Summary
Background
The role of radiology is of utmost importance not only in diagnosing s-OHSS but also in ruling out other cystic ovarian diseases and to determine the underlying etiology and course of the disease. We presented a radiological algorithm for diagnosing the various causes of s-OHSS.
Case Report
A 26-year-old female, gravida one was referred to radiology department with history of lower abdominal pain, nausea and vomiting since 2 days which was gradual in onset and progression. The patient had no significant medical and surgical history.
Conclusions
This article illustrates and emphasizes that diagnosis of s-OHSS and its etiology can be completely evaluated radiologically. Biochemical markers will confirm the radiological diagnosis.
MeSH Keywords: Choriocarcinoma, Ovarian Hyperstimulation Syndrome, Ultrasonography
Background
Ovarian hyperstimulation syndrome (OHSS) is a rare, potentially life-threatening systemic complication caused by iatrogenic and spontaneous (non-iatrogenic) ovarian stimulation occurring due to little-known patho-physiological mechanisms. The vast majority of OHSS cases are usually iatrogenic and are seen to complicate about 1 percent of all assisted reproductive technologies, and are induced by exogenous hormonal therapy [1], whereas the extremely rare, non-iatrogenic cases, which are also known as spontaneous OHSS (s-OHSS) are unrelated to exogenous hormones and are usually seen to complicate pregnancy (both singleton and multiple) [2–8], gestational trophoblastic neoplasms [9–12], pituitary adenomas [13–19], and hypothyroidism [20–23]. Clinicoradiological spectrum ranges from mild to severe [24] OHSS which needs prompt evaluation and management.
The role of radiology is of utmost importance not only in diagnosing s-OHSS but also in ruling out other cystic ovarian diseases; it is also used to determine the underlying etiology of this complication and monitor the course of the disease. We presented a radiological algorithm for diagnosing the various causes of s-OHSS. We also highlighted an unusual and never reported case of mediastinal choriocarcinoma causing s-OHSS.
Case Report
Case report 1
A 26-year-old female, gravida one was referred to the radiology department with a history of lower abdominal pain, nausea and vomiting for the last 2 days, gradual in onset and progression. The patient had no significant medical or surgical history. She neither received any medication for 6 months before pregnancy nor underwent any assisted reproduction therapy. Menarche occurred at the age of 15 years, and her menstrual periods were regular. Her family history was negative for tuberculosis or gynecological malignancy. The vital signs were as follows: blood pressure of 100/60 mmHg, pulse rate100 bpm, body temperature 36.2°C, and respiration rate 26/min with normal preliminary blood counts.
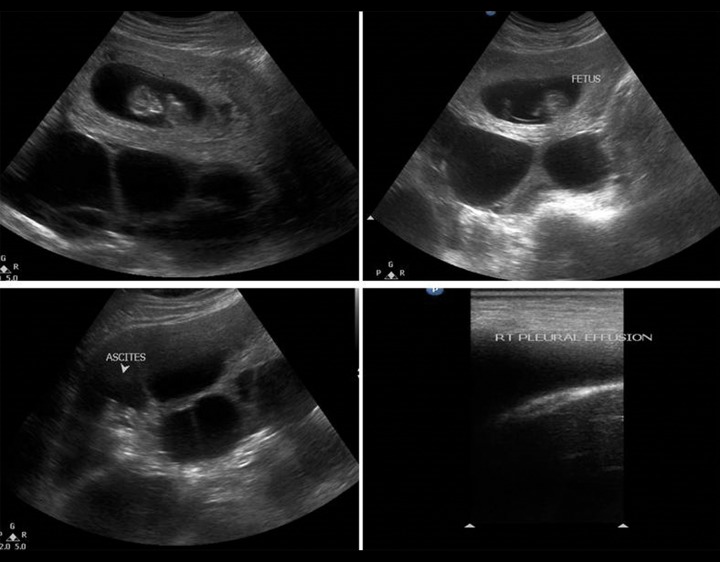
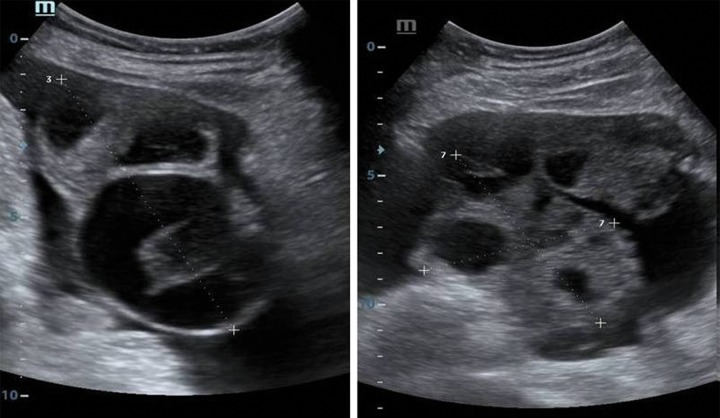
Ultrasonographic examination revealed a live intrauterine gestational sac at 6 weeks gestation, with bilaterally enlarged, multicystic ovaries measuring 15×12 cm on the right side and 14×11 cm on the left side with ascites (Figure 1). The rest of the abdomen was unremarkable. Chest US examination revealed bilateral mild pleural effusion with no pericardial effusion.
Figure 1.
A 26-year-old female with spontaneous OHSS. Ultrasonography B-mode images show intrauterine fetus with multiple cysts involving both the right and the left ovary. There is mild free fluid in the intra-peritoneal cavity with pleural effusion.
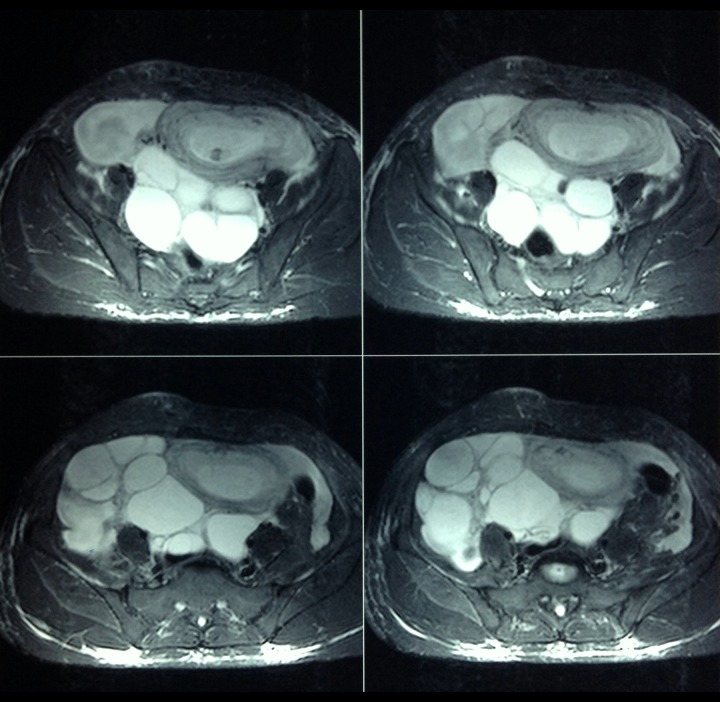
A provisional diagnosis of severe OHSS in spontaneous pregnancy was made. Differential diagnosis of tuberculosis and malignancy was also considered. Further imaging with MRI confirmed OHSS (Figure 2). The β hCG level corresponded to the weeks of gestation. Estradiol level was elevated and amounted to 3000 pg/mL (reference value for the 1st trimester is 188–2479 pg/mL); CA 125 was normal. The patient was managed with intravenous fluid and 5% albumin, and subjected to regular physical, biochemical, and radiological monitoring. The patient was discharged and advised to undergo weekly follow-ups. The pregnancy progressed to term without any other complications. A follow-up MRI after 6 weeks post-partum revealed a simple ovarian cyst in the left ovary with complete regression of s-OHSS (Figure 3).
Figure 2.
A 26-year-old female with spontaneous OHSS. The pelvis T2W MRI images show a T2 hypointense lesion in the intrauterine cavity suggestive of a fetus. There are hyperintense multiple cysts seen without any mural nodule arising from either the right (in the right iliac fossa) or the left ovary (in the pouch of Douglas).
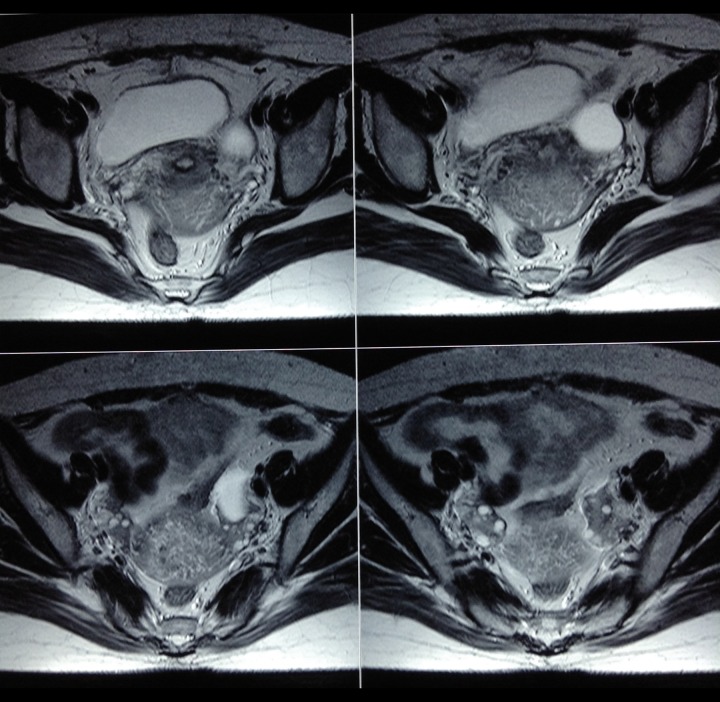
Figure 3.
A 26-year-old female with spontaneous OHSS. The pelvis T2W MRI images at 6 weeks post-partum show complete resolution of bilateral cystic lesions with a single follicular cyst in the left ovary and multiple small follicles in the right ovary.
Case report 2
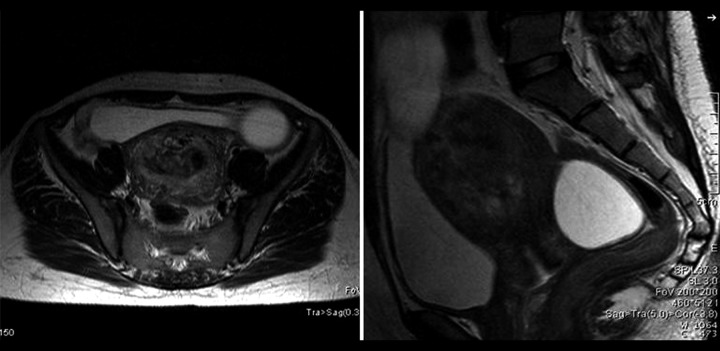
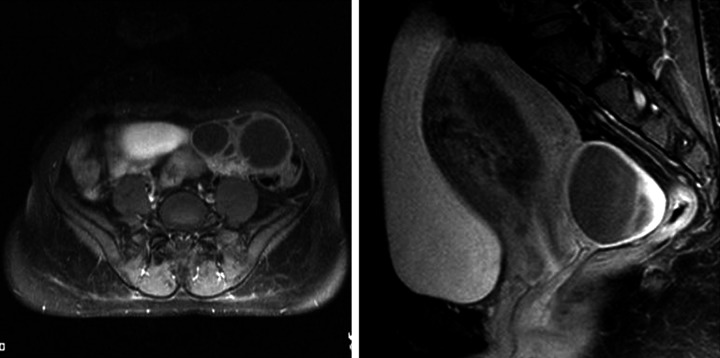
A 29-year-old female, gravida one presented to the emergency department with acute abdominal pain, nausea for the last 2 days and amenorrhea for the last 4 months. She had never undergone assisted reproduction and gave no history of any drug intake. General examination revealed stable vitals. The abdomen was mildly distended. Ultrasonography revealed an ill-defined heterogeneous, predominantly hyper-echoic mass with multiple scattered tiny hypo-echoic areas in the uterine cavity with loss of the endometrial-myometrial interface. Colour Doppler revealed prominent vessels within the lesion with invasion of the myometrium. Both ovaries were enlarged and multicystic. The abdominal and chest examination was otherwise normal. MRI confirmed deep myometrial invasion and no pelvic adenopathy (Figures 4 and 5). Laboratory investigations revealed an elevated β hCG level, of 210467 mIU/mL (normal reference value 4060–165400 mIU/mL).
Figure 4.
A 29-year-old female with an invasive mole. The pelvis T2W axial and sagittal MRI images show a T2 heterogeneous lesion in the endometrial cavity causing invasion of the anterior myometrium with T2 hypointense flow voids in the myometrium. There are hyperintense cysts arising from both the left and the right ovary.
Figure 5.
A 29-year-old female with an invasive mole. the T1 post-contrast fat-saturated axial and sagittal pelvic images show multiple cysts arising from the left ovary in the left iliac fossa without any obvious solid component, and a right ovarian cystic lesion in the pouch of Douglas. There is a heterogeneously-enhancing lesion within the endometrial cavity with invasion of the anterior myometrium and the overlying serosa.
A working diagnosis of an invasive mole with mild spontaneous OHSS was made. The patient was immediately started on methotrexate with regular β hCG monitoring and ultrasonographic studies. There was resolution of the symptoms and a repeat MRI after 6 months revealed a normal endometrial cavity and ovarian follicle.
Case report 3
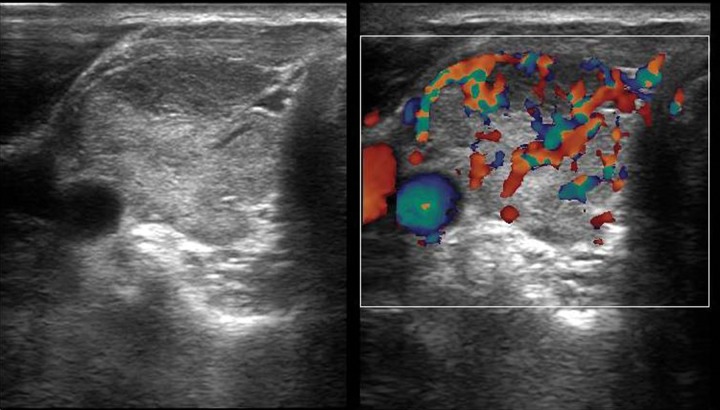
A 17-year-old female presented with lower abdominal pain, fatigue and nausea for 10 days. Menarche occurred at the age of 15 years and there was oligomenorrhea. She also had a long history of fatigue with weight gain of 8 kg in the last 2 months. Her family history was noncontributory. She was afebrile, with vitals within normal limits. Her weight was 69 kg. The abdomen was mildly distended, not tender. An abdominal ultrasound showed bilaterally enlarged ovaries with multiple cysts, without free fluid in the abdomen. The uterus was normal in size. Endometrial thickness amounted to 8 mm. Her history of chronic fatigue and oligomenorrhea prompted us to examine the thyroid. Ultrasonography of the thyroid revealed a mildly enlarged thyroid gland with heterogeneous echotexture and increased vascularity on Doppler, consistent with thyroiditis (Figure 6). Hormonal evaluation revealed high TSH levels, i.e. of 12.3 Uu/mL (normal reference value: 5–6 uU/mL), low T3–76 ng/dL (80–180 ng/dL), and T4–3.1 ug/dL (4.6–12 ug/dL), mildly elevated prolactin and estrogen – 170 (reference 30–100 pg/mL).
Figure 6.
A 17-year-old female with bilateral enlarged ovaries with multiple cysts. Ultrasonography images of the thyroid show heterogeneous appearance of the right lobe with increased vascularity on Doppler examination.
Based on the clinical, laboratory and radiological findings, a diagnosis of hypothyroidism-induced mild s-OHSS was made. The patient was started on levothyroxine (100 ug/day). Follow-up imaging after 3 months revealed complete resolution of the abnormal cysts.
Case report 4
A 24-year-old female presented to the emergency department with acute pelvic pain and lower abdominal fullness. A detailed history reveled irregular menstrual cycles and chronic headaches. Urine pregnancy test was negative. Pelvic ultrasound showed bilaterally enlarged ovaries with multiple cysts, without intraperitoneal free fluid. The uterus was normal in contour with normal endometrial cavity. Chest X-ray was normal.
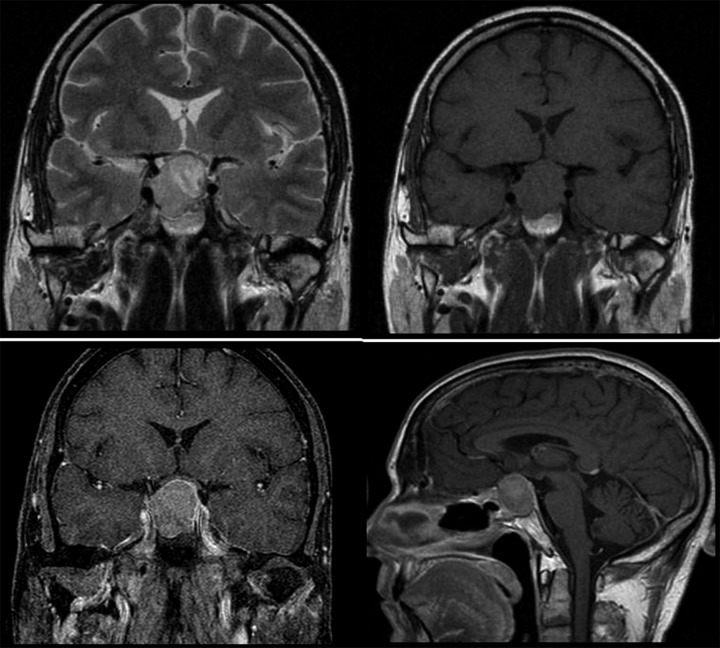
Lab investigations revealed elevated FSH, of 24.0 IU/L (reference range 3–10 IU/L) and decreased LH – 0.8 IU/U (reference range 2–8 IU/L), and normal β hCG levels. Her estradiol was elevated to 5430 pmol/L (reference value <1,094.0 pmol/L). ACTH and thyroid profile were within normal limits. MRI of the pituitary was carried out and revealed a 3.3×3.7-cm macroadenoma abutting the right ICA and lifting the optic chiasma (Figure 7).
Figure 7.
A 24-year-old female with gonadotropin-secreting macroadenoma. Brain MRI T2 coronal image shows a hyperintense lesion arising from the pituitary fossa which is abutting the right ICA and lifting the optic chiasma. The lesion appears hypo-isointense on a T1 coronal image. Post-contrast fat-saturated coronal and sagittal images show homogeneous enhancement of the lesion.
A diagnosis of s-OHSS induced by gonadotropin-secreting macroadenoma was made. The patient underwent surgical resection by trans-sphenoidal route and medical management with a somatostatin agonist – Octreotide. GNRH agonist was not given as it causes a paradoxical increase in estradiol level and exacerbates OHSS. There was symptomatic and radiological improvement after 4 weeks and the patient is on medications during follow-up.
Case report 5
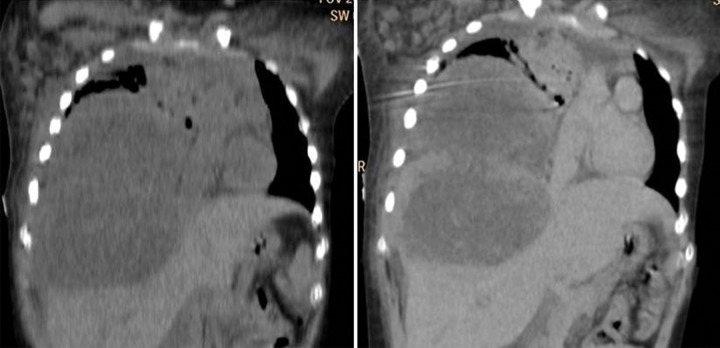
A 12-year-old female came to our hospital with complaints of dyspnea and severe abdominal pain. The patient was a biopsy-proven diagnosed case of extragonadal choriocarcinoma of the mediastinum on chemotherapy. Chest CT revealed a large heterogeneously enhancing mass occupying the right hemithorax causing invasion of the diaphragm and the right side of the liver (Figures 8 and 9). On examination, she was tachypneic with tachycardia and tender abdomen. A clinical diagnosis of tumor lysis syndrome or disseminated metastasis was made.
Figure 8.
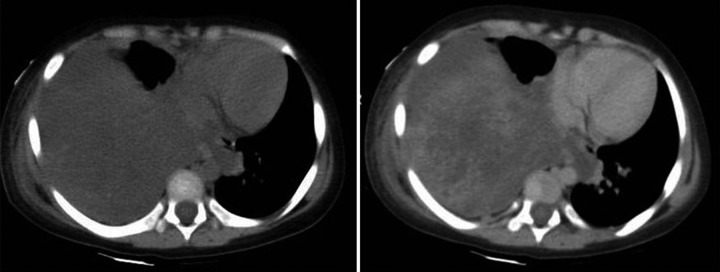
A 12-year-old female with mediastinal choriocarcinoma. CT axial plain and contrast-enhanced images show hypodense lesions occupying the right hemithorax causing a mediastinal shift to the left and showing heterogeneous post-contrast enhancement.
Figure 9.
A 12-year-old female with mediastinal choriocarcinoma. CT coronal plain and contrast-enhanced images show a hypodense lesion in the right hemithorax with invasion of the diaphragm and the right lobe of the liver, and causing heterogeneous post-contrast enhancement.
Bedside pelvic ultrasound revealed bilaterally enlarged ovaries with multiple enlarged follicles and a normal uterine cavity (Figure 10). Investigation revealed elevated β hCG level, of 61230IU/mL, and non-elevated alpha-fetoprotein level, with normal TSH, LH, and FSH. A diagnosis of s-OHSS due to mediastinal choriocarcinoma (biopsy-proven) was made. Elevated β hCG levels due to mediastinal choriocarcinoma triggered the ovaries. The patient was continued on a standard BEP (bleomycin, etoposide, cisplatinum) regimen but due to the large intrathoracic mass lesion and invasion, the patient did not improve symptomatically and succumbed to illness a few weeks later.
Figure 10.
A 12-year-old female with mediastinal choriocarcinoma. Ultrasonography B-mode images show multiple cysts involving both the right and the left ovary. There is mild free fluid in the intra-peritoneal cavity.
Discussion
Spontaneous OHSS is an uncommon, potentially life-threatening systemic complication caused by non-iatrogenic ovarian stimulation in spontaneous cycles due to little-known mechanisms. The clinical and radiological spectrum is similar to the one of much more common, ovulation therapy-induced OHSS, which complicates 1% of assisted reproduction techniques [1]. The s-OHSS is usually seen to complicate pregnancy (both singleton and multiple) [2–8], gestational trophoblastic tumor [9–12], pituitary adenomas [13–19], hypothyroidism [20–23], and β hCG-secreting tumors. A single case of OHSS due to ectopic FSH secretion by a neuro-endocrine tumor in the thorax has also been reported [25], with no case yet reported in the literature of non-gonadal β hCG -secreting tumor causing OHSS, as in our case.
High levels of glycoprotein hormones like β hCG, TSH, LH, and FSH with/without FSH receptor mutation are seen as the trigger for most cases of spontaneous OHSS [26]. These hormones are believed to release unknown mediators and vasoactive substances such as histamine, serotonin, prostaglandins, interleukins, estrogen, prolactin, the ovarian renin-angiotensin system, and vascular endothelial growth factor (VEGF) leading to increased vascular permeability, extravascular fluid accumulation, hemoconcentration, deep vein thrombosis, and other complications observed in OHSS. The latest evidence suggests a key role of VEGF, presumably of a follicular origin, in the pathogenesis of s-OHSS [27].
Clinical manifestation of OHSS includes rapid weight gain, abdominal distention accompanied by pain, vomiting and dehydration with hypovolaemia, oliguria, and hydroelectrolytic imbalance. In the most severe cases, hemoconcentration and coagulation disturbances can lead to thromboembolic phenomena [28].
Clinical suspicion is high in cases of iatrogenic OHSS as the history is provided by the patient, but clinical suspicion of spontaneous OHSS is difficult due to rare incidence and symptoms resembling intestinal obstruction. Thus, radiological imaging plays the key role in diagnosing spontaneous OHSS. Imaging findings reveal bilateral enlarged ovaries with multiple cysts which may be simple or hemorrhagic with no abnormal solid component or vascularity within the cystic lesions. Other features include free fluid in the peritoneal, pleural and pericardial cavity.
There are many clinico-radiological classifications of s-OHSS but one, proposed by Golan in 1989, is commonly used now. Golan used clinical parameters such as abdominal distension, gastrointestinal symptoms, biochemical parameters such as hemoconcentration, coagulopathy, electrolyte disorders, creatinine level, and radiological parameters such as the size of the ovary, ascites, pleural effusion, and pericardial effusion for grading OHSS into mild, moderate, and severe [24] (Table 1).
Table 1.
Grading of OHSS.
| Mild OHSS | Grade 1 – Abdominal distension and discomfort |
| Grade 2 – Grade 1 + vomiting, nausea + ovarian size (5–12 cm) | |
| Moderate OHSS | Grade 3 – Mild OHSS + ascites |
| Severe OHSS | Grade 4 – Moderate OHSS + effusion (pleural and pericardial) |
| Grade 5 – Grade 4 + biochemical imbalance in the form of hemoconcentration, coagulaopathy, deranged LFT, RFT |
Biochemical classification by Deleeners [29] is most commonly used for systematic grouping of spontaneous OHSS of various etiology. Nikoletta Panagiotopoulou et al. performed an exhaustive literature search of spontaneous OHSS cases and tabulated the data of the various case reports of s-OHSS until November 2012 according to their own modified Deleeners classification, and carried out a synthesis of each case based upon the presence/absence of FSHR mutation, and biochemistry profile regarding the β hCG/TSH/FSH/LH levels [7] (Table 2).
Table 2.
Modified Deleeners classification.
| Modified Deleeners classification | Primary abnormality | Underlying trigger |
|---|---|---|
| I | Mutated FSHR |
|
| II | Elevated β hCG |
|
| III | Elevated TSH |
|
| IV | Elevated FSH/LH |
|
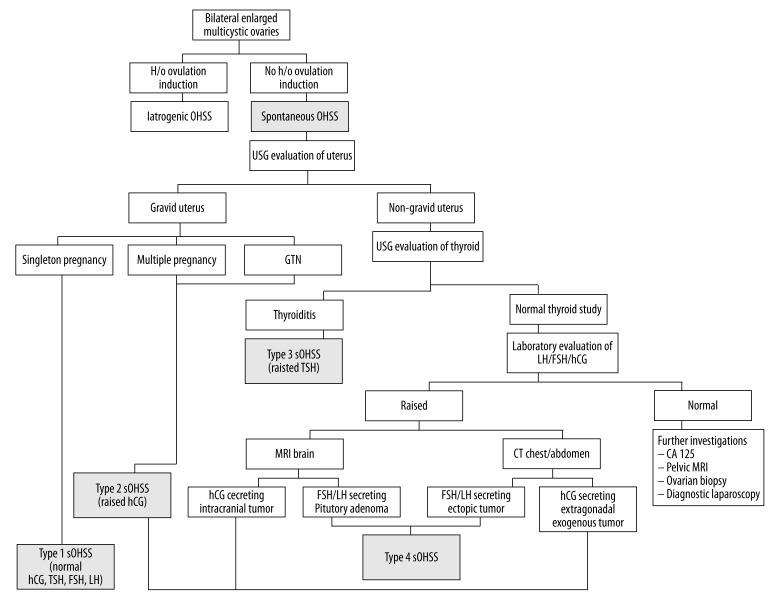
Ultrasonography plays the main role not only in diagnosing OHSS but also in diagnosing its etiology. At our center, to improve the detection of s-OHSS and find the underlying trigger, we proposed a radiological algorithm (Figure 11). An underlying history of drug intake rules out the iatrogenic cause of OHSS. Then, the uterine cavity is scanned to rule out gestation or persistent gestational disease. A single live pregnancy with OHSS is labeled as Type I s-OHSS and is confirmed by normal β hCG, TSH, and LH levels, and raised estradiol levels. Such patients should undergo DNA analysis for a mutated FSH receptor. Multiple live pregnancies cause a substantial increase in β hCG levels which in turn leads to OHSS. Abnormal multiple scattered cystic spaces with or without fetal parts should raise the suspicion of hydatidiform mole or persistent gestational trophoblastic disease, which is confirmed by exceptionally elevated β hCG levels. This is classified as Type 2 s-OHSS because OHSS is triggered by raised β hCG levels. Subsequently, if the patient is non-gravid, the thyroid gland is evaluated in the same setting to rule out thyroiditis as the underlying cause of OHSS. Thyroiditis-induced OHSS, also called Type 3 OHSS, is confirmed by raised TSH and normal to decreased T4 and T3 levels. A triple hormonal assay of LH, FSH, and β hCG is carried out if all the above mentioned investigations are negative. Raised values of any hormone will raise the suspicion of a hormone-secreting tumor. Multimodality imaging in the form of brain MRI and CECT of the abdomen and chest is performed. Generally, brain MRI is the preferred non-ionizing modality over CECT of the abdomen and pelvis. Brain MRI may reveal a sellar, suprasellar or pineal gland mass, which is commonly a pituitary adenoma, and is confirmed by raised LH and FSH levels, whereas raised β hCG levels with an intracranial mass lesion support the diagnosis of a β hCG-secreting tumor. Chest and abdomen CT is advised to rule out an ectopic LH/FSH-secreting tumor or extragonadal β hCG-secreting tumor, as in our case. Thus β hCG-secreting tumors are classified as Type 2 s-OHSS, and LH/FSH-secreting tumors are labeled as Type 4 s-OHSS. Further investigations, like CA-125, pelvic MRI, laparoscopy/biopsy can be done for inconclusive cases.
Figure 11.
Radiological algorithm for diagnosing the various causes of s-OHSS.
Type 1 s-OHSS
Type 1 s-OHSS is usually seen to be triggered by pregnancy in spite of normal β hCG, TSH, and FSH levels. The patho-physiology is explained by a recent discovery of mutations in the FSH receptor gene, which shows an increased sensitivity towards β hCG [29]. The symptoms of spontaneous OHSS usually develop around 8 weeks’ amenorrhea and culminate at the end of the first trimester of pregnancy when the level of β hCG drops [30]. Follow-up ultrasonography revealing reduction in ovarian volume and regression of detected cysts can differentiate OHSS from other differentials. A follow-up of such a patient is necessary, especially in the next pregnancy, as there are high chances of recurrence due to mutated FSH receptors which get triggered.
Type II OHSS
OHSS triggered due to supra-physiological levels of β hCG are classified as Type II. Excessive secretion of β hCG is commonly seen in GTN and multiple pregnancies. It has been suggested that high levels of β hCG overcome the specificity of the hormone receptor, leading to a hypothesis that wild-type FSH receptors have a higher affinity for β hCG [31] and even TSH [32]. Alternative theories suggest the presence of a variant of β hCG that exhibits higher biological activity causing supra-maximal stimulation of the receptors [7]. Ultrasonography coupled with MRI gives a complete detail of the disease. A chest X-ray may be useful in case of a metastasis from choriocarcinoma, which is the most severe form in the spectrum of Gestational Trophoblastic Disease (GTD). Chemotherapy is the mainstay of treatment; rarely hysterectomy is required for invasive cases. Follow-up USG and β hCG levels of such a patient is imperative for complete resolution of the disease in the ongoing and future pregnancies, as they are prone to foster gestational trophoblastic diseases in subsequent pregnancies. The β hCG-secreting intracranial lesions are also seen, but it is unknown whether they can result in s-OHSS (there is no reported case in literature). Extragonadal β hCG-secreting tumors are rare, but associated s-OHSS may be seen due to ovarian stimulation, as in our case of mediastinal choriocarcinoma causing s-OHSS. Extragonadal mediastinal choricarcinoma has a very poor prognosis compared to its gonadal counterpart, and combined surgical and medical management is used to prolong patients’ life span [33,34].
Type III OHSS
Few reports of s-OHSS associated with hypothyroidism are reported in pregnant, non-pregnant, and young females. Hypothyroidism has deleterious effects on the ovary through reduced levels of sex-hormone binding globulin, increased level of thyrotropin-releasing hormone and estradiol, with a weak suppressive effect on gonadotropin. Hyperstimulation of the ovaries can be explained by high levels of TSH acting on wild-type (normal) FSH receptors as TSH shares an identical beta subunit with other glycoprotein hormones like LH, FSH, and β hCG [32]. Other mechanisms suggest preferential formation of estriol which results in excessive gonadotropin release (with estriol being a weaker suppressor of gonadotropin release than estradiol) [23]. Patients generally present with mild to moderate OHSS with a good prognosis on Levothyroxine monotherapy [22,35].
Type IV s-OHSS
Few cases of s-OHSS associated with gonadotropin-secreting pituitary adenomas and only a single case of an ectopic FSH-secreting neuroendocrine tumor [25] have been reported till date. The adenomas can be FSH- or LH-secreting tumors [16,19] and are both implicated in causing OHSS (majority of the reported cases are FSH-secreting adenomas). In their vast majority, these tumors secrete biologically inactive gonadotropin monomer subunits, and thus they do not cause a recognizable clinical syndrome. However, exceptionally, some tumors secrete high levels of intact bioactive heterodimers of FSH or LH which stimulate the FSH receptors. However, a characteristic feature of adenoma-induced s-OHSS, as compared to the rest of other etiologies, is the absence of increased systemic vascular permeability [17], and therefore absence of ascites, and pleural and pericardial effusion. Diagnosis of these rare lesions is possible with multimodality imaging combined with biochemical investigations. Medical management using somatostatin analogues such as octreotide and dopamine agonists is commonly conducted [15,36]. The GNRH agonist is contraindicated in gonadotropin-secreting tumors associated with s-OHSS as they may paradoxically cause increased ovarian stimulation [14] Surgical removal of the tumor is the preferred treatment especially in large and refractory pituitary adenomas.
Early recognition and prompt intervention will avoid serious complications. OHSS usually requires hospital admission and prompt volume resuscitation with continuous monitoring to avoid fatal complications like renal failure and thrombo-embolic episodes [37–39]. Since clinical suspicion of s-OHSS is difficult, the diagnosis of s-OHSS lies in the hand of a radiologist. Further evaluation of its cause is with radiological imaging. We proposed a radiological algorithm as practiced in our center for diagnosing the causes of OHSS. We also highlighted an unusual and never-reported case of s-OHSS caused by β hCG-secreting mediastinal choriocarcinoma.
Conclusions
Spontaneous OHSS is an uncommon identity due to variable number of causes, with gestation and gestational disease being more common than thyroiditis and hormone-secretory tumors. We not only highlighted the importance of diagnosis of s-OHSS but also illustrated a radiological algorithm for diagnosing the etiology of s-OHSS. Thus, we emphasized that diagnosis of s-OHSS and its etiology can be completely evaluated radiologically. Biochemical markers will confirm the radiological diagnosis.
References
- 1.Delvinge A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): A review. Hum Reprod Update. 2002;8(6):c 559–77. doi: 10.1093/humupd/8.6.559. [DOI] [PubMed] [Google Scholar]
- 2.Irvine LM. Spontaneous ovarian hyperstimulation syndrome (OHSS): a rare but important differential diagnosis for abdominal distension in early pregnancy. J Obstet Gynaecol. 2011;31(4):338–39. doi: 10.3109/01443615.2011.560299. [DOI] [PubMed] [Google Scholar]
- 3.Chae HD, Park EJ, Kim SH, et al. Ovarian hyperstimulation syndrome complicating a spontaneous singleton pregnancy: a case report. J Assist Reprod Genet. 2001;18(2):120–23. doi: 10.1023/A:1026543027300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oztekin O, Soylu F, Tatli O. Spontaneous ovarian hyperstimulation syndrome in a normal singleton pregnancy. Taiwan J Obstet Gynecol. 2006;45(3):272–75. doi: 10.1016/S1028-4559(09)60241-2. [DOI] [PubMed] [Google Scholar]
- 5.Sridev S, Barathan S. Case report on spontaneous ovarian hyperstimulation syndrome following natural conception associated with primary hypothyroidism. J Hum Reprod Sci. 2013;6(2):158–61. doi: 10.4103/0974-1208.117164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He C, Huang H, Song Y. Spontaneous and severe ovarian hyperstimulation syndrome after delivery: a case report. Gynecol Endocrinol. 2008;24(8):450–51. doi: 10.1080/09513590802246075. [DOI] [PubMed] [Google Scholar]
- 7.Panagiotopoulou N, Byers H, Newman WG, Bhatia K. Spontaneous ovarian hyperstimulation syndrome: case report, pathophysiological classification and diagnostic algorithm. Eur J Obstet Gynecol Reprod Biol Elsevier Ireland Ltd. 2013;169(2):143–48. doi: 10.1016/j.ejogrb.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal NR, Gupta G, Verma K, Varyani N. Spontaneous ovarian hyperstimulation syndrome in a triplet pregnancy. Case Rep Crit Care. 2012;2012:189705. doi: 10.1155/2012/189705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rachad M, Chaara H, Zahra Fdili F, et al. Ovarian hyperstimulation syndrome in a spontaneous pregnancy with invasive mole: report of a case. Pan Afr Med J. 2011;9:23. doi: 10.4314/pamj.v9i1.71198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X, Duan Z. A case of ovarian hyperstimulation syndrome following a spontaneous complete hydatidiform molar pregnancy. Gynecol Endocrinol. 2012;28(11):850–52. doi: 10.3109/09513590.2012.683063. [DOI] [PubMed] [Google Scholar]
- 11.Arora R, Merhi ZO, Khulpateea N, et al. Ovarian hyperstimulation syndrome after a molar pregnancy evacuation. Fertil Steril. 2008;90(4):1 197.e5–7. doi: 10.1016/j.fertnstert.2007.09.067. [DOI] [PubMed] [Google Scholar]
- 12.Diness M, Nilas L. Course of mole pregnancy complicated by ovarian hyperstimulation syndrome. Ugeskr Laeger. 2012;174(21):1465–67. [PubMed] [Google Scholar]
- 13.Baba T, Endo T, Kitajima Y, et al. Spontaneous ovarian hyperstimulation syndrome and pituitary adenoma: incidental pregnancy triggers a catastrophic event. Fertil Steril. 2009;92(1):390.e1–3. doi: 10.1016/j.fertnstert.2009.02.071. [DOI] [PubMed] [Google Scholar]
- 14.Ceccato F, Occhi G, Regazzo D, et al. Gonadotropin secreting pituitary adenoma associated with erythrocytosis: case report and literature review. Hormones. 2014;13(1):131–39. doi: 10.1007/BF03401328. [DOI] [PubMed] [Google Scholar]
- 15.Karapanou O, Tzanela M, Tamouridis N, Tsagarakis S. Gonadotroph pituitary macroadenoma inducing ovarian hyperstimulation syndrome: successful response to octreotide therapy. Hormones. 2012;11(2):199–202. doi: 10.14310/horm.2002.1347. [DOI] [PubMed] [Google Scholar]
- 16.Knoepfelmacher M, Danilovic DLS, Rosa Nasser RHR, Mendonça BB. Effectiveness of treating ovarian hyperstimulation syndrome with cabergoline in two patients with gonadotropin-producing pituitary adenomas. Fertil Steril. 2006;86(3):719.e15–8. doi: 10.1016/j.fertnstert.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 17.Macchia E, Simoncini T, Raffaelli V, et al. A functioning FSH-secreting pituitary macroadenoma causing an ovarian hyperstimulation syndrome with multiple cysts resected and relapsed after leuprolide in a reproductive-aged woman. Gynecol Endocrinol. 2012;28(1):56–59. doi: 10.3109/09513590.2011.588758. [DOI] [PubMed] [Google Scholar]
- 18.Murakami T, Higashitsuji H, Yoshinaga K, et al. Management of ovarian hyperstimulation due to follicle-stimulating hormone-secreting gonadotroph adenoma. BJOG. 2004;111(11):1297–300. doi: 10.1111/j.1471-0528.2004.00409.x. [DOI] [PubMed] [Google Scholar]
- 19.Castelo-Branco C, del Pino M, Valladares E. Ovarian hyperstimulation, hyperprolactinaemia and LH gonadotroph adenoma. Reprod Biomed Online. 2009;19(2):153–55. doi: 10.1016/s1472-6483(10)60065-x. [DOI] [PubMed] [Google Scholar]
- 20.Emami MHH, Langroudi RM. ovarian hyperstimulation syndrome and autoimmune primary hypothyroidism in two members of a family. J Clin Case Reports. 2012;02(04):2–4. [Google Scholar]
- 21.Kanza RE, Gagnon S, Villeneuve H, et al. Spontaneous ovarian hyperstimulation syndrome and pituitary hyperplasia mimicking macroadenoma associated with primary hypothyroidism. World J Radiol. 2013;5(1):20–24. doi: 10.4329/wjr.v5.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langroudi RM, Amlashi FG, Emami MHH. Ovarian cyst regression with levothyroxine in ovarian hyperstimulation syndrome associated with hypothyroidism. Endocrinol Diabetes Metab Case Rep. 2013;2013:130006. doi: 10.1530/EDM-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sultan A, Velaga MR, Fleet M, Cheetham T. Cullen’s sign and massive ovarian enlargement secondary to primary hypothyroidism in a patient with a normal FSH receptor. Arch Dis Child. 2006;91(6):509–10. doi: 10.1136/adc.2005.088443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golan A, Ron-el R, Herman A, et al. Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv. 1989;44(6):430–40. doi: 10.1097/00006254-198906000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Burgos J, Cobos P, Vidaurrazaga N, et al. Ovarian hyperstimulation secondary to ectopic secretion of follicle-stimulating hormone. Literature review prompted by a case. Fertil Steril. 2009;92(3):1168.e5–8. doi: 10.1016/j.fertnstert.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 26.Delbaere A, Smits G, De Leener A, et al. Understanding ovarian hyperstimulation syndrome. Endocrine. 2005;26(3):285–90. doi: 10.1385/ENDO:26:3:285. [DOI] [PubMed] [Google Scholar]
- 27.Kasum M, Oresković S. New insights in prediction of ovarian hyperstimulation syndrome. Acta Clin Croat. 2011;50(2):281–88. [PubMed] [Google Scholar]
- 28.Delvigne A, Rozenberg S. Review of clinical course and treatment of ovarian hyperstimulation syndrome (OHSS) Hum Reprod Update. 2003;9(1):77–96. doi: 10.1093/humupd/dmg005. [DOI] [PubMed] [Google Scholar]
- 29.De Leener A, Caltabiano G, Erkan S, et al. Identification of the first germline mutation in the extracellular domain of the follitropin receptor responsible for spontaneous ovarian hyperstimulation syndrome. Hum Mutat. 2008;29(1):91–98. doi: 10.1002/humu.20604. [DOI] [PubMed] [Google Scholar]
- 30.Delbaere A, Smits G, Olatunbosun O, et al. New insights into the pathophysiology of ovarian hyperstimulation syndrome. What makes the difference between spontaneous and iatrogenic syndrome? Hum Reprod. 2004;19(3):486–89. doi: 10.1093/humrep/deh124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smits G, Campillo M, Govaerts C, et al. Glycoprotein hormone receptors: determinants in leucine-rich repeats responsible for ligand specificity. EMBO J. 2003;22(11):2692–703. doi: 10.1093/emboj/cdg260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schubert RL, Narayan P, Puett D. Specificity of cognate ligand-receptor interactions: fusion proteins of human chorionic gonadotropin and the heptahelical receptors for human luteinizing hormone, thyroid-stimulating hormone, and follicle-stimulating hormone. Endocrinology. 2003;144(1):129–37. doi: 10.1210/en.2002-220829. [DOI] [PubMed] [Google Scholar]
- 33.Gaude GS, Patil P, Malur PR, et al. Primary mediastinal choriocarcinoma. South Asian J Cancer. 2013;2(2):79. doi: 10.4103/2278-330X.110495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kathuria S, Jablokow VR. Primary choriocarcinoma of mediastinum with immunohistochemical study and review of the literature. J Surg Oncol. 1987;34(1):39–42. doi: 10.1002/jso.2930340112. [DOI] [PubMed] [Google Scholar]
- 35.Katulande P, Kariyawasam SSM, Senanayake HM, Weerakkodi M. Multicystic ovaries and pituitary pseudo-adenoma associated with primary hypothyroidism. J Obstet Gynaecol. 2013;33(1):17–19. doi: 10.3109/01443615.2011.565388. [DOI] [PubMed] [Google Scholar]
- 36.Paoletti AM, Depau GF, Mais V, et al. Effectiveness of cabergoline in reducing follicle-stimulating hormone and prolactin hypersecretion from pituitary macroadenoma in an infertile woman. Fertil Steril. 1994;62(4):882–85. doi: 10.1016/s0015-0282(16)57021-6. [DOI] [PubMed] [Google Scholar]
- 37.Stewart JA, Hamilton PJ, Murdoch AP. Thromboembolic disease associated with ovarian stimulation and assisted conception techniques. Hum Reprod. 1997;12(10):2167–73. doi: 10.1093/humrep/12.10.2167. [DOI] [PubMed] [Google Scholar]
- 38.Abramov Y, Elchalal U, Schenker JG. Pulmonary manifestations of severe ovarian hyperstimulation syndrome: a multicenter study. Fertil Steril. 1999;71(4):645–51. doi: 10.1016/s0015-0282(98)00528-7. [DOI] [PubMed] [Google Scholar]
- 39.Navot D, Bergh PA, Laufer N. Ovarian hyperstimulation syndrome in novel reproductive technologies: prevention and treatment. Fertil Steril. 1992;58(2):249–61. doi: 10.1016/s0015-0282(16)55188-7. [DOI] [PubMed] [Google Scholar]