Summary
Background
Restricted diffusion is the second most common atypical presentation of PRES. This has a very important implication, as lesions with cytotoxic edema may progress to infarction. Several studies suggested the role of DWI in the prediction of development of infarctions in these cases. Other studies, however, suggested that PRES is reversible even with cytotoxic patterns. Our aim was to evaluate whether every restricted diffusion in PRES is reversible and what factors affect this reversibility.
Material/Methods
Thirty-six patients with acute neurological symptoms suggestive of PRES were included in our study. Inclusion criteria comprised imaging features of atypical PRES where DWI images and ADC maps show restricted diffusion. Patients were imaged with 0.2-T and 1.5-T machines. FLAIR images were evaluated for the severity of the disease and a FLAIR/DWI score was used. ADC values were selectively recorded from the areas of diffusion restriction. A follow-up MRI study was carried out in all patients after 2 weeks. Patients were classified according to reversibility into: Group 1 (reversible PRES; 32 patients) and Group 2 (irreversible changes; 4 patients). The study was approved by the University’s research ethics committee, which conforms to the declaration of Helsinki.
Results
The age and blood pressure did not vary significantly between both groups. The total number of regions involved and the FLAIR/DWI score did not vary significantly between both groups. Individual regions did not reveal any tendency for the development of irreversible lesions. Similarly, ADC values did not reveal any significant difference between both groups.
Conclusions
PRES is completely reversible in the majority of patients, even with restricted diffusion. None of the variables under study could predict the reversibility of PRES lesions. It seems that this process is individual-dependent.
MeSH Keywords: Diffusion Magnetic Resonance Imaging, Eclampsia, Posterior Leukoencephalopathy Syndrome
Background
Posterior Reversible Encephalopathy syndrome (PRES) is a common syndrome that occurs primarily due to an alteration of the cerebro-vascular auto-regulatory mechanisms. Patients may present with acute recent-onset seizures, blindness, disturbed consciousness and headache. This syndrome is usually completely reversible with mild permanent morbidity, if any. After the introduction of diffusion weighted imaging (DWI) as a routine sequence in brain MRI studies, there has been an increasing recognition of atypical patterns of PRES, with diffusion restriction instead of the classically described enhanced diffusion due to vasogenic edema.
Several theories have been proposed for its pathophysiology but none of them has been fully confirmed. There are two widely accepted theories. The first one describes a failure of the cerebro-vascular auto-regulatory mechanism, with consequent vasodilatation, hyper-perfusion and vasogenic brain edema. The second theory describes a “vasculopathy” pattern with consequent diffuse or focal vasospasm or even alternating areas of vascular spasm and dilatation, and hypo-perfusion [1].
Restricted diffusion was the second most common atypical presentation of PRES in a study by McKinney et al., accounting for 17.3%. According to his study, atypical patterns of PRES may be more frequent than commonly perceived [2]. This has a very important implication, as lesions with cytotoxic edema may progress to infarction. Several studies also suggested the role of DWI in the prediction of irreversibility of PRES lesions and their progression into infarctions [3].
Other studies, however, suggested that PRES is reversible even with cytotoxic patterns [4,5]. No definite criteria have, however, been established to predict which lesions are likely to be reversible and which lesions are likely to progress to infarction.
Aim of study
Our aim was to evaluate the atypical cases of PRES, presenting with restricted diffusion, and whether they were reversible, and which factors affected that reversibility.
Material and Methods
Thirty-six patients were included in our study. Patients presented to the Radiology Department with acute neurological symptoms suggestive of PRES. Most of the patients were referred from the Obstetrics and Gynecology Department with peri-partum pre-eclampsia/eclampsia manifestations. Only 1 male child was included. He presented with neurological manifestations due to hypertension secondary to the nephritic syndrome. The age of patients ranged from 14 to 39 years with a mean age of 22.9±5.4 years. Inclusion criteria comprised imaging features of atypical PRES with DWI images and ADC maps showing restricted diffusion suggestive of cytotoxic changes. Exclusion criteria included inability to have an MRI scan and studies with no evidence of diffusion restriction on the ADC maps.
All patients had an MRI performed after the end of fits and discharge from the intensive care unit (ICU). The range of days between presentation and the MRI study was 3–5 days. Clinical data on recorded blood pressure and patients’ age were evaluated.
MR imaging
Patients were imaged with 0.2-T GE Signa Excite machine (GE Healthcare, Waukesha, WI). All patients had a customized brain MRI study composed of axial FLAIR, sagittal T1-weighted and axial Line Scan DWI. That MRI study was customized to have the shortest scan time possible with enough imaging sequences. The scan parameters for the FLAIR sequence were: TR/TE/TI: 7575/110/1593 msec, slice thickness/inter-slice gap: 8/1 mm, matrix: 224×160, for the sagittal T1W sequence: TR/TE: 560/15 msec, slice thickness/inter-slice gap: 6/7 mm, matrix: 224×192, and for the DWI: line scan DWI sequence, axial slices with TR/TE: 280/118 msec, slice thickness/inter-slice gap: 8/8 mm, b-value: 750 s/mm2, matrix: 112×96. The sagittal T1W sequence was added to evaluate the pituitary gland for peri-partum complications, e.g. pituitary apoplexy. None of our patients had abnormalities in the pituitary gland.
Two patients were imaged on a 1.5-T Philips Achieva machine (Philips, Netherlands), following the same standard sequences. DWI parameters were as follows: single-shot echo-planar imaging sequence (SS-EPI), axial slices with TR/TE: 6000/90 msec, slice thickness/inter-slice gap: 4/1 mm, b-value: 1000 s/mm2, matrix: 128×128.
Image analysis
Images were transferred to a personal computer. First, FLAIR images were evaluated for the severity of the disease. The total number of regions affected was counted, regardless of bilaterality, as PRES is commonly bilateral. The evaluated regions included: infra-tentorial area, basal ganglia/thalami, temporal lobes, occipital lobes, parietal lobes, and frontal lobes. Then, a FLAIR/DWI score was also used to assess the extent of abnormalities, as previously described [6]. This score measures the extent of disease using T2/FLAIR and DWI sequences on a scale of 0–4. Normal findings were scored as 0, small areas of abnormal signal intensity were scored as 1, easily perceptible abnormalities were scored as 2, and large confluent areas of abnormal high signal intensity were scored as 3. A score of 4 was assigned to each region with high DWI signal intensity. The total T2/DWI score for each patient is the sum of scores in each region [6].
The ADC maps were generated by the local software of the machine Functool (GE Healthcare, Waukesha, WI) and similarly the local software of the Philips machine. Images are automatically co-registered. Regions of interest (ROIs) were placed on the DWI image and the corresponding ADC value was recorded. The ROIs were small enough to encompass only the restricted diffusion area. This was done to avoid intra-voxel averaging of ADC values with adjacent areas of vasogenic edema.
Follow-up
Follow-up MRI studies were carried out in all patients after 2 weeks. The same machine and same sequences were used for all patients. The presence of residual lesions, suggestive of an infarct, was evaluated. Patients were then classified according to the reversibility of lesions into: Group 1 (reversible PRES; 32 patients) and Group 2 (irreversible changes; 4 patients) – Figures 1–4.
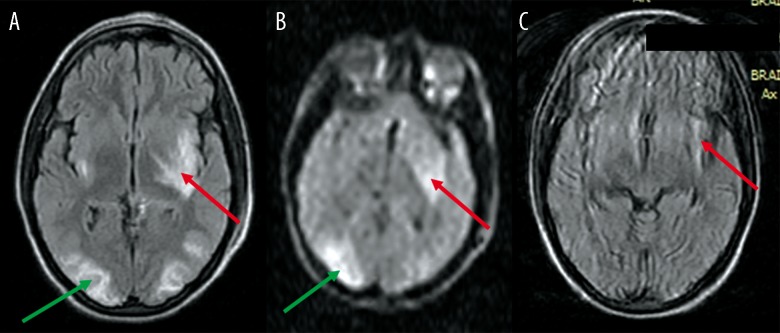
Figure 1.
A 28-year-old female presented with pre-partum fits (eclampsia). (A) FLAIR image reveals typical signal-intensity changes suggestive of PRES in both occipital lobes (green arrow), as well as in the insular cortex bilaterally (red arrow), left internal capsule and left lentiform nucleus. (B) DW image reveals high signal in the left lentiform and left insular cortex (red arrow) as well as the right. occipital lobe cortex (green arrow). (C) FLAIR image at follow-up reveals persistent infarction in the left insular cortex (red arrow).
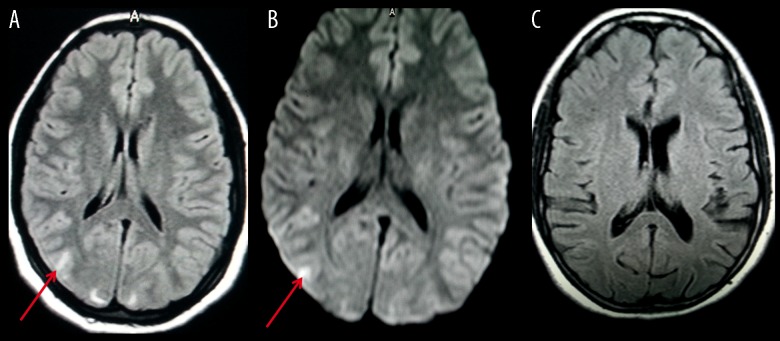
Figure 4.
A 23-year-old female presented with pre-partum fits. (A) FLAIR image (1.5 T) reveals lesions of abnormal signal intensity, suggestive of PRES, bilaterally in the occipital lobes (red arrow). (B) DW image reveals increased signal intensity in the right occipital lobe cortex (red arrow), suggesting cytotoxic edema. (C) FLAIR image at follow-up reveals complete resolution.
Ethical issues
The study was approved by the University’s local research ethics committee, which conforms to the declaration of Helsinki. All patients provided a written consent prior to their inclusion in the study.
Statistical analysis
An independent sample T-test was used to compare the difference of means in quantitative data, i.e. age, systolic and diastolic blood pressure, and mean ADC values. The Mann-Whitney U test was used to compare the number of regions affected and the FLAIR/DWI score between both groups. The chi-square test was used to compare the individual affection of different cerebral regions between both groups. Spearman’s correlation test was used to study the relation between reversibility of lesions and the clinical and radiological data under study. Linear regression analysis was also run to detect any influence of these factors on reversibility. The level of significance was determined as P≤0.05.
Results
The patients’ age and blood pressure did not vary significantly between both groups. This is displayed in Table 1.
Table 1.
Differences in age and blood pressure between both groups.
| Group 1 (Reversible) | Group 2 (Irreversible) | T-test | |
|---|---|---|---|
| Age | 22.4±5.5 | 27±1.2 | 0.113 |
| Systolic blood pressure | 176.3±19.6 | 181.5±31.8 | 0.769 |
| Diastolic blood pressure | 99.3±6.8 | 96.5±1.7 | 0.074 |
Comparison of radiological findings between both groups
Number of regions involved: The total number of regions involved (regardless of bilaterality) ranged from 1 to 4 for group 1 (median: 3 regions) while it ranged from 1 to 3 for group 2 (median: 2 regions). There was no significant difference between both groups (P=0.127).
FLAIR/DWI score: The score ranged from 4 to 52 for both groups with a median of 19. No significant difference was noted between both groups (P=0.713). This is displayed in Table 2.
Regions involved: The individual regions did not reveal any tendency for the development of irreversible lesions. This is displayed in Table 3.
ADC value: The mean ADC values of restricted diffusion lesions were compared between both groups. The results are represented in Table 4.
Table 2.
Differences in the extent of the disease between both groups.
| Extent of disease | Group 1 (Reversible) | Group 2 (Irreversible) | MW test |
|---|---|---|---|
| Number of regions | 1–4 (Median: 3) | 1–3 (Median: 2) | 0.127 |
| FLAIR/DWI score | 12–52 (Median: 19) | 4–42 (Median: 23) | 0.713 |
MW test – Mann-Whitney U test.
Table 3.
Comparison of regional involvement between both groups.
| Regions affected | Group 1 (Reversible) | Group 2 (Irreversible) | Chi-square test |
|---|---|---|---|
| Occipital lobe | 24 | 2 | 0.293 |
| Parietal lobe | 30 | 4 | 0.607 |
| Frontal lobe | 22 | 2 | 0.453 |
| Temporal lobe | 10 | 0 | 0.188 |
| Basal ganglia | 4 | 0 | 0.453 |
| Infra-tentorial | 4 | 0 | 0.453 |
Table 4.
Comparison of ADC value between both groups.
| Group 1 (Reversible) | Group 2 (Irreversible) | T-test | |
|---|---|---|---|
| ADC | 4.46±3.62×10−3 | 6.2 ± 6.36×10−3 | 0.627 |
ADC – apparent diffusion coefficient.
Correlation of imaging variables with reversibility of lesions and regression analysis
Using Spearman’s correlation, we found a positive correlation between the age of patients and the reversibility (R=0.428, P=0.009; which was significant at P=0.01 level). There was another positive correlation between the systolic blood pressure and ADC values (R=0.581, P<0.0005; which was significant at P=0.01 level).
Regression analysis revealed no significant contribution of any of the imaging variables to the reversibility of lesions.
Discussion
Posterior reversible encephalopathy syndrome (PRES) is probably the most common neurological complication noted in pre-eclampsia/eclampsia. However, this is not the sole clinical condition associated with PRES. It may also be seen with hypertensive encephalopathy, immune-suppressive therapy, solid organ or bone marrow transplantation, as well as other clinical situations [7]. Classically, PRES is described as bilateral vasogenic edema affecting preferentially the parieto-occipital lobes bilaterally. This preferential posterior circulation distribution has been suggested to be due to the decreased sympathetic innervation of the posterior circulation [1,8,9]. Vasogenic edema occurs due to failure of the protective cerebro-vascular auto-regulatory mechanism in situations of high blood pressure or circulating toxins, leading to consequent vasodilatation and leakage of fluid into the interstitial tissue.
The atypical patterns of PRES are being increasingly recognized. This has led many researchers to postulate a vaso-constrictive theory for the explanation of PRES. Whether this vaso-constrictive theory is a different entity, or it occurs along with vasodilatation, is still unknown. In our study, we selected patients with PRES-associated restricted diffusion as this is presumed to progress to infarction. Most of these patients (88.9%) were completely reversible. Only 4 patients (11.1%) revealed residual infarcts. We studied some clinical and imaging features to find out whether they affect reversibility. Although the age and systolic blood pressure were higher for the irreversible group, the difference was not significant. Spearman’s correlation test revealed conflicting results regarding the age of patients, showing a fair positive relation between the patients’ age and the reversibility of lesions. This conflicting result may have occurred because of a low number of patients, particularly in group 2, and we did not find any supporting evidence in literature. We agree with other authors that the age and blood pressure do not have a major impact on the reversibility of lesions [10,11].
The progression of vasogenic edema into cytotoxic edema and infarction is a poorly understood process. Ay et al. suggested that the increased tissue pressure due to vasogenic edema might eventually alter the micro-circulation and lead to ischemia [12]. Accordingly, we evaluated whether the severity or extent of PRES might be related to the development of infarction. Neither the total sum of regions affected nor the individual regions revealed any relation to the reversibility of PRES. This is in contradiction with some authors, who stated that the brain stem and deep white matter lesions revealed less reversibility [13]. In our study, the irreversible group of patients did not reveal any brain stem involvement and the location of infarcts was mainly in the cortex and sub-cortical white matter. The contradiction between our results and that of Pande et al. may be due to the low number of patients from the irreversible group in our study [13]. It may also be due to the fact that his study sample included patients with PRES due to different etiologies, i.e. hypertension, drug-induced and eclampsia, whereas in our study, nearly all patients were eclamptic. Pande et al. concluded that pre-eclampsia-induced PRES revealed maximum reversibility compared to hypertension- or drug-induced PRES [13]. We also used a semi-quantitative score to quantify the extent of PRES abnormalities in our study. Using the same score, Covarrubias et al. concluded that the incidence of complications and irreversibility increased with that score increased [6]. Our results disagreed with his and, again, this may have been due to the difference in the study sample, i.e. his group of patients included PRES induced by causes other than pre-eclampsia, whereas in our group of patients, it was predominantly due to pre-eclampsia.
Diffusion weighted MRI is not a new sequence. It is now a routine in clinical practice. Because of its sensitivity to proton diffusion, this sequence proved extremely valuable in the evaluation of tissue micro-structure. Normally, protons diffuse freely in the interstitial compartment and through the cell membranes. This motion causes these protons to lose signal on DWI. During an acute ischemic insult, there is loss of function of the Na+/K+-ATPase pump, leading to redistribution of protons from extracellular to intracellular compartments, with consequent cytotoxic edema. The motion of protons is thus limited in both compartments. Accordingly, no loss of signal occurs and the affected area appears hyperintense on the DWI scan and hypointense on the corresponding ADC map. The appearance of vasogenic edema on DWI scans is variable because of the well-known T2-shine-through artifact. It may appear hypo-, iso-, or hyper-intense [14,15], but it is confirmed on the ADC map, where the regions of vasogenic edema reveal increased ADC values.
The ADC values measured from areas of cytotoxic edema did not reveal any significant difference between reversible and irreversible lesions. In this we disagree with many authors who suggested that ADC values are significantly lower in irreversible lesions and are a good predictor of irreversibility [3,16–18]. This cause of disagreement between our study and other studies may be unclear, especially in comparison to Loureiro et al.(3) In the study by Loureiro et al., the ADC values were compared between normal brain tissue and vasogenic edema, but the authors did not compare the ADC values of the restricted diffusion areas with vasogenic edema or normal brain tissue. Also, the restricted diffusion accurately predicted infarction in 3 out of 4 patients, so there was 1 patient who had restricted diffusion that was completely reversible. But that was not discussed by the authors. In the study by Watanabe, the study sample was much smaller than ours (only 5 patients) [16]. This may have led to less reliable findings. Koch et al. described a single case, in which the patient had 2 regions of restricted diffusion that were confirmed later on to be infarcts [17]. Similarly, in the study by Mukherjee et al., the developing infarction was noted in 1 patient [18]. This confirms that the progression of eclamptic PRES to ischemic changes is an uncommon event. Finally, the low number of irreversible patients in our study may have also contributed to this disagreement. On the other hand, many authors have also described a restricted pattern of PRES that is fully reversible [4,5,13,19–21]. In addition, Chen et al. also suggested that the prediction of the outcome of PRES based on DWI and ADC values should be used with caution [4]. On the other hand, Covarrubias et al. suggested that the temporal evolution of ischemic changes in PRES needs further study before conclusions can be made on the development of ischemic changes [6].
A fair positive correlation was noted in our study between the systolic blood pressure and ADC values. This is probably an expected relation, which agrees with the first theory of PRES pathogenesis, i.e. the failure of the cerebro-vascular auto-regulatory mechanism with consequent vasodilatation and interstitial edema. To the authors’ best knowledge, this relation has not been described much in literature. Only animal reports were found describing the relation of hypertension and ADC values but in situations other than PRES [22–24].
The regression analysis in our study confirmed all the previous results that none of the variables under study could predict the reversibility of PRES lesions. It seems that this process is individual-dependent. In a study by the CYPRESS group, the determinants of recovery from severe PRES were the level of glycaemia and the time to causative-factor control. None of the imaging features predicted reversibility [11].
The pathogenesis of diffusion restriction in PRES is still unclear. Reversible diffusion restriction in patients with Influenza-associated encephalopathy/encephalitis (IAEE) syndrome has been suggested to be due to intra-myelinic edema [25–28]. This was similarly suggested in some cases of toxic leuko-encephalopathy and epilepsy [29–31]. This may be the case in PRES patients with reversible diffusion restriction. It is possible that prompt and rapid management of patients led to the reversibility of white matter lesions that were previously believed to be non-reversible.
Our study had some limitations. First, the limited number of patients in the study as a whole and in the irreversible group particularly, was a drawback. We still need large-scale studies to confirm our findings. Second, the low-field MRI machine obliged us to use an uncommon DWI sequence. However, every effort was made to utilize only the best-quality images. Also, 2 patients were imaged with a 1.5-T machine and were fully reversible, thus confirming the results of the study. Finally, patients in our study were mainly eclamptic females. Pre-eclampsia-induced PRES is suggested to have the best prognosis compared to that induced by other causes [13]. We still need more studies to evaluate whether PRES induced by other causes would have similar results.
Conclusions
Imaging still plays an important role in patients with suspected PRES, primarily to exclude other neurological disorders that clinically mimic PRES. PRES is completely reversible in the majority of patients. Still, many authors suggest more aggressive treatment for patients with cytotoxic changes, e.g. calcium channel blockers which are used in patients with cerebral vasoconstriction secondary to subarachnoid hemorrhage [32]. Large-scale studies are needed to confirm whether or not the imaging findings can alter the choice of treatment for better patient care.
Figure 2.
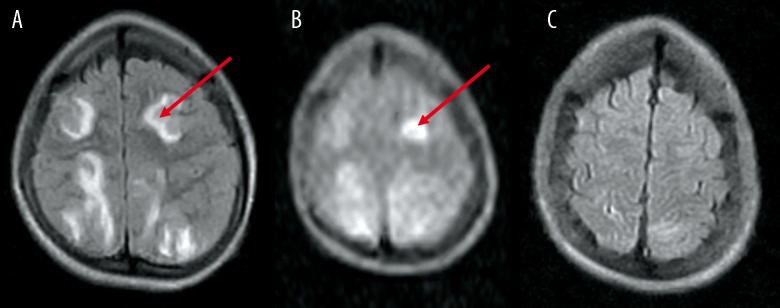
A 14-year-old boy presented with hypertensive encephalopathy secondary to nephritic syndrome. (A) FLAIR image reveals bilateral frontal (red arrow) and parietal lesions with abnormal signal intensity, suggestive of PRES. (B) DW image reveals increased signal intensity in the left frontal lobe (red arrow). (C) Follow-up image reveals complete resolution of the left frontal lobe.
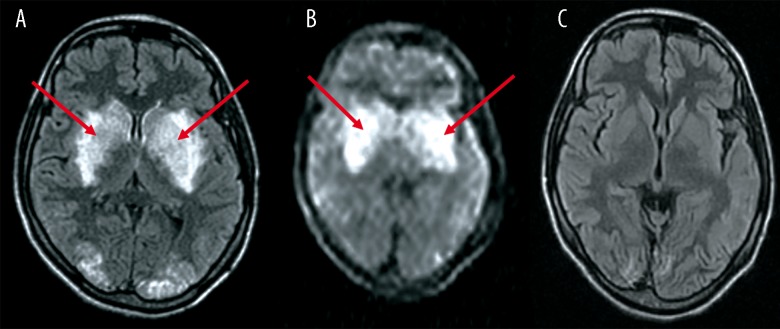
Figure 3.
A 24-year-old female presented with pre-partum fits. (A) FLAIR image reveals lesions of abnormal signal intensity, suggestive of PRES, within bilateral basal ganglia (red arrows) and in the occipital lobe. (B) DW image reveals increased signal intensity in the basal ganglia bilaterally (red arrows). (C) FLAIR image at follow-up reveals complete resolution.
Footnotes
Conflict of interest
The authors report no conflict of interest.
References
- 1.Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. Am J Neuroradiol. 2008;29(6):1043–49. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKinney AM, Short J, Truwit CL, et al. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. Am J Roentgenol. 2007;189(4):904–12. doi: 10.2214/AJR.07.2024. [DOI] [PubMed] [Google Scholar]
- 3.Loureiro R, Leite CC, Kahhale S, et al. Diffusion imaging may predict reversible brain lesions in eclampsia and severe preeclampsia: Initial experience. Am J Obstet Gynecol. 2003;189:1350–55. doi: 10.1067/s0002-9378(03)00651-3. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Xu M, Shang D, Luo B. A case of reversible splenial lesions in late postpartum preeclampsia. Intern Med. 2012;51(7):787–90. doi: 10.2169/internalmedicine.51.6500. [DOI] [PubMed] [Google Scholar]
- 5.Benziada-Boudour A, Schmitt E, Kremer S, et al. Posterior reversible encephalopathy syndrome: a case of unusual diffusion-weighted MR images. J Neuroradiol. 2009;36(2):102–5. doi: 10.1016/j.neurad.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. Am J Neuroradiol. 2002;23(6):1038–48. [PMC free article] [PubMed] [Google Scholar]
- 7.Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. Am J Neuroradiol. 2008;29(6):1036–42. doi: 10.3174/ajnr.A0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edvinsson L, Owman C, Sjöberg NO. Autonomic nerves, mast cells, and amine receptors in human brain vessels. A histochemical and pharmacologic study. Brain Res. 1976;115(3):377–93. doi: 10.1016/0006-8993(76)90356-5. [DOI] [PubMed] [Google Scholar]
- 9.Beausang-Linder M, Bill A. Cerebral circulation in acute hypertension – protective effects of sympathetic nervous activity. Acta Physiol Scand. 1981;111(2):193–99. doi: 10.1111/j.1748-1716.1981.tb06724.x. [DOI] [PubMed] [Google Scholar]
- 10.Demirtaş O, Gelal F, Vidinli BD, et al. Cranial MR imaging with clinical correlation in preeclampsia and eclampsia. Diagn Interv Radiol. 2005;11(4):189–94. [PubMed] [Google Scholar]
- 11.Legriel S, Schraub O, Azoulay E, et al. on behalf of the Critically Ill Posterior Reversible Encephalopathy Syndrome Study group (CYPRESS) Determinants of Recovery from Severe Posterior Reversible Encephalopathy Syndrome. PLoS One. 2012;7(9):e44534. doi: 10.1371/journal.pone.0044534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ay H, Buonanno FS, Schaefer PW, et al. Posterior leukoencephalopathy without severe hypertension: utility of diffusion-weighted MRI. Neurology. 1998;51(5):1369–76. doi: 10.1212/wnl.51.5.1369. [DOI] [PubMed] [Google Scholar]
- 13.Pande AR, Ando K, Ishikura R, et al. Clinicoradiological factors influencing the reversibility of posterior reversible encephalopathy syndrome: a multicenter study. Radiat Med. 2006;24(10):659–68. doi: 10.1007/s11604-006-0086-2. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology. 2000;217(2):331–45. doi: 10.1148/radiology.217.2.r00nv24331. [DOI] [PubMed] [Google Scholar]
- 15.Doelken M, Lanz S, Rennert J, et al. Differentiation of cytotoxic and vasogenic edema in a patient with reversible posterior leukoencephalopathy syndrome using diffusion-weighted MRI. Diagn Interv Radiol. 2007;13(3):125–28. [PubMed] [Google Scholar]
- 16.Watanabe Y, Mitomo M, Tokuda Y, et al. Eclamptic encephalopathy: MRI, including diffusion-weighted images. Neuroradiology. 2002;44(12):981–85. doi: 10.1007/s00234-002-0867-y. [DOI] [PubMed] [Google Scholar]
- 17.Koch S, Rabinstein A, Falcone S, Forteza A. Diffusion-weighted imaging shows cytotoxic and vasogenic edema in eclampsia. Am J Neuroradiol. 2001;22(6):1068–70. [PMC free article] [PubMed] [Google Scholar]
- 18.Mukherjee P, McKinstry RC. Reversible posterior leukoencephalopathy syndrome: evaluation with diffusion-tensor MR imaging. Radiology. 2001;219(3):756–65. doi: 10.1148/radiology.219.3.r01jn48756. [DOI] [PubMed] [Google Scholar]
- 19.Na SJ, Hong JM, Park JH, et al. A case of reversible postpartum cytotoxic edema in preeclampsia. J Neurol Sci. 2004;221(1–2):83–87. doi: 10.1016/j.jns.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Ulrich K, Tröscher-Weber R, Tomandl BF, et al. Posterior reversible encephalopathy in eclampsia: diffusion-weighted imaging and apparent diffusion coefficient-mapping as prognostic tools? Eur J Neurol. 2006;13(3):309–10. doi: 10.1111/j.1468-1331.2006.01156.x. [DOI] [PubMed] [Google Scholar]
- 21.Ishikura K, Hamasaki Y, Sakai T, et al. Children with posterior reversible encephalopathy syndrome associated with atypical diffusion-weighted imaging and apparent diffusion coefficient. Clin Exp Nephrol. 2011;15(2):275–80. doi: 10.1007/s10157-010-0380-2. [DOI] [PubMed] [Google Scholar]
- 22.Su ZY, Li CS. The evaluation of cerebral function by diffusion weighted imaging after norepinephrine-induced hypertensive perfusion therapy in pig model of cardiac arrest. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013;25(2):92–95. doi: 10.3760/cma.j.issn.2095-4352.2013.02.010. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 23.Kang BT, Leoni RF, Kim DE, Silva AC. Phenylephrine-induced hypertension during transient middle cerebral artery occlusion alleviates ischemic brain injury in spontaneously hypertensive rats. Brain Res. 2012;1477:83–91. doi: 10.1016/j.brainres.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee TH, Liu HL, Yang ST, et al. Effects of aging and hypertension on cerebral ischemic susceptibility: evidenced by MR diffusion-perfusion study in rat. Exp Neurol. 2011;227(2):314–21. doi: 10.1016/j.expneurol.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Takanashi J, Barkovich AJ, Yamaguchi K, et al. Influenza-associated encephalitis/encephalopathy with a reversible lesion in the splenium of the corpus callosum: a case report and literature review. Am J Neuroradiol. 2004;25:798–802. [PMC free article] [PubMed] [Google Scholar]
- 26.Tada H, Takanashi J, Barkovich AJ, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology. 2004;63:1854–58. doi: 10.1212/01.wnl.0000144274.12174.cb. [DOI] [PubMed] [Google Scholar]
- 27.Engelbrecht V, Scherer A, Rassek M, et al. Diffusion-weighted MR imaging in the brain in children: findings in the normal brain and in the brain with white matter diseases. Radiology. 2002;222:410–18. doi: 10.1148/radiol.2222010492. [DOI] [PubMed] [Google Scholar]
- 28.Oster J, Doherty C, Grant PE, et al. Diffusion-weighted imaging abnormalities in the splenium after seizures. Epilepsia. 2003;44:852–54. doi: 10.1046/j.1528-1157.2003.40902.x. [DOI] [PubMed] [Google Scholar]
- 29.McKinney AM, Kieffer SA, Paylor RT, et al. Acute toxic leukoencephalopathy: Potential for reversibility clinically and on MRI with diffusion-weighted and FLAIR imaging. Am J Roentgenol. 2009;193:192–206. doi: 10.2214/AJR.08.1176. [DOI] [PubMed] [Google Scholar]
- 30.Sivasubramanian S, Moorthy S, Sreekumar KP, Kannan RR. Diffusion-weighted magnetic resonance imaging in acute reversible toxic leukoencephalopathy: A report of two cases. Indian J Radiol Imaging. 2010;20(3):192–94. doi: 10.4103/0971-3026.69354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oster J, Doherty C, Grant PE, et al. Diffusion-weighted imaging abnormalities in the splenium after seizures. Epilepsia. 2003;44(6):852–54. doi: 10.1046/j.1528-1157.2003.40902.x. [DOI] [PubMed] [Google Scholar]
- 32.Schneider JP, Krohmer S, Günther A, Zimmer C. Cerebral lesions in acute arterial hypertension: the characteristic MRI in hypertensive encephalopathy. Rofo. 2006;178(6):618–26. doi: 10.1055/s-2006-926631. [in German] [DOI] [PubMed] [Google Scholar]