Abstract
Background
Health care utilization in older adults (≥60) with acute myeloid leukemia (AML) has not been well-studied.
Methods
We conducted a retrospective analysis of 330 consecutive older patients diagnosed with AML between 5/1/2005 and 12/23/2011 at two hospitals in Boston to examine their health care utilization and end of life (EOL) care. Using multivariable logistic and linear regression models adjusting for covariates, we also compared health care utilization for patients undergoing intensive induction (n=197; cytarabine/ anthracyline combination) versus non-intensive chemotherapy (n=133; single-agent therapy).
Results
The median number of hospitalizations for the entire cohort was 4.2 (range 1–18). Patients who died spent a mean of 28.3% of their life from diagnosis in the hospital and 13.8% of their life attending outpatient clinic appointments. Although the majority (87.9%) of patients died during the 2-year follow-up period, a minority (16.2% and 23.1%) utilized palliative care or hospice services, respectively. Within 30 days of death, 84.5% of patients were hospitalized, with 61.0% dying in the hospital. Among patients who died, those treated with intensive induction (versus non-intensive therapy) spent 30% more of their life in the hospital (p < 0.0001), and were less likely to utilize hospice services (OR 0.45, P = 0.05).
Conclusions
These findings highlight the intensity of health care utilization of older patients with AML, regardless of treatment modality. Despite the poor prognosis, palliative care and hospice services are rarely used. Future work should study novel health-care delivery models to optimize care throughout the course of illness and at the EOL.
Introduction
Older patients (≥60) with acute myeloid leukemia (AML) face a life-threatening illness that carries a poor prognosis with a median survival of 8–10 months, and a long-term disease-free survival of less than 10%.1, 2 Factors such as poor performance status, comorbidities, biological parameters such as frequent expression of the multidrug resistance p-glycoprotein and association with unfavorable karyotypes, and the high proportion of therapy-related disease all contribute to these poor outcomes.1, 3, 4 Surprisingly, studies exploring health care utilization and end-of-life (EOL) care in this population are lacking.10,11 Data describing patients’ utilization of health services such as the time they spend in the hospital and clinic, and their care at the EOL would allow clinicians to communicate accurate information to their patients about the ramifications of their diagnosis and treatment. Ensuring that patients are well-informed about their illness is a key component of patient-centered care as it provides patients with the vital information they need to plan for the future.5,6
Additionally, there are a variety of treatment options available for older patients with AML. There is limited agreement among clinicians as to the optimal initial treatment, and there are no published data on how these treatment strategies impact patients’ health care utilization and EOL care. Treatment options include (1) intensive chemotherapy using a combination of cytarabine and an anthracycline (‘7+3’ regimen), a regimen commonly used to induce remission in younger adults with AML;4, 7 (2) less intensive therapy with low-dose cytarabine or the hypomethylating agents decitabine or azacitidine;7–10 (3) clinical trial enrollment;7 or (4) supportive care alone.7 Patients who are thought to be more medically fit commonly receive intensive therapy with 7+3 with the hope of attaining a complete remission and ultimately undergoing allogeneic hematopoietic stem cell transplantation (HCT), which is potentially curative.1, 11 Older individuals who are not fit for induction therapy are often treated with less intensive therapy or supportive care alone, after discussing the risks and benefits of various options with their oncologists.1, 11
Given the short life expectancy of many older patients with AML and the low likelihood of cure, patients may wish to consider the impact of cancer therapy on their quality of life, including the time spent in the hospital and the medical care received throughout the illness and at the EOL. One randomized study conducted in 1989 compared intensive induction versus supportive care with cytoreductive agents as needed (cytarabine, or hydroxurea) in older patients with AML.12 In this study, patients treated with intensive induction had a 10-week survival advantage compared to those treated with supportive care. Importantly, there were no significant differences in patients’ time spent in the hospital or their quality of life between the treatment arms. However, quality of life was assessed based upon time spent in the hospital, rather than with patient-reported measures. Moreover, with the introduction of hypomethylating agents as well as improvement in supportive care measures, it is unclear whether the findings of this study would be replicable in the modern era.
The aim of our study was to describe current health care utilization and EOL care in older patients (≥60) with AML. We also sought to examine the impact of the initial treatment strategy (intensive versus non-intensive) on health care utilization and EOL care.
Methods
Study Design
We conducted a retrospective analysis of all patients 60 years or older with a diagnosis of AML treated at the Dana-Farber Cancer Institute or Massachusetts General Hospital between 5/1/2005 and 12/31/2011. Patients were categorized as either receiving intensive induction therapy (n=197) or non-intensive therapy (n=133) at the time of diagnosis. We defined intensive induction as receiving standard ‘7+3’ with a combination of cytarabine and an anthracycline or a modification of this regimen on a clinical trial with other agents added to the 7+3 backbone. We defined non-intensive therapy as receiving hypomethylating agents, low-dose cytarabine, or single agent therapy on a clinical trial. Single agents included as non-intensive therapy were: SNS595 (topoisomerase II inhibitor), HSP90 inhibitor, Panobinostat (HDAC inhibitor), cloretazine, lenalidomide, NEDD-8 activating enzyme inhibitor, sorafenib, PKC-412 inhibitor, and bortezomib. We excluded patients with a diagnosis of acute promyelocytic leukemia, and those seen only for one-time consultation. As one of our goals was to compare health care utilization and EOL care among those receiving intensive induction versus non-intensive therapy, we excluded patients treated with supportive care alone and those who received non-intensive therapy, but were subsequently treated with 7+3.
We identified the eligible cohort using the Dana-Farber Cancer Institute and Massachusetts General Hospital Leukemia CRIS (Clinical Research Information Systems) database, which includes all patients with acute leukemia seen at these institutions. We then conducted a comprehensive chart review to obtain information regarding patients’ demographics, comorbidities as measured by the Sorror Comorbidity index,13 Eastern Cooperative Oncology Group (ECOG) performance status, AML cytogenetics and molecular profile, subsequent consolidation therapy, and health services utilization and care at the EOL. We used the European Leukemia Net risk stratification to classify disease risk.4, 14
Health Services Utilization and End-of-Life Care
We obtained information regarding frequency of hospitalizations and clinic visits, hospital length-of-stay, palliative care consultations, and intensive care unit (ICU) admissions from the electronic medical record. For patients who died by 12/31/2013 (a minimum of 2-year follow-up), we determined the number of days spent hospitalized and the number of days patients visited the outpatient clinic. We then calculated the percent of life spent in the hospital (inpatient days/ total survival days × 100%), the percent of life spent in the outpatient clinic (outpatient clinic visit days/ total survival days × 100%), and the percent of life spent outside the hospital or clinic. We measured total survival days from the date of AML diagnosis. Each clinic visit was calculated as one full survival day. 70/330 (21.2%) of patients were missing data on the time spent in clinic as they were receiving care both locally and at our institutions concurrently. We conducted all analyses using observed data without imputation for missing data.
We determined patients’ place of death, cause of death, hospice utilization, and length-of-stay in hospice using the electronic medical record and the Social Security Death Index. We also determined whether patients were hospitalized (yes versus no), received chemotherapy (yes versus no), or were admitted to the ICU (yes versus no) within 30 days of death.
Statistical methods
We used descriptive statistics to summarize patient, and disease characteristics of all study subjects and stratified by initial treatment strategy. We used the chi-square test or two-sample t-test, as appropriate, to compare demographic and clinical characteristics between patients receiving intensive induction and those receiving non-intensive therapy.
We used Kaplan-Meier survival analysis to estimate overall survival and the log-rank test to assess univariate association between induction strategy and overall survival. Using multivariable Cox proportional hazards regression analysis, we assessed the independent effect of demographic and clinical variables (including induction strategy used) on overall survival. We calculated overall survival from the time of diagnosis, with patients censored at the date last known alive. The model included the following covariates: age, gender, marital status, education, Sorror comorbidity index, ECOG performance status, AML risk stratification, and the receipt of allogeneic HCT.
We used descriptive statistics to describe health care utilization and EOL care for all patients in this cohort. To compare health care utilization and EOL care between patients receiving intensive induction versus non-intensive therapy in the univariate analysis, we used Fisher’s exact test or two-sample t-test, as appropriate depending on the outcome of interest. Using multivariable logistic regression models, we examined the association between initial treatment strategy and the binary outcomes of interest (yes versus no: palliative care consultation; ICU admission; receipt of chemotherapy, hospitalization, ICU admissions within 30 days of death; hospice utilization, and length of stay in hospice ≤ 7 days). We used multivariable linear regression models to examine the association between the initial treatment strategy and continuous outcomes of interest (total number of hospitalizations, percent survival spent in the hospital, percent survival spent in the clinic, and percent survival spent outside the hospital or clinic). We included the following covariates in the multivariable linear and logistic regression models: age, gender, marital status, education, Sorror comorbidity index, ECOG performance status, AML risk stratification,4, 14 and the receipt of allogeneic HCT. We chose these covariates given their potential role as confounders as they are associated both with the treatment (intensive versus non-intensive) and the outcome of interest (health care utilization). These covariates are also commonly controlled for in multivariable analyses comparing intensive versus non-intensive therapy. All reported P values are two-sided with a P < 0.05 considered statistically significant.
Results
Patients Characteristics
Table 1 depicts the clinical characteristics of all patients (n=330) included in this study. The median age of the cohort was 70 years, and 42% had high risk disease.4, 14 Compared to patients receiving non-intensive therapy, those undergoing intensive induction were more likely to be younger (median age 66.3 versus 75.2, P < 0.0001), more educated (postgraduate education 18.3% versus 6.0%, P = 0.04), have fewer comorbidities (Median Sorror Comorbidity score 1.7 versus 2.2, P = 0.02) and a better performance status (0.77 versus 1.0, P < 0.0001).
Table 1.
Patients characteristics
| Variable | All (n=330) | Intensive (n=197) |
Non-intensive (n=133) |
P-Value |
|---|---|---|---|---|
| Age (SD) | 69.9 (6.8) | 66.3 (4.6) | 75.2 (6.2) | < 0.0001 |
| Male gender (%) | 195 (59.1%) | 110 (55.8%) | 85 (63.9%) | 0.14 |
| White Race (%) | 321 (97.3%) | 190 (96.4%) | 131 (98.5%) | 0.47 |
| Marital Status (%) | 0.05 | |||
| Single | 36 (10.9%) | 22 (11.2%) | 14 (10.5%) | |
| Married | 243 (73.6%) | 147 (74.6%) | 96 (72.2%) | |
| Divorced | 26 (7.9%) | 19 (9.6%) | 7 (5.3%) | |
| Widow | 25 (7.6%) | 9 (4.6%) | 16 (12.0%) | |
| Education (%) | 0.04 | |||
| High school or less | 136 (41.2%) | 75 (38.1%) | 61 (45.9%) | |
| College | 150 (45.5%) | 86 (43.7%) | 64 (48.1%) | |
| Post-Grad | 44 (13.3%) | 36 (18.3%) | 8 (6.0%) | |
| Therapy-related disease? (%) | 48 (14.6%) | 29 (14.7%) | 19 (14.3%) | 0.91 |
| Comorbidity Index (SD) | 1.9 (1.8) | 1.7 (1.6) | 2.2 (2.1) | 0.02 |
| Disease Risk (%) | 0.40 | |||
| Favorable | 25 (7.6%) | 18 (9.1%) | 7 (5.3%) | |
| Intermediate | 165 (50.0%) | 99 (50.3%) | 66 (49.6%) | |
| High Risk | 140 (42.4%) | 80 (24.2%) | 60 (45.1%) | |
| ECOG performance status (SD) | 0.88 (0.56) | 0.77 (0.55) | 1.0 (0.55) | < 0.0001 |
| Achieved complete remission | 171 (51.8%) | 140 (71.1%) | 31 (23.3%) | < 0.0001 |
| Received allogeneic HCT | 109 (33.0%) | 102 (51.8%) | 7 (5.3%) | < 0.0001 |
| Median survival in days [95%CI] | 340 [280–391] | 390 [309–487] | 255 [198–354] | < 0.0001 |
SD = Standard deviation; ECOG = Eastern Cooperative Oncology Group; HCT = Hematopoietic Stem Cell Transplantation.
The median survival for the entire cohort was 340 days [95% CI 280–391]. Patients receiving intensive induction were more likely to achieve complete remission (71.1% vs. 22.3%, P < 0.0001), and receive an allogeneic HCT (51.8% vs. 5.3%, P < 0.0001). In the unadjusted analysis, the median survival for patients who received intensive induction was 390 days [95% CI 309–487] and 255 days [95% CI 198–354] for those who received non-intensive therapy (P = 0.0003). In multivariable analyses, there was no significant difference in overall survival between patients treated with intensive induction vs. non-intensive therapy (HR 1.36, 95% CI 0.98–1.87, P = 0.06). Receiving an allogeneic HCT (HR 0.30, 95% CI 0.21–0.41, P < 0.0001) was associated with better overall survival.
Health Care Utilization in Older Patients with AML
The median number of hospitalizations for the entire cohort was 4.2 [range = 0–18], and 27.9% (92/330) of patients were admitted to the ICU at some point during their care [Table 2]. With a minimum follow-up of two years, 87.9% (290/330) of patients had died. Patients who died spent a mean of 28.3% of their life from diagnosis in the hospital and 13.8% of their life attending outpatient clinic appointments. Overall, patients who died spent a mean of 57.9% of their life from diagnosis outside of the hospital or clinic [Table 2]. Only 14.2% (47/330) of all patients and 16.2% (47/290) of those who died were seen by palliative care. The median time from palliative care consultation to death was 7 days [range = 0–364].
Table 2.
Health care utilization in older patients with AML
| Variable | All Patients (n = 290) n (frequency or SD) |
|---|---|
| Number of hospitalizations (SD) | 4.2 (3.0) |
| Percent of life in hospital (SD) | 28.3% (28.2) |
| Percent of life in clinic (SD) | 13.8% (14.7) |
| Percent of life outside hospital or clinic (SD) | 57.9% (33.3) |
| ICU Admissions (%) | 92 (31.7%) |
| Palliative care consults (%) | 49 (16.2%) |
| Time in days from palliative care consult to death (SD) | 7 (58.9) |
SD = Standard deviation; ICU = Intensive Care Unit.
EOL Care Outcomes in Older Patients with AML
Among patients who died (n=290), 61% died in the hospital, 31.0% died at home, and 8% died in a skilled nursing facility or hospice home [Table 3]. The main causes of death were: disease (68.6%), infection (13.4%), and treatment-related complications (12.4%). Within 30 days of death, 84.5% of patients were hospitalized, 44.5% had received chemotherapy, and 26.6% were admitted to the ICU. Only 22.1% received hospice services, and 11.3% has a hospice length of stay > 7 days.
Table 3.
End of life outcomes in older patients with AML
| Variable | All Patients (n = 290) n (frequency) |
|---|---|
| Cause of death | |
| Disease | 199 (68.6%) |
| Infection | 39 (13.4%) |
| Treatment Complications | 36 (12.4%) |
| Other | 7 (2.4%) |
| Place of death | |
| Home without hospice | 49 (16.9%) |
| Hospice (home or facility) | 64 (22.1%) |
| Hospital | 177 (61.0%) |
| Received hospice services | 67 (23.1%) |
| Hospice Length of Stay > 7 days | 12 (6.1%) |
| Chemotherapy within 30 days of death | 80 (49.4%) |
| Hospitalization within 30 days of death | 144 (88.9%) |
| ICU admissions within 30 days of death | 59 (36.4%) |
ICU = Intensive Care Unit.
Health Care Utilization and EOL Care Based on Initial Treatment Strategy
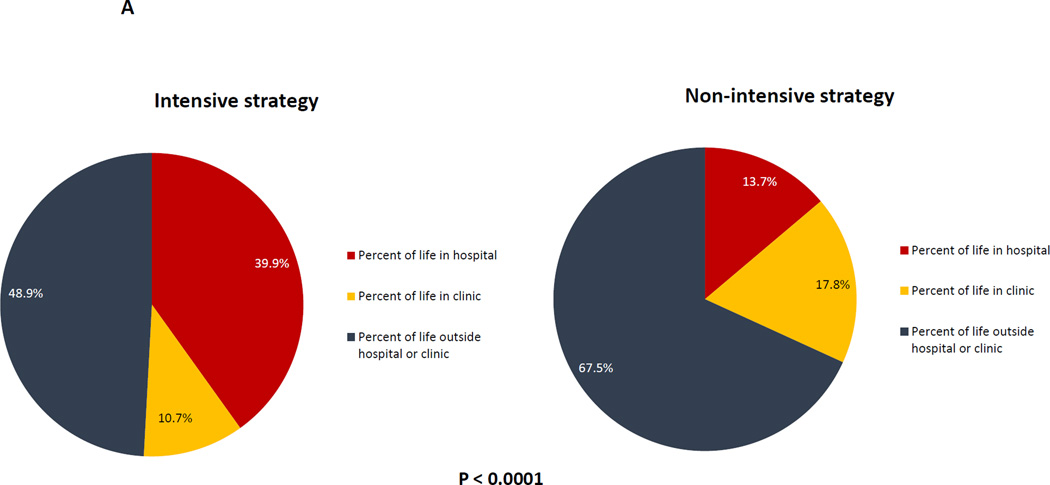
In the unadjusted analyses, patients treated with intensive therapy had more frequent hospitalizations (5.0 versus 3.1, P < 0.0001), and were more likely to be admitted to the ICU (45.1% versus 14.8%, P < 0.0001) at some point during their care. Among patients who died, those treated with intensive therapy spent more of their life from diagnosis until death in the hospital (39.9% versus 13.7%, P < 0.0001), but less of their life in clinic (10.7% versus 17.8%, P < 0.0001) [Figure 1A].
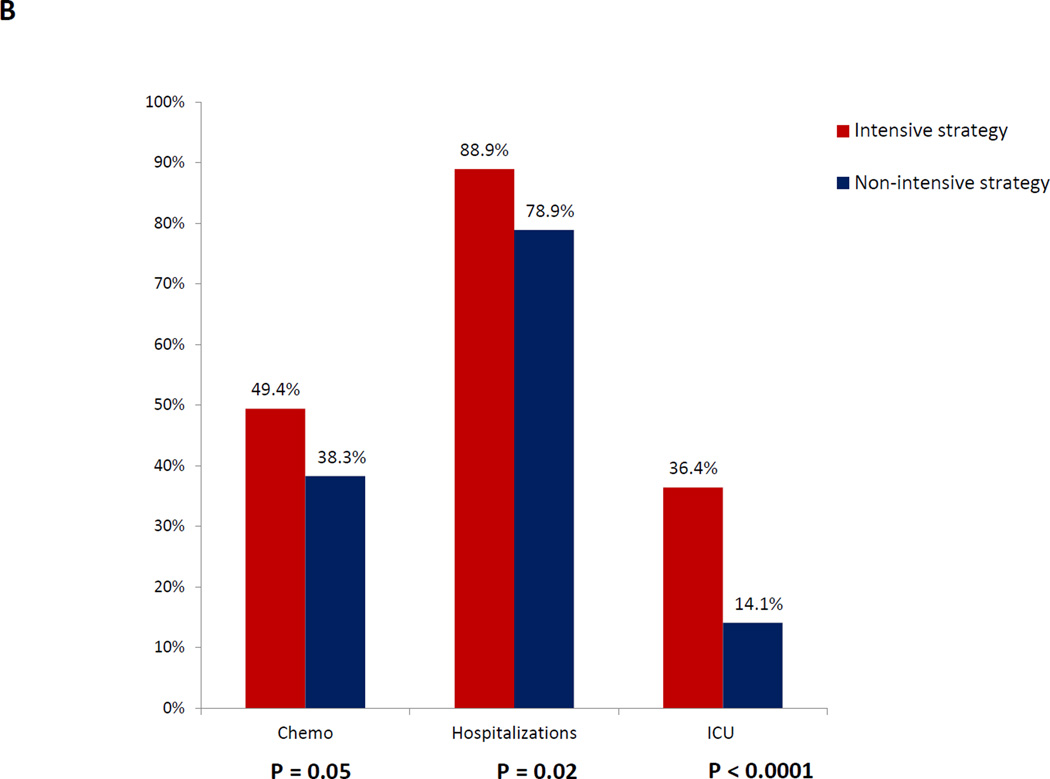
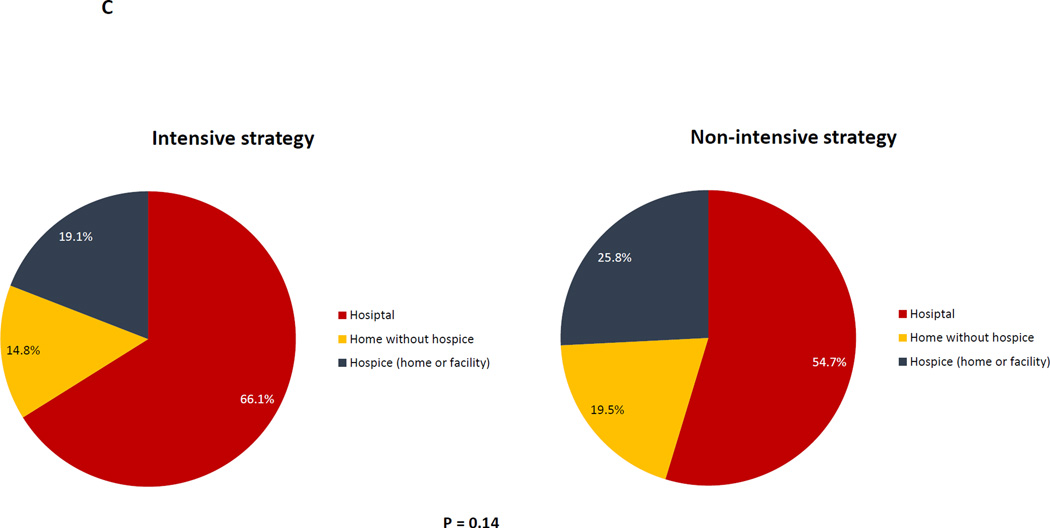
Figure 1. Univariable analyses of health services utilization and end-of-life care based on initial treatment strategy.
Figure 1A: Time spent at home, hospital, and clinic based on initial treatment strategy; Figure 1B: Health services utilization within 30 days of death based on initial treatment; Figure 1C: Place of death based on initial treatment strategy
In unadjusted analyses examining EOL care within 30 days of death, patients treated with intensive induction were more likely to be hospitalized (88.9% vs. 78.9%, p= 0.02), receive chemotherapy (49.4% vs. 38.3%, P = 0.05), and be admitted the ICU (36.4% vs. 14.1%, p < 0.0001) [Figure 1B]. There were no significant differences in place of death [Figure 1C], hospice utilization, or palliative care consults between the two groups. However, patients treated with intensive induction were less likely to have a hospice length of stay >7 days (6.1% vs.15.8%, P = 0.004).
In multivariable analyses [Table 4], patients treated with intensive induction were more likely to be admitted to the ICU (OR 3.59, 95% CI 1.74–7.41, P = 0.0005] at some point during their care. Among patients who died, those treated with intensive induction (versus non-intensive) spent 30% more of their life in the hospital (P < 0.0001), but 9% less in clinic (P = 0.0001). Patients treated with intensive induction spent 20% less of their life from diagnosis until death out of the hospital and clinic (P < 0.0001) compared to those receiving non-intensive therapy. No differences were noted in hospitalization frequency between the two groups.
Table 4.
Multivariable analyses of health services utilization and end-of-life care based on initial treatment strategy
| Outcomes (Intensive versus non-intensive) |
Odds Ratio or β |
95% CI or SE | P-Value |
|---|---|---|---|
| Total number of hospitalizations (SE) | β = 0.69 | 0.42 | P = 0.10 |
| Percent of life spent in the hospital (SE) | β = 30% | 4% | P = < 0.0001 |
| Percent of life spent in clinic (SE) | β = −9% | 2% | P = 0.0001 |
| Percent of life spent outside hospital or clinic (SE) | β = −20% | 5% | P < 0.0001 |
| ICU Admissions | OR = 3.59 | [1.74, 7.41] | P = 0.0005 |
| Palliative care consults | OR = 0.53 | [0.23, 1.17] | P = 0.12 |
| Hospice utilization | OR = 0.45 | [0.20, 0.99] | P = 0.05 |
| Hospice LOS =< 7 days | OR = 2.96 | [1.03, 8.52] | P = 0.04 |
| Chemotherapy within 30 days of death | OR = 1.84 | [0.95, 3.55] | P = 0.07 |
| Hospitalization within 30 days of death | OR = 1.26 | [0.50, 3.14] | P = 0.62 |
| ICU admission within 30 days of death | OR = 2.89 | [1.3, 6.20] | P = 0.006 |
SE = Standard Error; 95%CI = 95% Confidence Interval; ICU = Intensive Care Unit; LOS = Length-of-Stay.
There were no differences in hospitalizations or chemotherapy administration within 30 days of death between the two groups in multivariable analyses. However, patients treated with intensive induction were more likely to be admitted to the ICU within 30 days of death (OR 2.89, 95% CI 1.3–6.2, P=0.006), less likely to utilize hospice services prior to death (OR 0.45, 95% CI 0.2–1.0, P = 0.05), and more likely to have a hospice length of stay of ≤ 7 days (OR 2.96, 95% CI 1.03–8.52, P = 0.04).
Discussion
This study depicts the substantial health care burden of AML and the therapy used to treat it on older patients as they navigate their illness and face the EOL. The data also highlights the intensity of health care utilization at the EOL in this population. Patients in our sample were hospitalized frequently during their illness and the majority were admitted to the hospital in the last month of life and died in the hospital. While not explicitly studied in patients with AML, data suggest that the majority of cancer patients and the general public express a strong preference to die at home and minimize time spent in the hospital at the EOL.15, 16 Unfortunately, there are many barriers to achieving this goal in patients with AML including uncertainty about prognosis, the rapid trajectory of decline at the EOL, the frequency of infectious and bleeding complications, and the intensity of supportive care measures needed, particularly blood product support.17, 18 Our findings highlight the need to develop novel health service delivery models to provide appropriate EOL care for patients with AML, taking into account the specific needs of this population.
Despite the proven benefits of palliative and hospice care for patients with cancer, these services were rarely utilized in our cohort. As early integration of palliative care has been shown to improve quality of life, symptom burden, and decrease health service utilization in patients with solid malignancies,19, 20 a similar strategy may prove valuable in improving quality of life and health care delivery in patients with AML. Early referral to palliative care in this population—known to have a high symptom burden21 and relatively short survival1, 2 –can occur concurrently while pursuing curative therapy. Such strategies should be evaluated in future studies, as they would allow the incorporation of supportive care interventions in spite of the unpredictable illness trajectory. In addition, specialized palliative care models must be developed with proper attention to the special needs of elderly patients with AML including methods to address their transfusion requirements and frequent complications such as bleeding and infections.
We found that only a minority of patients dying with AML utilized hospice services. Hospice utilization is associated with improvement in patients’ quality of life and family caregivers’ grief and satisfaction with care.22, 23 The absence of a clear transition between the curative and palliative phase of disease for older patients with AML may hamper the utilization of hospice services.17, 18 Additionally, the frequent need for blood product support, which many hospice organizations do not permit due to financial constraints, likely contributed to lower rates of hospice referrals.17, 24 Despite the challenges, studies should determine whether AML patients and their families receive benefit from an earlier and frequent use of hospice care in part via the adoption of alternative care models.
Our retrospective study was not designed to compare the survival outcomes of intensive versus non-intensive therapy in older patients with AML; indeed, this would be impossible given the high selection biases inherent in such treatment choices. However, our work represents the first study comparing health care utilization and EOL care in older patients with AML treated with various induction strategies. Our results highlight the significant amount of time spent hospitalized or interacting with the health care system after a diagnosis of AML, especially among patients treated with intensive induction. The intensity of health care utilization is likely driven by the nature of the disease and its complications as well as the therapies used to treat it. Patients treated with intensive induction who ultimately died spent over 50% of their life in the hospital or clinic. While intensive induction offers a minority of patients a potentially curative therapy, patients should also be informed of the likely outcome if cure is not achieved. This information can enable patients to make decisions that are aligned with their values, and it can be utilized to design supportive care interventions to improve the quality of life and care of this population.
Several of our findings are consistent with prior studies examining EOL care in patients with hematologic malignancies. In one study, patients with hematologic malignancies were more likely like to have hospital admissions, and chemotherapy use within 30 days of death compared to patients with solid malignancies.25 In another study, hematologic malignancy patients were referred late or never received hospice services.26 We focused on older patients with AML, as opposed to a heterogeneous population of hematologic malignancies patients. Given the drastic differences in illness trajectories for patients with various hematologic malignancies, it is important to examine patients with different hematologic malignancies separately.21
Our study has several important limitations. First, we conducted the study at two academic institutions in Boston, thereby limiting the generalizability of our findings to other settings. However, many older patients diagnosed with AML are referred to tertiary care centers for their care given their specialized needs. Thus, our data likely accurately reflects the patterns of care for many older patients with AML treated in the United States. Second, comparing health care utilization between patients receiving different induction strategies in a retrospective fashion can be challenging due to selection biases and the potential for unmeasured confounders. However, we controlled for known baseline differences between the two groups in all of our analyses. Additionally, compared to those receiving intensive induction, patients receiving non-intensive therapy are older, less fit, and have more comorbidities, which would bias the results towards higher health care utilization during therapy and at the EOL in this group, contrary to our findings. Third, we were unable to assess to what extent the intensity of health care utilization in this population was driven by the nature of the disease or the therapy used to treat it. This is an important issue as our findings should not be used to discourage clinicians from recommending therapy for this disease, but rather they should be incorporated into the discussions with patients when clinicians review treatment options. Finally, patients may have been hospitalized or received EOL care at facilities outside of our institutions, which might not have been entirely captured by our medical records.
In conclusion, older patients with AML spend a significant portion of their life after diagnosis in the hospital or clinic. They are also likely to die in the hospital and infrequently utilize hospice or palliative care services. While older patients with AML receiving intensive induction are more likely to achieve a complete remission and receive an allogeneic HCT, a potentially curative therapy for their disease, they are also more likely to spend time in the hospital and receive aggressive care at the EOL. While these findings are important for informed decision-making, they must be placed within the context of a larger discussion regarding the potential benefits and harms of treatment for this disease. Clinicians must integrate patients’ prognostic and disease risk stratification, as well as their values into their recommendation about the optimal therapy for each patient. Moreover, although quality of life data are limited in this population,27, 28 clinicians must also consider the impact of the various treatments on patients’ quality of life when discussing therapeutic options. Finally, clinicians must engage in an honest discussion with patients regarding how to best incorporate the pursuit of curative therapy into the decision-making process when cure is only a realistic possibility for a minority of older patients with AML. Importantly, our findings highlight the need for developing supportive care interventions to improve quality of life and care for patients with AML throughout the course of their illness, during hospitalizations, and at the EOL.
Acknowledgments
Funding: Dr. El-Jawahri is supported by funds from the NCI Federal Share Program and the National Palliative Care Research Center (NPCRC CDA). Dr. LeBlanc is supported by funds from the NPCRC. Dr. Temel is supported by funds from K24 CA 181253.
Footnotes
Financial Disclosures: None
References
- 1.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol. 2007;25:1908–1915. doi: 10.1200/JCO.2006.10.2731. [DOI] [PubMed] [Google Scholar]
- 3.Leith CP, Kopecky KJ, Chen IM, et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: a Southwest Oncology Group Study. Blood. 1999;94:1086–1099. [PubMed] [Google Scholar]
- 4.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 5.Weeks JC, Cook EF, O'Day SJ, et al. Relationship between cancer patients' predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709–1714. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 6.Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc Sci Med. 2000;51:1087–1110. doi: 10.1016/s0277-9536(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 7.Dombret H, Raffoux E, Gardin C. Acute myeloid leukemia in the elderly. Semin Oncol. 2008;35:430–438. doi: 10.1053/j.seminoncol.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lubbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29:1987–1996. doi: 10.1200/JCO.2010.30.9245. [DOI] [PubMed] [Google Scholar]
- 10.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 11.Sekeres MA, Stone RM, Zahrieh D, et al. Decision-making and quality of life in older adults with acute myeloid leukemia or advanced myelodysplastic syndrome. Leukemia. 2004;18:809–816. doi: 10.1038/sj.leu.2403289. [DOI] [PubMed] [Google Scholar]
- 12.Lowenberg B, Zittoun R, Kerkhofs H, et al. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: a randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J Clin Oncol. 1989;7:1268–1274. doi: 10.1200/JCO.1989.7.9.1268. [DOI] [PubMed] [Google Scholar]
- 13.Sorror ML, Appelbaum FR. Risk assessment before allogeneic hematopoietic cell transplantation for older adults with acute myeloid leukemia. Expert Rev Hematol. 2013;6:547–562. doi: 10.1586/17474086.2013.827418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone M. Acute Myeloid Leukemia in First Remission: To Choose Transplantation or Not? Journal of Clinical Oncology. 2012;21:1262–1266. doi: 10.1200/JCO.2012.43.4258. [DOI] [PubMed] [Google Scholar]
- 15.Higginson IJ. Priorities and preferences for end of life care. London: The Cicely Saunders Foundation Scottish Partnership for Palliative Care and National Council for Hospice and Specialist Palliative Care Services; 2003. [Google Scholar]
- 16.Higginson IJ, Sen-Gupta GJ. Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. J Palliat Med. 2000;3:287–300. doi: 10.1089/jpm.2000.3.287. [DOI] [PubMed] [Google Scholar]
- 17.Howell DA, Shellens R, Roman E, Garry AC, Patmore R, Howard MR. Haematological malignancy: are patients appropriately referred for specialist palliative and hospice care? A systematic review and meta-analysis of published data. Palliat Med. 2011;25:630–641. doi: 10.1177/0269216310391692. [DOI] [PubMed] [Google Scholar]
- 18.Epstein AS, Goldberg GR, Meier DE. Palliative care and hematologic oncology: the promise of collaboration. Blood Rev. 2012;26:233–239. doi: 10.1016/j.blre.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 20.El-Jawahri A, Greer JA, Temel JS. Does palliative care improve outcomes for patients with incurable illness? A review of the evidence. J Support Oncol. 2011;9:87–94. doi: 10.1016/j.suponc.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann C, Yuen D, Mischitelle A, et al. Symptom burden and supportive care in patients with acute leukemia. Leuk Res. 2013;37:731–736. doi: 10.1016/j.leukres.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright AA, Keating NL, Balboni TA, Matulonis UA, Block SD, Prigerson HG. Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers' mental health. J Clin Oncol. 2010;28:4457–4464. doi: 10.1200/JCO.2009.26.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGrath P. Palliative care for patients with hematological malignancies--if not, why not? J Palliat Care. 1999;15:24–30. [PubMed] [Google Scholar]
- 25.Hui D, Didwaniya N, Vidal M, et al. Quality of end-of-life care in patients with hematologic malignancies: a retrospective cohort study. Cancer. 2014;120:1572–1578. doi: 10.1002/cncr.28614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sexauer A, Cheng MJ, Knight L, Riley AW, King L, Smith TJ. Patterns of hospice use in patients dying from hematologic malignancies. J Palliat Med. 2014;17:195–199. doi: 10.1089/jpm.2013.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliva EN, Nobile F, Alimena G, et al. Quality of life in elderly patients with acute myeloid leukemia: patients may be more accurate than physicians. Haematologica. 2011;96:696–702. doi: 10.3324/haematol.2010.036715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alibhai SM, Leach M, Kermalli H, et al. The impact of acute myeloid leukemia and its treatment on quality of life and functional status in older adults. Crit Rev Oncol Hematol. 2007;64:19–30. doi: 10.1016/j.critrevonc.2007.07.003. [DOI] [PubMed] [Google Scholar]