elevated in both the ileum and colon following the combined insult. There was a ~7-fold increase in IL-6 levels in the colon as compared with the duodenum after the combined insult. Levels of IL-18 were increased by ~1.5-fold in the colon as compared to the ileum following alcohol and burn injury. The data suggest that pro-inflammatory mediators are differentially expressed in the intestine following alcohol and burn injury.
Keywords: inflammation, cytokines, chemokines, ethanol, gut
Introduction
Approximately 450,000 burn injuries are reported each year in the United States (“Burn incidence and treatment in the US: 2013 fact sheet”, 2013). Nearly half of the reported burn injuries occur under the influence of alcohol (Choudhry et al., 2004; Haum et al., 1995; Jones, Barber, Engrav, & Heimbach, 1991). Multiple studies demonstrate that the combined insult results in delayed wound healing, longer hospitalization, and increased susceptibility to infection. Additionally, intoxicated patients also have significantly higher mortality rates and die from smaller burns (Choudhry et al., 2004; Haum et al., 1995; Jones et al., 1991). Experimental models of alcohol and burn injury corroborate clinical data illustrating that the combined insult results in adverse effects, including an increased innate response, particularly resulting in excess inflammation (Bird & Kovacs, 2008; Li, Akhtar, Kovacs, Gamelli, & Choudhry, 2011). Alternatively, alcohol and burn injury result in depression of the adaptive immune response with a decreased T cell response (Choudhry, Fazal, Goto, Gamelli, & Sayeed, 2002; Choudhry et al., 2004). Experimental data have also established that the combined insult further exacerbates production of inflammatory mediators (Li et al., 2011; Zahs, Bird, Ramirez, Choudhry, & Kovacs, 2013). Such an increase in inflammatory mediators following combined alcohol and burn injury is likely to contribute to multiple organ dysfunction/failure (Choudhry et al., 2004).
The intestine is the second largest immunological organ consisting of functionally discrete compartments – duodenum, jejunum, ileum, and colon. Additionally, the intestine is the largest bacterial reservoir within the body. Collectively, the intestine is responsible for nutrition absorption and maintaining an interface to prevent gut bacterial translocation (Mowat & Agace, 2014). Regions of the intestine (duodenum, jejunum, ileum, and colon) are functionally distinct and contain regional variations in antigen-presenting cells and bacterial content (Denning et al., 2011; Hao & Lee, 2004; Mowat & Agace, 2014). It is well established that the intestinal bacterial content increases progressively from the duodenum to the colon, where the colon contains the largest bacterial population (Hakansson & Molin, 2011; O’Hara & Shanahan, 2006). Both alcohol and burn injury alone perturb intestinal structural and functional integrity (Costantini et al., 2009; Gosain & Gamelli, 2005; Magnotti & Deitch, 2005). Similarly, alcohol combined with burn injury has been demonstrated to cause increased intestinal permeability and bacterial translocation (Choudhry et al., 2004; Li, Akhtar, & Choudhry, 2012; Rendon, Li, Akhtar, & Choudhry, 2013; Zahs et al., 2013). Previously, our lab also showed that acute alcohol exposure prior to burn injury results in significantly increased levels in inflammatory mediators in the terminal ileum (Li, Schwacha, Chaudry, & Choudhry, 2008; Li et al., 2011). However, regional variations of cytokine production following alcohol and burn injury have not been examined. The present study examined whether alcohol combined with burn injury differently influences the expression of cytokines in various parts of the intestine.
Materials and Methods
Animals
Adult 8–10-week-old male C57BL/6 mice (22–25 g) were obtained from Harlan Laboratories (Indianapolis, IN, USA). Mice were housed and acclimated for 2 weeks prior to experimentation. All animal procedures were conducted in accordance with the Animal Care and Use Committee at Loyola University Chicago Health Sciences Division.
Mouse model of acute alcohol intoxication and burn injury
Mice were randomly divided into four groups: sham vehicle (n = 11–12), sham alcohol (n = 10–12), burn vehicle (n = 5–8), and burn alcohol (n = 6–8). As described previously, alcohol- or water-treated mice were gavaged with 0.4 mL of 25% alcohol in water (~2.9 g/kg) or water, respectively (Li et al., 2011). Four hours after the gavage, mice were anesthetized by intraperitoneal injection with a cocktail of ketamine and xylazine (80 mg/kg and 1.25 mg/kg, respectively). The dorsal surface was shaved and mice were transferred to a template, which is fabricated to expose ~12.5% of the total body surface area (TBSA). Mice in the burn group were immersed in 85–90 °C water for 7–8 sec. Mice in the sham group were immersed in lukewarm water for 7–8 sec. Mice were then dried and resuscitated with an intraperitoneal injection of 1.0 mL physiological saline (Li et al., 2011). Mice were returned to their cages and given water and food ad libitum.
Tissue harvesting
Mice were euthanized 1, 3, and 7 days after injury. The duodenum, jejunum, ileum, and colon were removed and immediately transferred into liquid nitrogen (Li et al., 2011).
Preparation of tissue homogenates
For the measurement of inflammatory mediators, tissue from the various groups was sonicated in phosphate-buffered saline (PBS) containing a protease inhibitor cocktail (Sigma Chemical Co., St. Louis, MO). Homogenates were cleared by centrifuging at 9,000 rpm at 4 °C for 30 min and stored at −80 °C.
Measurement of cytokines in tissue homogenates
IL-6, IL-18, and KC levels in tissue homogenates were determined by enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions. Mouse IL-6 and KC ELISA kits were purchased from R&D Systems (Minneapolis, MN), and mouse IL-18 ELISA kit was purchased from eBioscience (Santa Clara, CA).
Statistical analysis
All statistical analysis was performed using ANOVA with Tukey-Kramer’s post hoc test (GraphPad InStat Software, San Diego, CA). A p < 0.05 was considered statistically significant.
Results
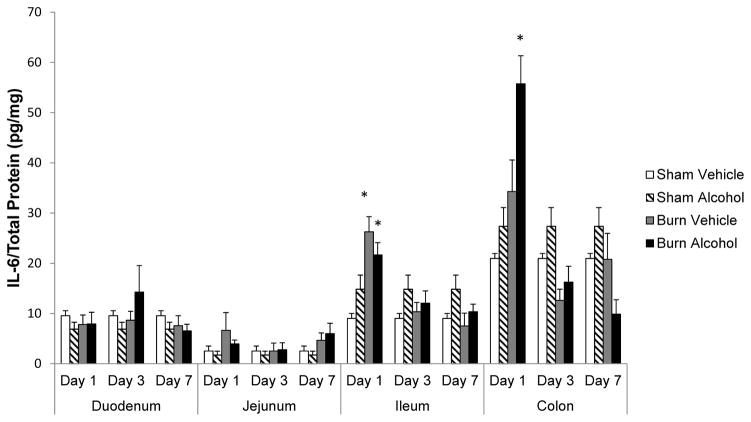
IL-6 levels significantly elevated in the ileum and colon following alcohol and burn injury
No change was observed in IL-6 levels in duodenum and jejunum following alcohol and burn injury compared to shams. IL-6 was significantly elevated in the ileum 1 day following burn injury alone compared to sham animals. A similar increase in IL-6 levels was noted in ileum and colon 1 day after a combined insult of alcohol and burn injury compared to sham. While the increases in IL-6 levels were similar (2.5-fold) in both ileum and colon after alcohol and burn injury compared to shams, the net IL-6 amount was much higher in colon compared to small intestine (e.g., duodenum, jejunum, and ileum). An analysis of the data revealed a ~7-fold increase in IL-6 levels in the colon following alcohol and burn injury as compared to duodenum and ~3-fold compared to ileum. IL-6 levels were normalized to shams by days 3 and 7 after alcohol and burn injury.
IL-18 significantly elevated in ileum and colon following alcohol and burn injury
IL-18 is a pro-inflammatory cytokine and is associated with intestinal tissue damage (Akhtar, Li, Chaudry, & Choudhry, 2009; Akhtar, Li, Kovacs, Gamelli, & Choudhry, 2011). There was no change in IL-18 levels in jejunum after alcohol and burn injury compared to shams. Although IL-18 tended to increase in duodenum 1 day following the combined insult of alcohol and burn injury, this was not found to be significant compared to shams. IL-18 levels were only significantly elevated in the ileum and colon following alcohol and burn injury compared to shams. Furthermore, in contrast to IL-6, the net elevation in IL-18 levels was only 1.5-fold higher in the colon as compared with the ileum following alcohol and burn injury. Similar to IL-6, there was no change in IL-18 levels in the intestine on days 3 and 7 after alcohol and burn injury compared to shams.
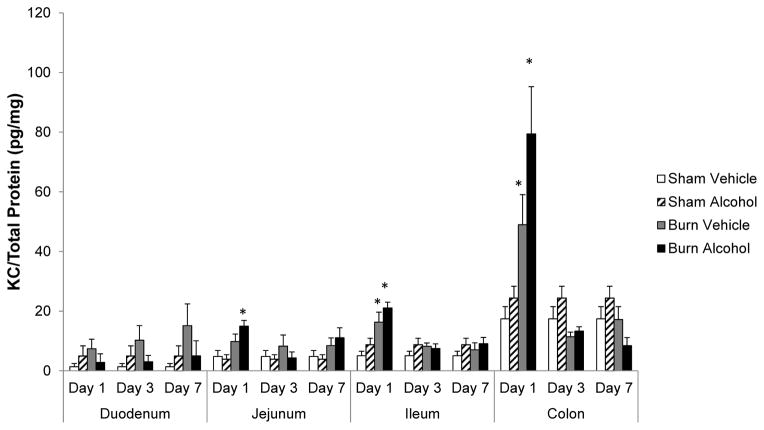
KC levels increase proximal to the colon after combined alcohol and burn insult
To establish whether chemokine levels are also differentially affected in different parts of the gut following alcohol and burn injury (similar to cytokines), we analyzed KC levels in different parts of the intestine on days 1, 3, and 7 after alcohol and burn injury. KC levels were not significantly elevated in duodenum in any of the days following alcohol and burn injury. We observed a significant increase in KC levels following burn injury alone in the ileum and colon. Furthermore, KC levels were significantly elevated in the jejunum, ileum, and colon 1 day following alcohol and burn injury as compared to sham animals. KC levels progressively increased following alcohol and burn injury, the more proximal to the colon. KC levels increased ~28-fold in the colon following alcohol and burn injury as compared with duodenum and ~4-fold compared to ileum. The elevation in KC, however, was not observed in the jejunum, ileum, and colon on days 3 and 7 after alcohol and burn injury compared to shams.
Discussion
The intestine facilitates water and nutrient absorption. The intestine also functions in the maintenance of an immunological and physical barrier which prevents the gut bacteria from entering into extra-intestinal sites. The intestine is compartmentalized into four anatomically and functionally distinct segments (Mowat & Agace, 2014). These compartments of the intestine have different bacterial content, with the bacterial number increasing progressively from the duodenum to the colon. The duodenum and jejunum contain 102–104 colony forming units (cfu)/g, whereas the ileum’s bacterial content is 1010 cfu/g. The colon’s bacterial content is 1010–1012 cfu/g (Hakansson & Molin, 2011; O’Hara & Shanahan, 2006). Alcohol and burn injury have been associated with increased bacterial translocation (Choudhry & Chaudry, 2006). Alcohol exposure has also been demonstrated to increase epithelial cell susceptibility to infection (Wood et al., 2013). Due to the regional variations in bacterial content and increased bacterial translocation because of alcohol and burn injury, it is important to determine if there are any differences in the inflammatory response in different parts of the intestine.
In our study, although we observed an increase in the pro-inflammatory cytokine levels in the jejunum, ileum, and colon, levels were several-fold higher in the colon compared to jejunum or ileum as a consequence of alcohol and burn injury. While the actual cause for differential expression of these cytokines/chemokines in various parts of the intestine remains to be established, the progressive increase in bacterial density from the duodenum to the colon is likely to contribute to an excess inflammatory response in the distal part (i.e., colon) compared to jejunum or ileum. In addition to bacteria, bacterial products such as endotoxins may also vary in different parts of the GI tract, and such differences in endotoxins are likely to contribute to regional differences in the inflammatory response in the GI tract. These findings implicate a role of bacterial overgrowth and endotoxins in intestinal inflammation.
The neutrophil chemokine, KC, increases more proximal to the colon following alcohol and burn injury. Circulating neutrophil sequestration to the site of injury following the combined insult is believed to be a major contributing factor to multiple organ failure. Neutrophils are the immediate responders following injury. An earlier study from our laboratory has shown an increase in neutrophil infiltration into the tissue following a combined insult of alcohol and burn injury (Akhtar et al., 2009). We observed that combined alcohol and burn insult significantly elevated KC levels in the jejunum, ileum, and colon compared to shams within 24 h after injury. Such an increase in KC is likely to cause neutrophil recruitment to the intestine, as observed in our previous studies. Similarly, the increase in IL-6 levels following alcohol exposure has also been associated with increased intestinal permeability (Al-Sadi et al., 2014). Additionally, findings from both patients and animal models indicate a relationship between the elevated levels of IL-6 and the development of septic complications (Hack et al., 1989; Remick, Bolgos, Copeland, & Siddiqui, 2005).
IL-18 has also been demonstrated to have a role in increased gut leakiness following combined alcohol and burn injury. Results from that study showed a role of IL-18 in decreased claudin-1 phosphorylation and occludin expression and phosphorylation (Li et al., 2012). Similarly, occludin dephosphorylation has been demonstrated to increase intestinal permeability (Dunagan, Chaudhry, Samak, & Rao, 2012). IL-18 levels were elevated in the duodenum, ileum, and colon following alcohol and burn injury. The elevation in duodenum, however, was not found to be significantly different from shams. We have previously demonstrated a role of IL-18 in increased neutrophil O2− production and their recruitment to the intestinal tissue. IL-18 has also been shown to delay neutrophil apoptosis (Akhtar et al., 2011). Such an increase in IL-18-dependent neutrophil infiltration into the intestinal bed may contribute to intestinal tissue damage and intestinal barrier disruption, leading to gut bacterial translocation following alcohol and burn injury (Rendon et al., 2013). This increase in translocation of bacteria or endotoxin is likely to contribute to sepsis and organ dysfunction under those conditions.
Together, the present study provides evidence that alcohol combined with burn injury causes inflammation throughout the intestinal tract but the colon or large intestine exhibits a more severe inflammatory response after combined alcohol and burn injury. Further studies are necessary to examine how regional changes in bacterial content in the gut lead to induction of the inflammatory response following alcohol and burn injury.
Figure 1.
Effects of combined alcohol and burn injury on IL-6 production in intestinal tissue 1, 3, and 7 days post-injury, *p < 0.05 by ANOVA compared to respective sham vehicle. The data shown are mean ± SEM of n = 5–12 animals per group. All sham vehicle and sham alcohol animals from all the days tested were pooled together.
Figure 2.
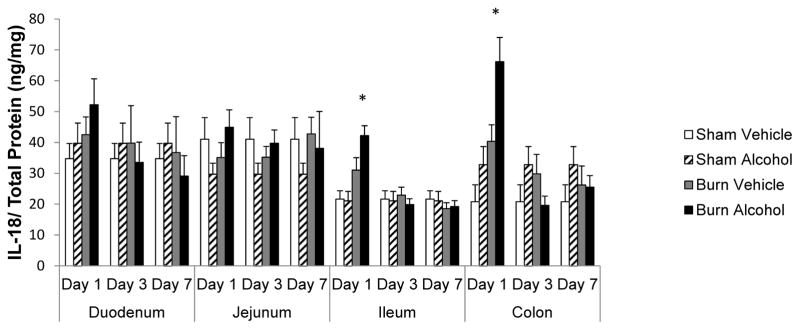
Effects of combined alcohol and burn injury on IL-18 production in intestinal tissue 1, 3, and 7 days post-injury, *p < 0.05 by ANOVA compared with respective sham vehicle. The data shown are mean ± SEM of n = 5–12 animals per group. All sham vehicle and sham alcohol animals from all the days tested were pooled together.
Figure 3.
Effects of combined alcohol and burn injury on KC production in intestinal tissue 1, 3, and 7 days post-injury, *p < 0.05 by ANOVA compared with respective sham vehicle. The data shown are mean ± SEM of n = 5–12 animals per group. All sham vehicle and sham alcohol animals from all the days tested were pooled together.
Highlights.
Alcohol combined with injury results in increased inflammation in the intestine.
The results further add that inflammatory mediators are differentially expressed in various parts of the intestine following alcohol and burn injury.
The levels of inflammatory mediators were several fold higher in the colon compared to jejunum or ileum as a consequence of alcohol and burn injury.
Acknowledgments
This study was supported by R01AA015731 (MAC), R01AA015731-08S1 (MAC), T32AA013527-12(EKJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhtar S, Li X, Chaudry IH, Choudhry MA. Neutrophil chemokines and their role in IL-18-mediated increase in neutrophil O2− production and intestinal edema following alcohol intoxication and burn injury. American Journal of Physiology Gastrointestinal and Liver Physiology. 2009;297:G340–347. doi: 10.1152/ajpgi.00044.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar S, Li X, Kovacs EJ, Gamelli RL, Choudhry MA. Interleukin–18 delays neutrophil apoptosis following alcohol intoxication and burn injury. Molecular Medicine. 2011;17:88–94. doi: 10.2119/molmed.2010.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadi R, Ye D, Boivin M, Guo S, Hashimi M, Ereifej L, et al. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PloS One. 2014;9:e85345. doi: 10.1371/journal.pone.0085345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MD, Kovacs EJ. Organ-specific inflammation following acute ethanol and burn injury. Journal of Leukocyte Biology. 2008;84:607–613. doi: 10.1189/jlb.1107766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry MA, Chaudry IH. Alcohol intoxication and post-burn complications. Frontiers in Bioscience. 2006;11:998–1005. doi: 10.2741/1857. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Fazal N, Goto M, Gamelli RL, Sayeed MM. Gut-associated lymphoid T cell suppression enhances bacterial translocation in alcohol and burn injury. American Journal of Physiology Gastrointestinal and Liver Physiology. 2002;282:G937–947. doi: 10.1152/ajpgi.00235.2001. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Rana SN, Kavanaugh MJ, Kovacs EJ, Gamelli RL, Sayeed MM. Impaired intestinal immunity and barrier function: a cause for enhanced bacterial translocation in alcohol intoxication and burn injury. Alcohol. 2004;33:199–208. doi: 10.1016/j.alcohol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Costantini TW, Loomis WH, Putnam JG, Drusinsky D, Deree J, Choi S, et al. Burn-induced gut barrier injury is attenuated by phosphodiesterase inhibition: effects on tight junction structural proteins. Shock. 2009;31:416–422. doi: 10.1097/SHK.0b013e3181863080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Norris BA, Medina-Contreras O, Manicassamy S, Geem D, Madan R, et al. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. Journal of Immunology. 2011;187:733–747. doi: 10.4049/jimmunol.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunagan M, Chaudhry K, Samak G, Rao RK. Acetaldehyde disrupts tight junctions in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. American Journal of Physiology Gastrointestinal and Liver Physiology. 2012;303:G1356–1364. doi: 10.1152/ajpgi.00526.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosain A, Gamelli RL. Role of the gastrointestinal tract in burn sepsis. The Journal of Burn Care & Rehabilitation. 2005;26:85–91. doi: 10.1097/01.bcr.0000150212.21651.79. [DOI] [PubMed] [Google Scholar]
- Hack CE, De Groot ER, Felt-Bersma RJ, Nuijens JH, Strack Van Schijndel RJ, Eerenberg-Belmer AJ, et al. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989;74:1704–1710. [PubMed] [Google Scholar]
- Hakansson A, Molin G. Gut microbiota and inflammation. Nutrients. 2011;3:637–682. doi: 10.3390/nu3060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao WL, Lee YK. Microflora of the gastrointestinal tract: a review. Methods in Molecular Biology. 2004;268:491–502. doi: 10.1385/1-59259-766-1:491. [DOI] [PubMed] [Google Scholar]
- Haum A, Perbix W, Häck HJ, Stark GB, Spilker G, Doehn M. Alcohol and drug abuse in burn injuries. Burns: Journal of the International Society for Burn Injuries. 1995;21:194–199. doi: 10.1016/0305-4179(95)80008-c. [DOI] [PubMed] [Google Scholar]
- Jones JD, Barber B, Engrav L, Heimbach D. Alcohol use and burn injury. The Journal of Burn Care & Rehabilitation. 1991;12:148–152. doi: 10.1097/00004630-199103000-00012. [DOI] [PubMed] [Google Scholar]
- Li X, Akhtar S, Choudhry MA. Alteration in intestine tight junction protein phosphorylation and apoptosis is associated with increase in IL-18 levels following alcohol intoxication and burn injury. Biochimica Et Biophysica Acta. 2012;1822:196–203. doi: 10.1016/j.bbadis.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Akhtar S, Kovacs EJ, Gamelli RL, Choudhry MA. Inflammatory response in multiple organs in a mouse model of acute alcohol intoxication and burn injury. Journal of Burn Care & Research. 2011;32:489–497. doi: 10.1097/BCR.0b013e3182223c9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Schwacha MG, Chaudry IH, Choudhry MA. Acute alcohol intoxication potentiates neutrophil-mediated intestinal tissue damage after burn injury. Shock. 2008;29:377–383. doi: 10.1097/shk.0b013e31815abe80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnotti LJ, Deitch EA. Burns, bacterial translocation, gut barrier function, and failure. The Journal of Burn Care & Rehabilitation. 2005;26:383–391. doi: 10.1097/01.bcr.0000176878.79267.e8. [DOI] [PubMed] [Google Scholar]
- Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nature Reviews Immunology. 2014;14:667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Reports. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remick DG, Bolgos G, Copeland S, Siddiqui J. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infection and Immunity. 2005;73:2751–2757. doi: 10.1128/IAI.73.5.2751-2757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendon JL, Li X, Akhtar S, Choudhry MA. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock. 2013;39:11–18. doi: 10.1097/SHK.0b013e3182749f96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S, Pithadia R, Rehman T, Zhang L, Plichta J, Radek KA, et al. Chronic alcohol exposure renders epithelial cells vulnerable to bacterial infection. PloS One. 2013;8:e54646. doi: 10.1371/journal.pone.0054646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahs A, Bird MD, Ramirez L, Choudhry MA, Kovacs EJ. Anti-IL-6 antibody treatment but not IL-6 knockout improves intestinal barrier function and reduces inflammation after binge ethanol exposure and burn injury. Shock. 2013;39:373–379. doi: 10.1097/SHK.0b013e318289d6c6. [DOI] [PMC free article] [PubMed] [Google Scholar]