Abstract
Background
Parkinson’s disease (PD) is histopathologically characterized by the loss of dopamine neurons in the substantia nigra pars compacta. The depletion of these neurons is thought to reduce the dopaminergic function of the nigrostriatal pathway, as well as the neural fibers that link the substantia nigra to the striatum (putamen and caudate), causing a dysregulation in striatal activity that ultimately leads to lack of movement control. Based on diffusion tensor imaging, visualizing this pathway and measuring alterations of the fiber integrity remain challenging. The objectives were to: 1) develop a diffusion tensor tractography protocol for reliably tracking the nigrostriatal fibers on multicenter data; 2) test whether the integrities measured by diffusion tensor imaging of the nigrostriatal fibers are abnormal in PD; 3) test if abnormal integrities of the nigrostriatal fibers in PD patients are associated with the severity of motor disability and putaminal dopamine binding ratios.
Methods
Diffusion tensor tractography was performed on 50 drug naïve PD patients and 27 healthy control subjects from the international multicenter Parkinson’s Progression Marker Initiative.
Results
Tractography consistently detected the nigrostriatal fibers, yielding reliable diffusion measures. Fractional anisotropy, along with radial and axial diffusivity of the nigrostriatal tract, showed systematic abnormalities in patients. In addition, variations in fractional anisotropy and radial diffusivity of the nigrostriatal tract were associated with the degree of motor deficits in PD patients.
Conclusion
Taken together, the findings imply that the diffusion tensor imaging characteristic of the nigrostriatal tract is potentially an index for detecting and staging of early PD.
Keywords: Parkinson’s disease, MRI, diffusion tensor imaging, diffusion tensor tractography, nigrostriatal pathway
Introduction
Parkinson’s disease (PD) is histopathologically characterized by loss of dopamine neurons in the substantia nigra (SN) pars compacta (SNc). The depletion of these neurons is thought to reduce the dopaminergic function of the nigrostriatal pathway, a bundle of nerve fibers that links the SN to the striatum, causing a dysregulation in striatal activity that ultimately leads to lack of movement control.1,2 Assessing the nigrostriatal pathway is therefore useful for aiding the diagnosis and treatment interventions of PD.
Previous studies in PD using diffusion tensor imaging (DTI)3 by evaluating the nigral fractional anisotropy (FA)4 have reported mixed results,5 though a recent study – attempting the separation between free and tissue water diffusion using a bi-tensor model – found a prominent free water increase in PD.6 DTI tractography7 of the nigrostriatal pathway has been demonstrated in healthy subjects,8 though this tract was termed “striatomesencephalic fiber”. Studies analyzing the nigrostriatal tract in PD patients are rare, presumably due to the difficulties in reliably identifying this small tract in the presence of crossing or joining fibers.9 Two recent studies10,11 have analyzed the connectivity profile, which was defined as the number of the most probable fibers that connect SN and putamen using probabilistic tractography. One10 study reported a significant reduction in this connectivity, whereas the other11 found no significant abnormality in PD. To our knowledge, no previous study reports variations in DTI measures of the nigrostriatal tract in PD.
In this study, DTI measures were used for assessing the integrity of the nigrostriatal fibers based on data from the Parkinson’s Progression Marker Initiative (PPMI),11 an international multicenter study. The main goals were to: 1) demonstrate that tractography of the nigrostriatal tract can reliably be performed in PD patients and HC subjects; 2) test if DTI measures along the nigrostriatal tract differ between PD patients and HC subjects; and 3) determine the degree to which DTI variations along the nigrostriatal tract in PD are associated with the severity of motor symptoms and striatal dopaminergic deficits.
Methods
Population
The data were obtained through the PPMI study12 which has been approved by the respective Institutional Review Boards of all participating sites, and all subjects provided written informed consent. After initial screening, the subjects were fully assessed at the baseline visit for clinical (motor, neuropsychologic and cognitive) performance by the site investigators. Specifically, motor function was assessed using Part III of the Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (UPDRS).13 Patients who received a diagnosis of PD less than 2 years before the initial visit without receiving any PD medication, and with a Hoehn and Yahr (H&Y)14 stage of I or II, were enrolled. Demographically comparable HC subjects, free of a neurological disorder, were recruited into the PPMI. All subjects had dopamine transporters (DAT) imaging, as routinely measured in the striatum using Ioflupane (123I) single-photon emission computed tomography (SPECT). SPECT imaging data were centrally reconstructed (http://www.indd.org/), attenuation corrected, and analyzed with a standardized volume of interest template for extraction of regional count densities in the left and right putamen. Putaminal DAT binding ratios were calculated using the occipital lobe as reference region. Since a reduced DAT binding ratio is usually considered an indicator of dopaminergic neuron loss,15 PD patients were excluded if: 1) there was no evidence of an abnormal DAT binding ratio, the so-called Scans Without Evidence of Dopaminergic Deficit (SWEDD); 2) subject withdrew from the study; or 3) tractography of the nigrostriatal fiber was not identifiable on at least one side due to poor DTI quality. In all, 77 DTI sets (PD=50, HC=27) were selected in the order the data became available for conducting tractography from an initial count of 178 DTI sets (PD=123, HC=55) from 10 PPMI sites. Further details of the PPMI recruitments and subject selection of the current study are described in Supplemental Materials. Demographic information, clinical characteristics, and the putaminal DAT binding ratios of the study population are listed in Table 1.
Table 1.
Group demographics and clinical characteristics
| HC | PD | Group Difference (p) | |
|---|---|---|---|
| Number of tractography | 27 | 50 | — |
| Age (years) | 56.8 ± 10.7 | 59.7 ± 9.2 | n.s. |
| Sex (M / F) | 21 / 6 | 32 / 18 | n.s. |
| Handedness (L:R:M)(a) | 23 : 4 : 0 | 44 : 4 : 2 | — |
| Side of symptom onset (L:R:S)(b) | — | 27 : 22 : 1 | — |
| Total UPDRS (I–IV)(c) | 5.8 ± 2.5 | 38.8 ± 13.3 | <0.001 |
| UPDRS III(d) | 0.4 ± 0.8 | 21.9 ± 7.8 | <0.001 |
| Hoehn & Yahr(e) | 0 | 1.6 ± 0.5 | <0.001 |
| MoCA(f) | 28.1 ± 1.1 | 28.0 ± 1.7 | n.s. |
| Putaminal DAT (minimum side) | 1.25 ± 0.4 | 0.58 ± 0.2 | <0.001 |
L:R:M = Left : Right : Mixed handedness
L:R:S = Left : Right : Symmetric onset
Unified Parkinson’s Disease Rating Scale (Movement Disorder Society revision) Part I-IV, range 0 to 200
Unified Parkinson’s Disease Rating Scale Part III, range 0 to 72
Hoehn and Yahr scale, range 0 to 5
MoCA: Montreal Cognitive Assessment test, range 0 to 30
MRI Acquisition
MRI was performed using a standardized protocol for 3 Tesla machines. The key acquisitions of the protocol included a 3D magnetization prepared rapid gradient echo (MPRAGE) sequence and a 2D single-shot echo-planar DTI sequence (TR/TE = 900/88ms, 2 mm3 resolution; 72 contiguous slices, twofold acceleration, axial-oblique aligned along the anterior-posterior commissure, diffusion-weighting along 64 gradient directions with a b-value of 1000s/mm2). Further details of the MRI acquisition and processing are summarized at (http://www.ppmi-info.org/). The majority of DTI scans were obtained with cardiac-gating except a few cases (less than 5%) when a clear cardiac signal was not available. The MRI protocol was electronically distributed to each site to guarantee consistent installations.
DTI Processing
The DTI data were post-processed in the laboratory of the senior author (N.S), using a standard processing pipeline.16 In brief, image quality was evaluated based on the signal-to-noise ratio where the background noise uniformity was measured using the Kullback-Leibler divergence.17 Diffusion-weighted images were corrected for eddy-currents and head motion. To maintain the original orientation of the principle eigenvector, no spatial distortion correction was performed. To achieve successful visualization of small fibers, the original DTI resolution was up-sampled to a 1 mm3 resolution through trilinear interpolation. In addition to analyzing a specific tract, an unbiased whole-brain voxel-based analysis using a high-dimensional normalization (DARTEL toolbox) in SPM8 was performed as a comparison.
Nigrostriatal Tractography
Tractography of the nigrostriatal tract was performed using TrackVis software (http://www.trackvis.org/). The ‘seed disk’ function,18 which computes DTI values along the fiber curvature point-by-point, was used to initiate streamlines starting from the SN. In detail, a circular seed disk of 1 mm2 size, small enough to avoid other fiber streamlines in neighboring area, with an angle perpendicular to the streamlines, was placed in the SN. Since only a few DTI voxels within SN produced visible fibers, the seed disk was slightly moved around until streamlines reached the putaminal and pallidal area. A default length threshold of 10 mm and an angular threshold of 35° were used to limit deviations from the main path. More detailed description of the fiber tracking method is given in the Supplemental Material. To avoid bias due to variable fiber lengths and terminations (see Supplemental Figure S1), measures of FA, radial diffusivity (rD) and axial diffusivity (aD) were extracted at every 1mm from a 30mm segment of the consistent portion of the streamlines.
Tracking and Measurement Reproducibility
Reproducibility tests of nigrostriatal tractography, i.e. the for intra-rater ROI placement as well as for track-based DTI measurements, were tested using mean FA along each tract as metric in 12 randomly selected subjects, who had DTI done twice on the same day. An experienced radiologist (Y.Z.), blinded to identity and diagnosis of the subjects, completed two tractography performances approximately a week apart on the duplicate DTI acquisitions of each subject. The reproducibility of intra-rater ROI placement was tested by comparing the two tractography results (i.e. mean FA) of the first DTI acquisition. The reproducibility of track-based DTI measurement was tested by comparing the first tractography result from the two DTI acquisitions. Reproducibility was quantified based on an intra-class correlation coefficient (ICC).19 Intra-rater ROI placement had an excellent reproducibility with an ICC of 0.93 overall (left = 0.91, right = 0.95). Track-based DTI measurements had an ICC of 0.76 overall (left = 0.73, right = 0.8).
Statistics
Mean values of FA, rD, and aD along the nigrostriatal tract segment as well as streamline numbers were analyzed. Since PD patients overwhelmingly (95%) presented asymmetric onsets of motor symptoms, we replaced the left and right brain hemisphere labels with contralateral and ipsilateral labels, according to each patient’s clinically predominated side of Parkinsonian symptoms onset (i.e. contralateral denotes the brain side opposite to the body side of symptoms; ipsilateral denotes the same brain side as the body side of symptoms). This approach is also consistent with general analysis procedures of the PPMI.16,20 For one patient with symmetrical symptom onset and for all HC subjects, the DTI values of left and right tracts were averaged, since there was neither a significant asymmetry (p > 0.6) between the left and right DTI variables, nor a significant effect due to handedness. Group effects on DTI measures were tested with a repeated measures analysis of variance (rmANOVA) model for each dependent variable (FA, rD, aD, and streamline number) separately. Diagnosis was included as a between-subject factor while ipsilateral/contralateral side was included as repeated measures. Following an overall test for diagnosis and a test for an interaction between diagnosis and side, post-hoc linear regressions were performed for each side. Linear mixed models were used to test relations of each DTI variable with the degree of motor or dopaminergic deficits in patients, as well an interaction between each DTI variable and the side of the tract. Every model included adjustments for age and gender. The significance level was α < 0.05 in all tests. All statistical tests were performed using the R-Project for statistical computing (http://www.r-project.org/).
Results
Demographic and clinical characteristics
PD patients and HC subjects were similar in their respective age (p = 0.2) and gender (χ2 = 1.83, p = 0.18) distributions as well as in the degree of cognitive performance (p = 0.7). As expected, PD patients had significant deficits on the motor tests as well as reduced DAT binding ratios in the putamen (all comparisons p < 0.001), compared to HC subjects (Table 1).
Visualization of the nigrostriatal fibers
Whole brain analysis did not show differences between PD and HC groups for any DTI measure at uncorrected p < 0.001. This result motivated the analysis specifically of the nigrostriatal fibers that connect the SN and putamen.
A small fiber tract that connected the SN and putaminal and pallidal area (Figure 1) was traceable in 77 subjects. This small fiber was clearly distinguishable from adjacent projection fibers that travel through the internal capsule and project to the superior frontal cortices. The anatomical regions where the nigrostriatal fibers travel through are illustrated in Figure 2. The fibers originate from the posterior part of SN, converge to the anterior dorsal part of SN, travel superiorly via the medial side of subthalamic nucleus (STN), and then turn laterally above the STN toward the ventral putaminal and pallidal area. This pattern is consistent with the known anatomy of the nigrostriatal pathway.21
Figure 1.
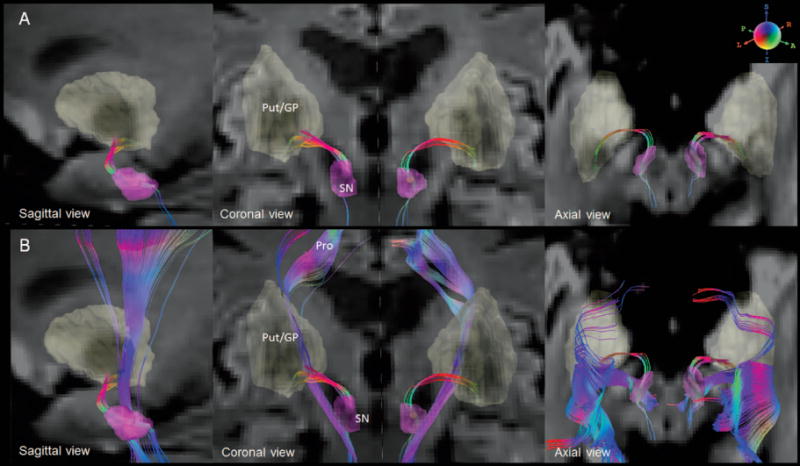
A. The reconstructed nigrostriatal fibers with color-encoded orientations: green for anterior-poster, red for transverse, and blue for superior-inferior directions. The connecting nuclei are highlighted on diffusion weighted images. B. The nigrostriatal fibers are distinguished from the neighboring fibers (Pro).
SN = substantia nigra (SN), Put/GP = putamen/globus pallidus, Pro = projection fibers
Figure 2.
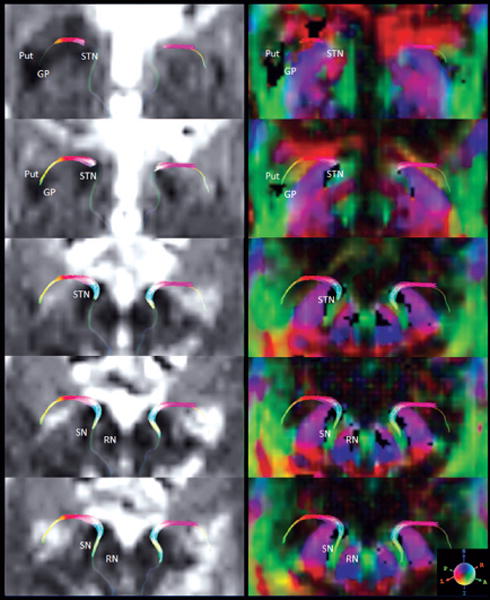
Bilateral nigrostriatal fibers are overlaid on diffusion weighted images (left) and color-coded anisotropic images (right). Five consecutive slices, starting from the level of Put/GP (top slices) until SN (bottom slices) are shown. Adjacent anatomical regions are also indicated.
SNc = pars compacta of substantia nigra, SNr = pars reticulata of substantia nigra, RN = red nucleus, STN = subthalamic nucleus, GP = globus pallidus, Put = putamen; Sphere indicates the color-coding.
DTI group differences
Group differences in each DTI measure, i.e. FA, rD and aD, of the nigrostriatal tract are illustrated in the scatter plots of Figure 3 and also numerically summarized in Supplemental Table S1. In comparison to HC subjects, FA of the nigrostriatal tract was on average markedly reduced in PD patients (F2, 150 = 12.7, p = 0.0007), regardless of the side of motor symptom onset (F1, 75 = 0.1, p = 0.7). rD was increased in PD patients (F2, 150 = 5.6, p = 0.02), regardless of the side of symptom onset (F1, 75 = 0.2, p = 0.6). aD was also increased in PD patients (F2, 150 = 4.0, p = 0.049), again with no side interaction (F1, 75 = 1.1, p = 0.3). Neither a significant group difference nor side interaction was found for streamline numbers.
Figure 3.
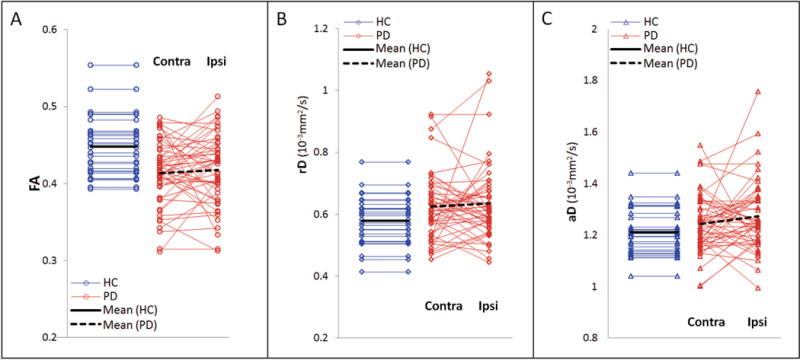
Scatter plots of FA (A), rD (B) and aD (C) of the nigrostriatal tracts in HC subjects and PD patients, separately for contralateral (Contra) and ipsilateral (Ipsi) side of symptom onset. Linear regressions are shown in black lines.
Relations between DTI and motor deficits
Relations between DTI variations of the nigrostriatal tracts in PD and motor deficits based on UPDRS part III scores (UPDRS-III) are illustrated in Figure 4, separately for FA, rD, and aD. A significant relationship was found for nigrostriatal FA and rD, but not for aD. There was a trend (p = 0.09 via likelihood ratio test) towards an interaction between UPDRS-III and ipsilateral/contralateral definition, with nigrostriatal FA decreasing more substantially for larger UPDRS-III on the contralateral side. Specifically, after controlling for age and gender, per unit increased in UPDRS-III corresponds with a 0.40% (CI95: 0.11% to 0.68%) decrease in contralateral FA and a 0.30% (CI95: −0.17%, 0.44%) decrease in ipsilateral FA. rD increased on average 1.05% (CI95: 0.47 to 1.64 %) as per UPDRS-III unit increase, without significant differences between the contralateral and ipsilateral side. aD was not significantly correlated with UPDRS-III (p = 0.2).
Figure 4.
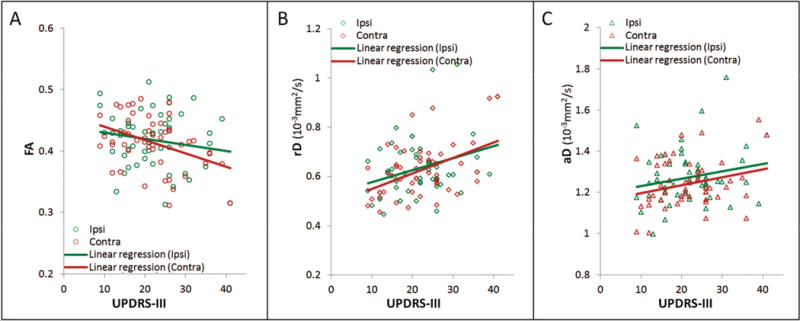
Relationships between FA (A), rD (B) and aD (C) of the nigrostriatal tracts and the degree of motor deficits based on UPDRS-III scores in PD patients, separately for contralateral (Contra) and ipsilateral (Ipsi) side of symptom onset.
Similar to UPDRS-III, significant relationships for FA and rD of the nigrostriatal tracts were also found with total UPDRS. There is a statistically significant (p = 0.009 via likelihood ratio test) interaction between total UPDRS and ipsilateral/contralateral definition, with nigrostriatal FA again decreasing more substantially for larger total UPDRS scores on the contralateral side. Specifically, after controlling for both age and gender, per unit increase in total UPDRS corresponds with a 0.25% (CI95: 0.08% to 0.42%) decrease in contralateral FA and a 0.01% (CI95: −0.17%, 0.20%) decrease in ipsilateral FA. rD increased 0.51 % (CI95: 0.13 to 0.89%) as per total UPDRS unit increase, with no difference between the contralateral and ipsilateral side. aD had no significant correlation with total UPDRS (p = 0.2).
Relations between DTI and DAT binding ratios
No significant relationships were found between any DTI measures of the nigrostriatal tract and putaminal DAT binding ratios. Interestingly, the putaminal DAT ratios also had no significant correlations with UPDRS-III (p = 0.12), whereas the ratios showed a trend of a negative correlation with total UPDRS (p = 0.2).
Discussion
Our study has three main findings: 1) The nigrostriatal tract can be visualized reliably in PD patients and HC subjects in a multicenter study using tractography. 2) The DTI characteristic of the nigrostriatal tract is abnormal in PD patients, suggesting a degradation of the microstructural integrity of the motor control circuitry. This finding is potentially important for new treatment interventions that target restoring the nigrostriatal circuitry. 3) The microstructural degradation of the nigrostriatal tract in PD patients is associated with the severity of motor symptoms, whereas a relationship to dopaminergic deficits is not apparent. This finding implies that structural deficits of the nigrostriatal circuit – aside from functional (i.e. dopaminergic) deficits – contribute to the severity of PD. Taken together, the findings suggest that the DTI characteristic of the nigrostriatal tract is potentially an index for detecting and staging of early PD.
The nigrostriatal pathway primarily innervates the dorsolateral striatum (putamen and caudate), an area that plays a central role in movement control via cortical activation and deactivation.1,2 The loss of the nigrostriatal projections, which comprises the dopaminergic neurons, results in dysfunctional regulations of the basal ganglia circuitry, which is presumed to cause the presence of bradykinesia, rigidity and other motor problems in PD.22 Postmortem studies have shown that striatal dopamine levels are reduced in PD and the level of reduction parallels the degree of nigral degeneration.23,24 Aside from common dopaminergic treatments of PD that restore striatal dopamine levels, new treatments that aim at restoring the nigrostriatal pathway, such as dopamine graft implantation25,26 and infusion of glial-cell-line-derived neurotrophic factors, also known as GDNF,27–29 have become major goals for potentially disease-modifying interventions. Our findings of abnormal microstructural integrities of the nigrostriatal tract in PD using DTI in a multisite MRI setting demonstrated that DTI assessment is potentially important for clinical trials that target restoring the nigrostriatal circuitry in PD.
It is noteworthy that we found a prominent disease effect on DTI measures of the nigrostriatal tract, while a previous DTI study of PPMI, focusing on the SN, reported only a trend of disease effect.16 Other single center DTI studies focusing on the SN in PD have also reported a disease effect,30 though magnitude and location of nigral abnormalities varied across the studies.5 More recently, another study using PPMI data6 and attempting the separation between free and tissue water diffusion based on a bi-tensor model, found a prominent disease effect selectively on increased free water diffusion in the SN while variations in tissue water were not significant. An increase of free water diffusion is generally interpreted as indication of atrophic or inflammatory fibers.31 Whether a bi-tensor analysis of the nigrostriatal tract data would also lead to similar findings of free water diffusion needs to be determined in the future. In addition, our findings imply that structural damage in PD can extend beyond the SN into the nigrostriatal tract. The disease effect also appears to impact the fibers in the vicinity of the SN more than at the opposite end toward the putamen, though these regional variations along the tract were not significant (Supplemental Table S2). Altogether, our results support the hypothesis that DTI alterations of the nigrostriatal tract are potentially relevant to the pathological characters of PD.
Given that one might generally expect a unilateral distribution of abnormal DTI values for unilateral symptoms, the finding of bilateral DTI abnormalities of the nigrostriatal tracts in PD is surprising. One interpretation for the bilateral abnormalities of the nigrostriatal tracts is that progression of PD symptoms can spread from one side to encompassing both within 20 months.32 Thus, finding bilateral DTI abnormalities of the nigrostriatal tract in these PD patients with reported unilateral domain of symptoms at onset might be a sign that the disease has already progressed to both hemispheres since the time when a PD diagnosis was given, e.g. within 2 years before enrollment in PPMI. Another possibility is that DTI abnormalities precede the appearance of clinical motor symptoms in PD. This can potentially be tested on a prodromal PD cohort, i.e. subjects with a genetic predisposition for PD, before subjects develop clinical motor symptoms. A sub-study of the PPMI is currently collecting DTI data in a prodromal PD cohort to test this possibility.33
The finding of strong relationships between motor deficits and FA as well as rD is consistent with several reports of nigral DTI variations in PD.34–36 In this context, the finding of a weak relationship between decreased putaminal DAT ratios and increased motor deficits in this cohort is interesting. These findings imply that microstructural damage of the nigrostriatal tract – and not functional (i.e. dopaminergic) deficits alone – contribute to motor deficits in PD. This result potentially has implications for the development of disease-modifying interventions. In particular, improvement of structural components of axons, such as myelin sheets and microtubules, through drug-induced increase of neural and glial grow factors, could be an important target for therapeutic interventions in PD.
Given the conventional concept of PD, in which reduced dopaminergic function of the nigrostriatal pathway is the underpinning of motor deficits, a relationship between nigrostriatal integrity and DAT might have been expected. However, our study didn’t reveal an apparent relationship between DTI measures and DAT. One explanation is that the DAT tracer uptake does not correlate with nigral neuron loss.37 DTI may therefore provide complementary information about PD pathology to DAT. Another explanation is that the selectivity of the nigostriatal fiber to dopaminergic neurons is diminished because fiber tracking may also have included the GABAnergic striato-pallido-nigral pathway aside from the dopaminergic nigrostriatal one. It is also possible that a bi-tensor analysis6 that separates free water from tissue water diffusion is more sensitive to detect DAT-DTI relationships. It should also not be overlooked that the relative sensitivity of DTI and DAT measures for PD may vary with disease progression. Therefore it cannot be excluded that DAT sensitivity exceeds that of nigrostriatal DTI for PD at other stages of the disease. An analysis of longitudinal DTI and DAT data from the PPMI is warranted to determine the value of each imaging modality for detecting PD pathology.
Some limitations of the study ought to be mentioned. One limitation is that the analysis was restricted to PPMI subjects enrolled at specific and selected centers that already had the technical capabilities to obtain DTI scans in a rigorous and highly standardized fashion. Although the difference between the DTI subset and the entire PPMI cohort may probably have no clinical relevance, a generalization of the DTI findings requires caution. Another technical limitation is that due to the nature of the multicenter study, we could not use the most sophisticated diffusion imaging methods, such as diffusion spectrum38 and high angular resolution diffusion imaging (HARDI),39 which overcome some pitfalls of DTI, especially the ambiguity from crossing or joining fibers. It is possible that these sophisticated diffusion imaging methods may lead to different results.
Supplementary Material
Acknowledgments
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
Study Funding: The study was supported in part by the funding partners of the PPMI group. PPMI – a public-private partnership – is funded by the Michael J. Fox Foundation (MJFF) for Parkinson’s Research and funding partners, including MJFF, Abbot, Avid Radiopharmaceuticals, Biogen Idec, Bristol-Myers Squibb, Covance, Elan Corporation, Eli Lilly & Co., F. Hoffman-La Roche, Ltd., GE Healthcare, Genentech, GlaxoSmithKline, Lundbeck, Merck, MesoScale, Pfizer and UCB. Full names of all of the PPMI funding partners can be found at www.ppmi-info.org/fundingpartners. The study was also supported by an NIH grant (P41 EB015904).
Footnotes
Authors’ Roles:
Dr. Yu Zhang: study conception and design, guarantors of integrity of entire study, literature research, MRI processing, data analysis/interpretation, drafting of the manuscript, critical revision of the manuscript, final version approval.
Ms. I-Wei Wu: study conception and design, data collection, MRI processing, literature research, data analysis/interpretation, critical revision of the manuscript, final version approval.
Mr. Shannon Buckley: study conception and design, MRI processing, data analysis/interpretation, critical revision of the manuscript, final version approval.
Dr. Christopher S. Coffey: study conception and design, data analysis/interpretation, statistical analysis, critical revision of the manuscript, final version approval.
Dr. Eric Foster: study conception and design, data analysis/interpretation, statistical analysis, critical revision of the manuscript, final version approval.
Ms. Susan Mendick: study conception and design, data collection, data analysis/interpretation, critical revision of the manuscript, final version approval.
Dr. John Seibyl: study conception and design, SPECT studies, data analysis/interpretation, critical revision of the manuscript; final version approval.
Dr. Norbert Schuff: study conception and design, guarantors of integrity of entire study, study supervision or coordination, literature research, data analysis/interpretation, statistical analysis, critical revision of the manuscript, final version approval.
Financial Disclosures of all authors for the past year
Dr. Yu Zhang has no disclosures to report.
Ms. I-Wei Wu has no disclosures to report.
Mr. Shannon Buckley has no disclosures to report.
Dr. Christopher S. Coffey has no disclosure to report.
Dr. Eric Foster has no disclosures to report.
Ms. Susan Mendick has no disclosures to report.
Dr. John Seibyl has received research funding from Michael J. Fox Foundation for Parkinson’s Research, consulting fees from GE Healthcare, Navidea Biopharmaceuticals, and Piramal Imaging. He has equity interest in Molecular Neuroimaging, LLC.
Dr. Norbert Schuff receives research funding from Michael J. Fox Foundation for Parkinson’s Research, National Institutes of Health, Department of Defense, and consulting honoraria from Eli Lilly.
References
- 1.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 2.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 3.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 4.Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 5.Cochrane CJ, Ebmeier KP. Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology. 2013;80:857–864. doi: 10.1212/WNL.0b013e318284070c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ofori E, Pasternak O, Planetta PJ, et al. Increased free water in the substantia nigra of Parkinson’s disease: a single-site and multi-site study. Neurobiol Aging. 2015;36:1097–1104. doi: 10.1016/j.neurobiolaging.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori S, van Zijl PC. Fiber tracking: principles and strategies – a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 8.Lehéricy S, Ducros M, Krainik A, et al. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Cereb Cortex. 2004;14:1302–1309. [Google Scholar]
- 9.Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Menke RA, Scholz J, Miller KL, Deoni S, Jbabdi S, Matthews PM, Zarei M. MRI characteristics of the substantia nigra in Parkinson’s disease: a combined quantitative T1 and DTI study. Neuroimage. 2009;47:435–441. doi: 10.1016/j.neuroimage.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Sharman M, Valabregue R, Perlbarg V, et al. Parkinson’s disease patients show reduced cortical-subcortical sensorimotor connectivity. Mov Disord. 2013;28:447–454. doi: 10.1002/mds.25255. [DOI] [PubMed] [Google Scholar]
- 12.Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI) Prog Neurobiol. 2011;95:629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 14.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 15.Varrone A, Marek KL, Jennings D, Innis RB, Seibyl JP. [(123)I]beta-CIT SPECT imaging demonstrates reduced density of striatal dopamine transporters in Parkinson’s disease and multiple system atrophy. Mov Disord. 2001;16:1023–1032. doi: 10.1002/mds.1256. [DOI] [PubMed] [Google Scholar]
- 16.Schuff N, Wu K, Buckley S, et al. Diffusion Tensor Imaging Of Nigral Alterations in Early Parkinson’s Disease With Dopamine Deficits. Mov Disord. 2014 doi: 10.1002/mds.26325. in press. [DOI] [PubMed] [Google Scholar]
- 17.Rohde GK, Barnett AS, Basser PJ, Pierpaoli C. Estimating intensity variance due to noise in registered images: applications to diffusion tensor MRI. Neuroimage. 2005;26:673–684. doi: 10.1016/j.neuroimage.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Wedeen VJ, Wang RP, Schmahmann JD, et al. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41:1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 19.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 20.Lebedev AV, Westman E, Simmons A, Lebedeva A, Siepel FJ, Pereira JB, Aarsland D. Large-scale resting state network correlates of cognitive impairment in Parkinson’s disease and related dopaminergic deficits. Front Syst Neurosci. 2014;8:45. doi: 10.3389/fnsys.2014.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith Y, Shink E, Sidibé M. Neuronal circuitry and synaptic connectivity of the basal ganglia. Neurosurg Clin N Am. 1998;9:203–222. [PubMed] [Google Scholar]
- 22.Tsui A, Isacson O. Functions of the nigrostriatal dopaminergic synapse and the use of neurotransplantation in Parkinson’s disease. J Neurol. 2011;258:1393–1405. doi: 10.1007/s00415-011-6061-6. [DOI] [PubMed] [Google Scholar]
- 23.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 24.Ehringer H, Hornykiewicz O. Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Klin Wochenschr. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- 25.Lindvall O, Björklund A. Cell therapeutics in Parkinson’s disease. Neurotherapeutics. 2011;8:539–548. doi: 10.1007/s13311-011-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pogarell O, Koch W, Gildehaus FJ, Kupsch A, Lindvall O, Oertel WH, Tatsch K. Long-term assessment of striatal dopamine transporters in Parkinsonian patients with intrastriatal embryonic mesencephalic grafts. Eur J Nucl Med Mol Imaging. 2006;33:407–411. doi: 10.1007/s00259-005-0032-z. [DOI] [PubMed] [Google Scholar]
- 27.Gill SS, Patel NK, Hotton GR, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 28.Manfredsson FP, Okun MS, Mandel RJ. Gene therapy for neurological disorders: challenges and future prospects for the use of growth factors for the treatment of Parkinson’s disease. Neurobiol Dis. 2007;25:35–44. doi: 10.2174/156652309789753400. [DOI] [PubMed] [Google Scholar]
- 29.Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005;102:216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- 30.Vaillancourt DE, Spraker MB, Prodoehl J, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72:1378–1384. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasternak O, Westin CF, Bouix S, et al. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci. 2012;32:17365–17372. doi: 10.1523/JNEUROSCI.2904-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao YJ, Wee HL, Au WL, et al. Progression of Parkinson’s disease as evaluated by Hoehn and Yahr stage transition times. Parkinsonism Relat Disord. 2011;17:194–197. doi: 10.1016/j.parkreldis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 33.The Parkinson Progression Marker Initiative (PPMI) The Parkinson’s progression marker initiative (PPMI) – Assessment of clinical, imaging and CSF PD biomarkers. Movement Disorders. 2014;29(Suppl 1):729. [Google Scholar]
- 34.Chan LL, Rumpel H, Yap K, et al. Case control study of diffusion tensor imaging in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:1383–1386. doi: 10.1136/jnnp.2007.121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prakash BD, Sitoh YY, Tan LC, Au WL. Asymmetrical diffusion tensor imaging indices of the rostral substantia nigra in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:1029–1033. doi: 10.1016/j.parkreldis.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 36.Zhang K, Yu C, Zhang Y, Wu X, Zhu C, Chan P, Li K. Voxel-based analysis of diffusion tensor indices in the brain in patients with Parkinson’s disease. Eur J Radiol. 2011;77:269–273. doi: 10.1016/j.ejrad.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 37.Karimi M, Tian L, Brown CA, et al. Validation of nigrostriatal positron emission tomography measures: critical limits. Ann Neurol. 2013;73:390–396. doi: 10.1002/ana.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med. 2005;54:1377–1386. doi: 10.1002/mrm.20642. [DOI] [PubMed] [Google Scholar]
- 39.Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48:577–582. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


