Abstract
Adenosine-to-inosine RNA editing modifies maturing mRNAs through the binding of adenosine deaminase acting on RNA (Adar) proteins to double-stranded RNA structures in a process critical for neuronal function. Editing levels at individual editing sites span a broad range and are mediated by both cis-acting elements (surrounding RNA sequence and secondary structure) and trans-acting factors. Here we aim to determine the roles cis-acting elements and trans-acting factors play in regulating editing levels. Using two closely related Drosophila species, D. melanogaster and D. sechellia, and their F1 hybrids, we dissect the effects of cis sequences from trans regulators on editing levels by comparing species-specific editing in parents and their hybrids. We report that cis sequence differences are largely responsible for editing level differences between these two Drosophila species. This study presents evidence for cis sequence and structure changes as the dominant evolutionary force that modulates RNA editing levels between these Drosophila species.
Keywords: RNA editing, A-to-I editing, Adar, cis regulation, trans regulation, Drosophila hybrids
INTRODUCTION
Adenosine-to-inosine (A-to-I) RNA editing is a co-transcriptional process mediated by adenosine deaminase acting on RNA (Adar) proteins that bind double-stranded RNA (dsRNA) structures to convert adenosines into inosines, which are recognized as guanosine by the cellular machinery (Bass, 2002; Gott and Emeson, 2000; Nishikura, 2010; Rodriguez et al., 2012). This process is critical for neuronal function in multiple species, including Drosophila (Li and Church, 2013; Rosenthal and Seeburg, 2012; Tariq and Jantsch, 2012), where over 5,000 RNA editing sites have been identified, many edited to different extents (Graveley et al., 2010; Ramaswami and Li, 2014; Ramaswami et al., 2013; Rodriguez et al., 2012; St Laurent et al., 2013). Mechanisms for maintaining editing levels at individual sites are not fully understood, although recent work demonstrates a role for both cis sequences and trans-acting factors.
Pre-mRNA sequence and secondary structure help determine editing levels as they control the ability of Adar to bind the substrate. Most editing sites are harbored in imperfect dsRNA structures which leads to editing only at specific adenosines (Rieder and Reenan, 2012; Tian et al., 2011). Distal tertiary structural elements can also be critical in establishing editing levels (Daniel et al., 2012; Reenan, 2005; Rieder et al., 2013). Furthermore, Adar proteins show primary sequence preferences in the bases adjacent to the edited adenosine (Eggington et al., 2011), suggesting that sequences both next to and far from the editing site contribute to establishing the editing levels at specific sites.
Editing levels may also be under the control of trans-acting factors. In mammals, numerous proteins are known to affect editing levels (Garncarz et al., 2013; Marcucci et al., 2011; Tariq et al., 2013; Wang et al., 2013). In flies, proteins Fmr1, Period and Maleless have been implicated as regulators of editing (Bhogal et al., 2011; Hughes et al., 2012; Reenan et al., 2000), and the overrepresentation of certain sequence motifs in edited RNAs suggests that the binding of sequence-specific factors may facilitate editing at some sites (Graveley et al., 2010).
Despite these previous findings demonstrating both the role of trans regulators and cis sequences in controlling editing levels at specific sites, the relative contribution of these factors in regulating editing levels on a genome-wide scale is not well understood. Interspecies hybrids provide a simple system to dissect the contribution of cis elements and trans-regulatory factors because, in hybrids, the cis environments of the parent species are confined to the same trans environment (Cowles et al., 2002). Therefore, allele-specific differences in editing levels in the hybrids can be attributed to the effects of cis sequence differences between the parent species while differences that are not accounted for by cis effects are then attributed to trans-regulatory differences (Wittkopp et al., 2004).
Here, we use Drosophila melanogaster, Drosophila sechellia and their F1 hybrid progeny to dissect the effects of cis sequences from trans factors on editing levels at hundreds of editing sites in the two species. We report that cis sequence effects play the largest role in modulating the editing levels between these two species, and we find that cis sequence changes promoting stability of edited dsRNA hairpins often correlate with higher editing levels. We further show that the majority of editing differences between the species are not a result of differences in Adar. Our data suggest a model where cis sequence changes surrounding editing sites play a critical role in determining RNA editing levels genome-wide and are largely responsible for the evolution of editing levels across these species.
RESULTS
Determining RNA editing levels in two Drosophila species and their F1 hybrids
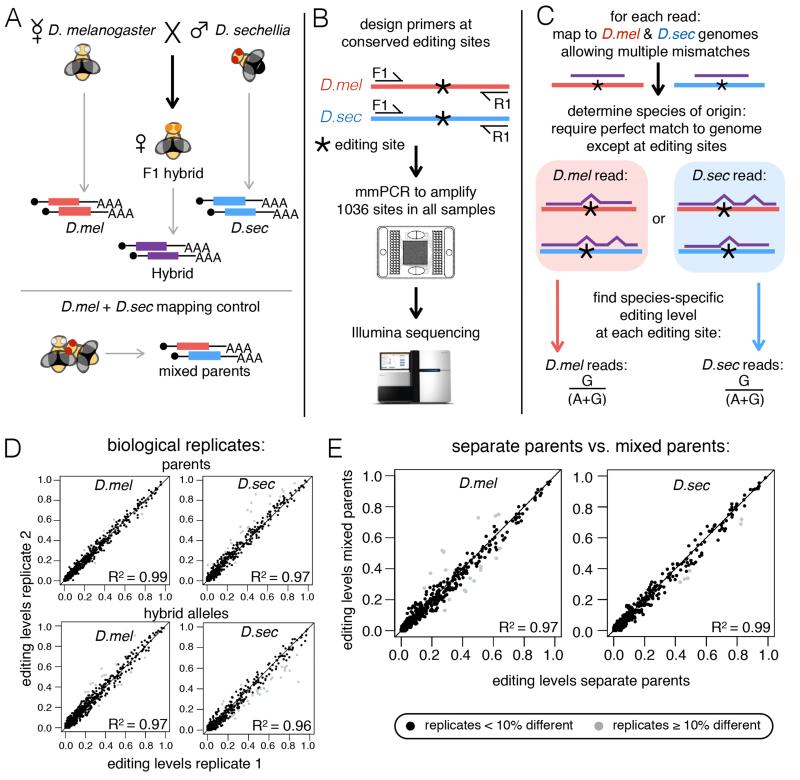
We extracted total RNA from the heads of 0-2 day old female flies from D. melanogaster, D. sechellia, their female F1 hybrid progeny and a mixture of equal numbers of female fly heads from the two parent species (termed “mixed parents”) (Fig 1A). To accurately measure RNA editing levels at a large number of sites, we used microfluidic multiplex PCR and sequencing (mmPCR-seq) (Zhang et al., 2014) (Fig 1B). This approach allows us to simultaneously PCR amplify hundreds of editing sites from dozens of cDNA samples and deeply sequence the amplicons with high coverage at all sites of interest. We designed 493 pairs of PCR primers that assayed a total of 1,036 editing sites in D. melanogaster that have conserved adenosines in D. sechellia, requiring high conservation within the primer sequences to allow amplification in both species (Table S1). We chose target sites with the intent to maximize observed differences in editing between the species (see Supplemental Experimental Procedures).
Figure 1. Determining RNA editing levels in D. melanogaster, D. sechellia, and their F1 hybrids.
(A) Cartoon illustrating the 4 samples collected and used in the study: 0-2 day old females from D. melanogaster (red), D. sechellia (blue), and their F1 hybrids (purple) and a mixed parent mapping control. Total RNA was extracted from 10 heads for each sample. (B) Schematic of primer design and mmPCR-seq workflow. (C) Schematic of mapping F1 hybrid reads to their species of origin to call species-specific editing levels. (D) Scatter plots comparing editing levels in biological replicates from D. melanogaster and D. sechellia parents and hybrid alleles (see also Fig S1). (E) Scatter plots comparing separate parent and mixed parent editing levels. Gray dot, replicates differ by ≥ 10% editing and site was excluded from subsequent analysis.
We developed a computational pipeline that mapped sequencing reads to both species’ genomes allowing for mismatches and then compared alignments at known sequence variants between the two species to find the perfect species match, ignoring editing sites where A to G mismatches were present (Fig 1C, see Supplemental Experimental Procedures). After assigning each read to its respective species, we determined species-specific editing levels by calculating the percentage of A to G mismatches at editing sites across reads derived from each species.
Our approach produced highly reproducible editing levels between biological replicates (R2 ≥ 0.96) (Fig 1D and Fig S1) as well as between separate parent samples and the corresponding alleles from the mixed parent samples (Fig 1E) demonstrating the reproducibility of mmPCR-seq and the accuracy of our mapping workflow in determining species-specific editing levels from a mixture of alleles from the two species. Only editing sites that showed reproducible editing levels (within 10% edited) in all biological replicates and between separate and mixed parents were used in downstream analyses.
Majority of editing level differences between D. melanogaster and D. sechellia are maintained in F1 hybrids
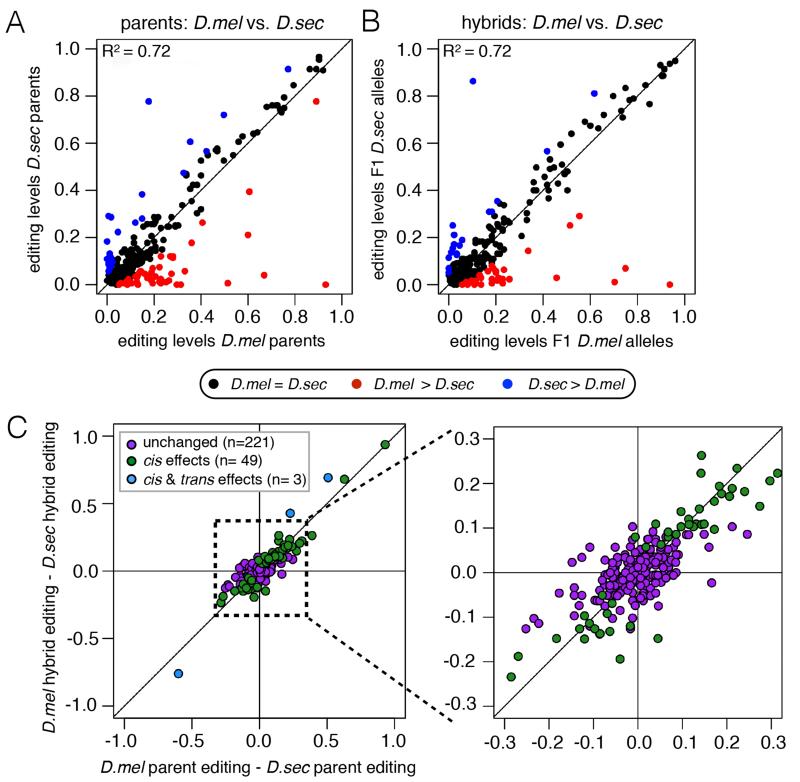
We first compared the editing levels between the two parent species at 273 editing sites with high coverage and reproducible editing levels of greater than 2% in at least one species (Fig 2A, Table S2). The 273 editing sites are found in 103 genes, with 143 (52%) leading to nonsynonymous changes, 38 (14%) causing synonymous changes, 87 (32%) altering 3′ UTRs and 5 (2%) altering 5′ UTRs. As expected, editing levels varied considerably more between species than between the biological replicates within species (R2 = 0.72 and R2 = 0.96-0.99 respectively), with a total of 69 sites differing significantly between species.
Figure 2. Differences in editing levels between parents are largely maintained in hybrid alleles.
(A) Scatter plot comparing editing levels between D. melanogaster and D. sechellia parents. (B) Scatter plot comparing editing levels between D. melanogaster and D. sechellia alleles in F1 hybrids. Red dot, D. melanogaster more highly edited. Blue dot, D. sechellia more highly edited. Black dot, no editing difference (Fisher’s exact tests, FDR=5%). (C) Scatter plots comparing the difference in editing between parents versus the difference in editing between hybrid species-specific alleles. Purple dot, no change between parent and hybrid differences. Green dot, evidence of cis divergence. Blue dot, evidence of cis and trans divergence (FDR=5%)(see Experimental Procedures for statistical analysis). Right plot, magnification of points for clarity.
We then measured species-specific editing levels in the F1 hybrid progeny, where editing differences are solely due to cis-acting effects. Interestingly, we observed similar results in the hybrid comparison as in the parent comparison, with 52 sites differing between D. melanogaster and D. sechellia alleles in the hybrids; 40 of these sites (77%) also differed between parents (Fig 2B, Table S2).
We classified the 52 sites with editing differences between hybrid alleles as cis- regulated sites. To determine trans-regulated sites, we looked for sites where the difference between the hybrid alleles did not account for the difference between the parents. We plotted the difference in editing level between the hybrid alleles versus the difference in editing between the parent species and estimated the editing divergence due to trans-regulatory differences for these sites as the residuals from the linear regression determined by all sites (Fig 2C, see Experimental Procedures for statistical analyses). We found that 3 sites showed statistically significant evidence of trans-regulation, although we note that our method for determining trans-regulated sites lacks power compared to that determining cis-regulated sites (see Fig S2 for alternative analyses). Of the 52 cis-affected sites, 24 (46%) were found in 3′ UTRs, 19 (37%) lead to nonsynonymous changes and 9 (17%) to synonymous changes. We classified the remaining 221 sites as unchanged. Thus, the majority of large editing level differences between these two closely related species are encoded in cis, suggesting changes in the genomic sequence of edited substrates are the primary drivers of the evolution of editing levels between these two species.
pre-mRNA sequence and structural changes around editing sites contribute to editing level differences between species
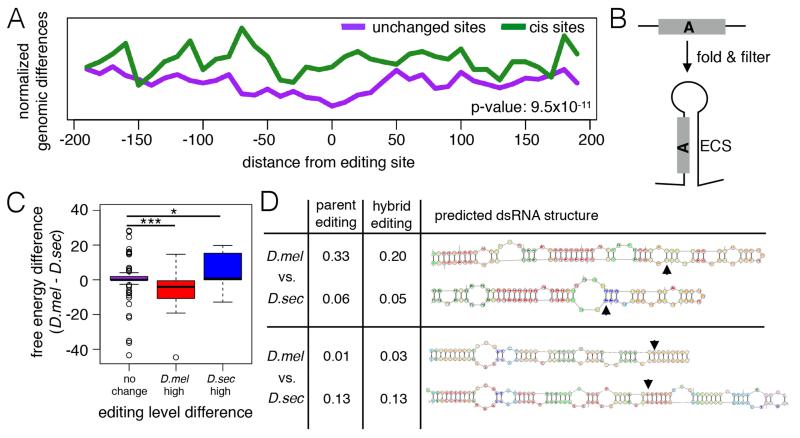
To characterize how cis effects alter editing levels, we looked at genomic sequence differences between the two species around the editing sites. Only 3 of 52 cis-affected sites showed a variant within the Adar triplet motif (Bass, 2002), suggesting changes immediately adjacent to the editing site are not the dominant mechanism altering editing levels at most sites. We next examined genomic variants in a broader region surrounding the editing sites. When examining 200 bases upstream and downstream of each of the 273 editing sites to encompass bases likely included in edited dsRNA hairpins, we saw an increase in sequence differences around cis-affected sites compared to unchanged sites that persisted across the entire 400 bases surrounding the editing sites, although it was greater in the 100 bases up and downstream of the editing sites (Fig 3A).
Figure 3. Cis sequence differences surrounding editing sites alter editing levels between species.
(A) Amount of genomic sequence variants surrounding cis-affected editing sites versus unchanged editing sites, normalized by number of sites in each group (p-value from one-sided Kolmogorov-Smirnov test). (B) Schematic of ECS (editing complementary sequence) prediction. ECS regions were predicted by folding the region around editing sites with RNAfold software (see Experimental Procedures). (C) Differences in free energy of RNA secondary structure between the two species. Purple, unchanged sites (n=115). Red, cis regulated sites with higher D. melanogaster editing (n=12). Blue, cis regulated with higher D. sechellia editing (n=9). p-values from one-sided Mann-Whitney-U test (* p-value = 0.1, *** p-value = 0.001). (D) Two examples of secondary structure and editing level changes between species at cis sites as determined by ECS prediction script. Top, chr2L@11796346. Bottom, chr3R@10642469. Arrows indicate edited adenosine.
To determine whether these sequence changes affect the stability of the dsRNA structure around the editing sites, which might alter Adar binding, we used the RNA secondary structure prediction software RNAstructure (Reuter and Mathews, 2010) to computationally predict the RNA secondary structure near our editing sites of interest and determined the editing complementary sequence (ECS) that pairs with the region around our editing site (Fig 3B, see Experimental Procedures). Based on these computational predictions, we compared the free energy of the edited hairpin between the two species. We found that the majority of editing sites that we categorized as unchanged (see Fig 2C) had similar predicted free energies for the edited hairpin in both species (Fig 3C). In contrast, in the set of cis-regulated sites, we saw a correlation between increased editing level and substrate stability, in that sites with higher editing in the D. melanogaster allele showed a hairpin with a lower free energy in D. melanogaster, while the opposite was true for sites that were more highly edited in D. sechellia (Fig 3C). Examples of predicted dsRNA hairpins around two cis-affected editing sites in both species and the corresponding editing levels are shown in Fig 3D. These examples highlight that fact that longer hairpins with more paired bases were often more highly edited. These data suggest that stability of the dsRNA hairpin containing the editing site is a major contributing factor to editing divergence between these two species.
Differences in D. melanogaster and D. sechellia Adar proteins are not responsible for editing level differences between the species
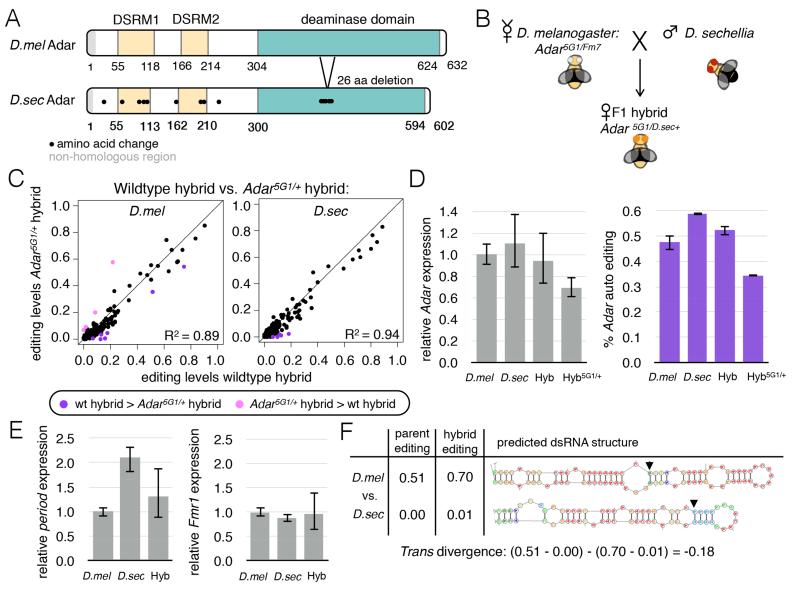
While most sites with large editing level differences between the two species appeared to be cis-regulated, a small subset of sites showed evidence of trans regulation. We first determined whether the trans-acting effects could be simply attributed to differences in sequence or expression between the species’ Adars, which while highly conserved, have protein-level differences including seven amino acid changes within the two RNA binding domains and a 26 amino acid deletion surrounded by six amino acid changes within the deaminase domain (Fig 4A).
Figure 4. Differences in D. melanogaster and D. sechellia Adar proteins are not responsible for editing level differences between the species.
(A) Schematic highlighting amino acid differences between D. melanogaster and D. sechellia Adar proteins. (B) Schematic depicting the cross to create hybrid flies with only D. sechellia Adar using the Adar5G1 mutant (Palladino et al., 2000). (C) Scatter plots comparing editing levels between wildtype hybrids and Adar5G1/D.sec+ hybrids in D. melanogaster (left) and D. sechellia (right) alleles (see also Fig S3). Purple dot, wildtype hybrid more highly edited. Pink dot, Adar5G1/D.sec+ hybrid more highly edited (Fisher’s exact tests, FDR=5%). (D) Adar expression by qPCR (left) and editing level measured from Sanger sequencing at Adar auto-regulatory editing site (right) in D. melanogaster and D. sechellia parents, wildtype hybrids and Adar5G1/D.sec+ hybrids, relative to D. melanogaster parents. Error bars = SEM. (E) Expression of period and Fmr1 mRNAs in D. melanogaster, D. sechellia and F1 hybrids measured by qPCR. Error bars = SEM. (F) Editing level and secondary structure changes between species at chr3R@1062097 which showed cis and trans effects. Arrows indicate edited adenosine.
To determine if the two Adars can edit alleles from either species, we crossed D. melanogaster Adar null mutant females (Adar5G1)(Palladino et al., 2000) to D. sechellia males to create a hybrid with only one copy of Adar from D. sechellia (Fig 4B). In the Adar5G1/D.sec+ hybrid, both D. melanogaster and D. sechellia alleles were similarly edited to the alleles in the wildtype hybrid at 145 sites analyzed (R2 = 0.89 and 0.95, respectively), suggesting that the two Adars have similar editing specificities (Fig 4C, Fig S3). Four editing sites were more highly edited in D. melanogaster alleles of the Adar5G1/D.sec+ hybrid than the wildtype hybrid, suggesting these sites may be sensitive to the differences between D. melanogaster and D. sechellia Adars; however, none showed significant evidence of trans regulation. Editing levels were slightly lower in the Adar mutant hybrid than the wildtype hybrid at many sites (Fig 4C), likely because with only one copy of Adar, the mutant hybrid had 70% of the Adar expression of the wildtype hybrid as measured by quantitative real-time PCR (qPCR) (Fig 4D). Despite the decrease in Adar expression, the editing levels between the wildtype and mutant hybrid were quite similar, which may be explained by a reduction in auto-editing of the Adar transcript. Editing within the Adar transcript itself is known to reduce Adar activity (Keegan et al., 2005; Savva et al., 2012), and the D. sechellia Adar transcript within the mutant hybrid was edited at 35%, compared to 60% in wildtype D. sechellia (Fig 4D).
We used qPCR to examine the expression of two known trans regulators of editing in flies, period and Fmr1, to determine whether they might regulate editing in trans between these species. We observed an increase in period, but not Fmr1 transcript expression in D. sechellia and the F1 hybrid compared to D. melanogaster (Fig 4E), suggesting that sites regulated by Period may show evidence of trans regulation in our study. Indeed, one of the three trans-regulated sites, a site in retinophilin (chr3R@1062097) (Fig 4F), is known to be highly edited in wildtype D. melanogaster but not edited in period mutant flies (Hughes et al., 2012). This result raises the possibility that the increase in period expression in the hybrid is responsible for the increase in editing of the D. melanogaster allele at this site.
DISCUSSION
Here we measure RNA editing level differences between the closely related species, D. melanogaster and D. sechellia. In F1 hybrids, many of these differences are also maintained in the species-specific alleles, suggesting that cis sequence differences are largely responsible for the changes in editing between these species. Many editing level differences caused by cis effects show differences in the stability of the dsRNA hairpin between the species; the presence of sequences that stabilize the dsRNA hairpin encompassing the editing site correlate with higher editing levels, while the opposite is true for sequences that destabilize the dsRNA hairpin.
Our data reinforce and greatly extend the findings of other studies that suggest a critical role of dsRNA structure in determining editing specificity and levels (Rieder and Reenan, 2012). We do not rule out other previously identified mechanisms of cis regulation, such as alterations to the Adar editing motif (although rare in this study) and regulation by distant cis-acting structural elements (Daniel et al., 2012; Rieder et al., 2013); in fact, these mechanisms may play a role in the cases where dsRNA stability around the editing site cannot account for changes in editing.
These data suggest that the evolution of editing levels across species is closely tied to sequence conservation surrounding the editing site. The fact that we see an enrichment for 3′ UTR editing sites regulated in cis reinforces the notion that non-protein-coding regions that are less likely to be under evolutionary constraints are more likely to vary in editing levels between these species. Conversely, an optimal editing level at a functional editing site could potentially drive sequence conservation surrounding the site in order to maintain beneficial editing.
In this study, we are unable to attribute a large number of editing differences to trans regulation. We are limited by the need for species to be closely related to create viable F1 hybrids. If mating of more distantly related species would yield F1 progeny, we would likely observe greater divergence in editing substrates as well as potential trans regulators. With these species, changes caused by trans regulators, such as RNA binding proteins, are far less likely to be observed as they may require a substantial sequence or expression change in what are likely to be highly conserved proteins between these species. Further exploration of trans regulation in flies is needed to appreciate the extent to which trans-acting factors affect editing levels.
Differences in Adar proteins between these two species do not appear to lead to major differences in editing levels, except at a few sites. This result is not unexpected, as human Adar2 is able to rescue the D. melanogaster Adar mutant, and dAdar has been shown to edit the mammalian GluR2 site in vitro (Keegan et al., 2011), providing strong evidence that Adar editing specificity across species is driven more by substrate sequence than the differences in Adar proteins.
This study expands the evidence that cis sequence differences between species are critical determinants of editing level divergence, yet does not rule out a role for trans regulators in addition to Adar in modulating editing levels. Future studies are needed to determine the types of cis sequence changes that have the largest effects on editing level and to identify additional proteins that act as trans regulators of editing.
EXPERIMENTAL PROCEDURES
Processing samples for mmPCR-seq
Fly heads from 0-2 day old female flies were flash frozen in liquid nitrogen. RNA extractions, cDNA synthesis, and mmPCR-seq were done following standard protocols (see Supplemental Experimental Procedures for details).
Statistical analysis of cis and trans regulation
We combined read counts for all replicates and downsampled to a uniform coverage of 175 reads across all samples. Fisher’s exact tests were performed in R using A and G counts for differences between the parent species and differences between the hybrid alleles. For trans analysis, we determined the linear regression and standardized residuals and calculated the two-tailed probability for observing the residuals in a standard normal distribution using pnorm(). Multiple hypothesis testing corrections were done using the Benjamini and Hochberg correction (Benjamini and Hochberg, 1995) with p.adjust() following both Fisher’s exact tests and regression analysis. Corrected p-values < 0.05 were considered significant. All statistical tests were performed using R version 2.15.1 (R Core Team, 2012).
Predicting RNA secondary structures around editing sites
Using the programs partition, MaxExpect, and ct2dot from the RNAStructure package (Reuter and Mathews, 2010), we predicted the secondary structure of the sequence 200 bases downstream and upstream of each editing site. We required a stem of at least 20 bases with a maximum bulge size of 8 bases to call the sequence base-paired to the edited side of the stem the ECS. To determine the energies of the edited stems, we joined the ECS with the edited part of the stem using a 100 base linker of adenosines and ran the fold program in the RNAStructure package.
Quantitative real time PCR
qPCR was performed with the same cDNA samples used in the mmPCR-seq experiments using KAPA SYBR FAST qPCR kit (Kapa Biosystems) following the standard protocol. Primers used in qPCR experiments are listed in Supplemental Experimental Procedures. Averaging three technical replicates, fold changes were calculated using the ΔΔCt method for the change between the gene of interest and reference gene GAPDH.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Li lab for comments, H. Tang and J. Davis III for help with statistical analysis, L. Keegan and M. O’Connell for Adar mutant flies, J. Coolon for advice on fly crosses and T. Clandinin, A. Fire and A. Gitler for their input. This work is funded by NIH R01 GM102484 and Ellison Medical Foundation. ALS and PD received funding from NSF GRFP fellowships and NIH training grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
ALS and JBL conceived of experiments. ALS performed experiments. PD and RZ designed ECS script. ALS wrote the manuscript.
ACCESSION NUMBERS
Next-generation sequencing data has been deposited at the NCBI Gene Expression Omnibus (GEO) under accession number: GSE67236.
The authors declare no conflict of interest.
REFERENCES
- Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu. Rev. Biochem. 2002;71:817–846. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Bhogal B, Jepson JE, Savva YA, Pepper AS-R, Reenan RA, Jongens TA. Modulation of dADAR-dependent RNA editing by the Drosophila fragile X mental retardation protein. Nat Neurosci. 2011;14:1517–1524. doi: 10.1038/nn.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles CR, Hirschhorn JN, Altshuler D, Lander ES. Detection of regulatory variation in mouse genes. Nat Genet. 2002;32:432–437. doi: 10.1038/ng992. [DOI] [PubMed] [Google Scholar]
- Daniel C, Venø MT, Ekdahl Y, Kjems J, Öhman M. A distant cis acting intronic element induces site-selective RNA editing. Nucleic Acids Research. 2012;40:9876–9886. doi: 10.1093/nar/gks691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggington JM, Greene T, Bass BL. Predicting sites of ADAR editing in double-stranded RNA. Nat Commun. 2011;2:319. doi: 10.1038/ncomms1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garncarz W, Tariq A, Handl C, Pusch O, Jantsch MF. A high-throughput screen to identify enhancers of ADAR-mediated RNA-editing. RNA Biol. 2013;10:192–204. doi: 10.4161/rna.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gott JM, Emeson RB. Functions and mechanisms of RNA editing. Annu. Rev. Genet. 2000;34:499–531. doi: 10.1146/annurev.genet.34.1.499. [DOI] [PubMed] [Google Scholar]
- Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, Yang L, Artieri CG, van Baren MJ, Boley N, Booth BW, et al. The developmental transcriptome of Drosophila melanogaster. Nature. 2010;471:473–479. doi: 10.1038/nature09715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ME, Grant GR, Paquin C, Qian J, Nitabach MN. Deep sequencing the circadian and diurnal transcriptome of Drosophila brain. Genome Res. 2012;22:1266–1281. doi: 10.1101/gr.128876.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan LP, Brindle J, Gallo A, Leroy A, Reenan RA, O’Connell MA. Tuning of RNA editing by ADAR is required in Drosophila. Embo J. 2005;24:2183–2193. doi: 10.1038/sj.emboj.7600691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan LP, McGurk L, Palavicini JP, Brindle J, Paro S, Li X, Rosenthal JJC, O’Connell MA. Functional conservation in human and Drosophila of Metazoan ADAR2 involved in RNA editing: loss of ADAR1 in insects. Nucleic Acids Research. 2011;39:7249–7262. doi: 10.1093/nar/gkr423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JB, Church GM. Deciphering the functions and regulation of brain-enriched A-to-I RNA editing. Nat Neurosci. 2013;16:1518–1522. doi: 10.1038/nn.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucci R, Brindle J, Paro S, Casadio A, Hempel S, Morrice N, Bisso A, Keegan LP, Del Sal G, O’Connell MA. Pin1 and WWP2 regulate GluR2 Q/R site RNA editing by ADAR2 with opposing effects. Embo J. 2011;30:4211–4222. doi: 10.1038/emboj.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K. Functions and regulation of RNA editing by ADAR deaminases. Annu. Rev. Biochem. 2010;79:321–349. doi: 10.1146/annurev-biochem-060208-105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MJ, Keegan LP, O’Connell MA, Reenan RA. A-to-I Pre-mRNA Editing in Drosophila Is Primarily Involved in Adult Nervous System Function and Integrity. Developmental Biology. 2000;102:437–449. doi: 10.1016/s0092-8674(00)00049-0. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna Austria: 2013. [Google Scholar]
- Ramaswami G, Li JB. RADAR: a rigorously annotated database of A-to-I RNA editing. Nucleic Acids Research. 2014;42:D109–D113. doi: 10.1093/nar/gkt996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami G, Zhang R, Piskol R, Keegan LP, Deng P, O’Connell MA, Li JB. Identifying RNA editing sites using RNA sequencing data alone. Nat Meth. 2013;10:128–132. doi: 10.1038/nmeth.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reenan RA, Hanrahan CJ, Ganetzky B. The mlenapts RNA helicase mutation in Drosophila results in a splicing catastrophe of the para Na+ channel transcript in a region of RNA editing. Neuron. 2000;25:139–149. doi: 10.1016/s0896-6273(00)80878-8. [DOI] [PubMed] [Google Scholar]
- Reenan RA. Molecular determinants and guided evolution of species-specific RNA editing. Nature. 2005;434:409–413. doi: 10.1038/nature03364. [DOI] [PubMed] [Google Scholar]
- Reuter JS, Mathews DH. RNAstructure: software for RNA secondary structure prediction and analysis. BMC Bioinformatics. 2010;11:129. doi: 10.1186/1471-2105-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder LE, Reenan RA. The intricate relationship between RNA structure, editing, and splicing. Developmental Biology. 2012;23:281–288. doi: 10.1016/j.semcdb.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Rieder LE, Staber CJ, Hoopengardner B, Reenan RA. Tertiary structural elements determine the extent and specificity of messenger RNA editing. Nat Commun. 2013;4:2232. doi: 10.1038/ncomms3232. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Menet JS, Rosbash M. Nascent-seq indicates widespread cotranscriptional RNA editing in Drosophila. Molecular Cell. 2012;47:27–37. doi: 10.1016/j.molcel.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal JJC, Seeburg PH. A-to-I RNA Editing: Effects on Proteins Key to Neural Excitability. Neuron. 2012;74:432–439. doi: 10.1016/j.neuron.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva YA, Jepson JE, Sahin A, Sugden AU, Dorsky JS, Alpert L, Lawrence C, Reenan RA. Auto-regulatory RNA editing fine-tunes mRNA re-coding and complex behaviour in Drosophila. Nat Commun. 2012;3:790. doi: 10.1038/ncomms1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Laurent G, Tackett MR, Nechkin S, Shtokalo D, Antonets D, Savva YA, Maloney R, Kapranov P, Lawrence CE, Reenan RA. Genome-wide analysis of A-to-I RNA editing by single-molecule sequencing in Drosophila. Nat Struct Mol Biol. 2013;20:1333–1339. doi: 10.1038/nsmb.2675. [DOI] [PubMed] [Google Scholar]
- Tariq A, Garncarz W, Handl C, Balik A, Pusch O, Jantsch MF. RNA-interacting proteins act as site-specific repressors of ADAR2-mediated RNA editing and fluctuate upon neuronal stimulation. Nucleic Acids Research. 2013;41:2581–2593. doi: 10.1093/nar/gks1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq A, Jantsch MF. Transcript diversification in the nervous system: a to I RNA editing in CNS function and disease development. Front. Neurosci. 2012;6:99. doi: 10.3389/fnins.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N, Yang Y, Sachsenmaier N, Muggenhumer D, Bi J, Waldsich C, Jantsch MF, Jin Y. A structural determinant required for RNA editing. Nucleic Acids Research. 2011;39:5669–5681. doi: 10.1093/nar/gkr144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IX, So E, Devlin JL, Zhao Y, Wu M, Cheung VG. ADAR regulates RNA editing, transcript stability, and gene expression. Cell Reports. 2013;5:849–860. doi: 10.1016/j.celrep.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkopp PJ, Haerum BK, Clark AG. Evolutionary changes in cis and trans gene regulation. Nature. 2004;430:85–88. doi: 10.1038/nature02698. [DOI] [PubMed] [Google Scholar]
- Zhang R, Li X, Ramaswami G, Smith KS, Turecki G, Montgomery SB, Li JB. Quantifying RNA allelic ratios by microfluidic multiplex PCR and sequencing. Nat Meth. 2014;11:51–54. doi: 10.1038/nmeth.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.