Abstract
The clinical manifestation of chronic subdural hematoma is not limited to neurological deficits or cognitive impairment. It may present with behavioral abnormalities. When the behavioral abnormalities present without obvious neurological deficits and in the absence of trauma, it leads to misdiagnosis. A trivial trauma may cause intracranial bleed that is either missed or ignored in the clinical history. This case report highlights the clinical picture of a homeless patient who presented with withdrawn and disorganized behavior, apathy and poverty of speech in the absence of any neurological deficit. His clinical presentation led to a possibility of psychosis and he was started on antipsychotics. He had developed extrapyramidal side effects in low dose of antipsychotic without any clinical benefit in his clinical picture. Neuroimaging done to rule out any possible organicity-revealed bilateral subdural hematoma, which was later evacuated by neurosurgical intervention in multiple settings and the patient had improved. This case report unfolds the mystery behind the psychotic presentation in a homeless adult.
Keywords: Chronic subdural hematoma, extrapyramidal side effects, neuroimaging, psychosis
INTRODUCTION
Subdural hematoma is a neurosurgical condition resulting from the collection of blood or remnants of blood elements in the subdural space following bleeding. It can be acute, subacute, or chronic depending on the duration since bleeding. Chronic subdural hematoma is characterized by the persistence of liquefied hematoma in the subdural space for more than 3 weeks by encapsulation.[1] Craniocerebral disproportion is a potential risk factor for subdural hematoma formation.[1] In the elderly, due to cerebral atrophy the disproportion between the cranial cavity and cerebral cortex is more that gives enough space for expansion of the hematoma. Currently, it is believed that recurrent bleeding in the subdural space due to series of events-local angiogenesis, inflammation, continuing coagulation, and fibrinolysis-results in persistence of the hematoma.[1]
Chronic subdural hematoma commonly present with features like alteration of the sensorium raised intracranial tension and motor weakness.[2] Common psychiatric manifestation in patients with chronic subdural hematoma is cognitive impairment which may mimic delirium or dementia.[3,4,5]
Trauma is an important cause of subdural hematoma and in elderly population even a trivial trauma is enough in causing subdural hematoma.[3] Spontaneous subdural hematoma without trauma is also reported in elderly population, but rare.[3] Spontaneous subdural hematoma usually occurs in the presence of risk factors such as vasculopathy, coagulopathy, and concurrent anticoagulation treatment.[6,7,8]
The clinical manifestation depends upon the extent of cerebral compression.[3] Chronic alcoholism and postoperative pneumocephalus are poor prognostic factors in chronic subdural hematoma.[2]
Computed tomography of the brain is diagnostic of chronic subdural hematoma; however, magnetic resonance imaging of the brain gives more structural details of hematoma.[9]
CASE REPORT
An adult, homeless male of approximately 40 years age was brought to psychiatric emergency by a voluntary organization. The patient was found wandering aimlessly in a disheveled state in the road. Detailed history of the patient was not available as there was no reliable informant. The patient was also not able to give any information related to his current and past psychiatric condition. He was also unable provide any history of seizure disorder, trauma, or any other physical illness.
At the time of first consultation to the psychiatrist at the emergency, he was having very poor hygiene with bad odor coming from his body. He was restless and not sitting on the chair on repeated request. There was poor eye to eye contact. He was conscious but his orientation could not be checked as he was not speaking at all. He was not following any verbal or nonverbal command.
In inpatient evaluation, it was found that he used to remain withdrawn to self. There was complete absence of speech. He had no concern for food, self-care including personal hygiene. He had little interest in the interaction with others, play activities, television, music, and things happening around. With adequate efforts, he could not be engaged in recreational activities or conversation. Though, he was attending the call of nature but most of the time he used to do it in clothes. His sleep was reported to be 3 to 4 h per day. When not asleep, he used to wander in the ward.
Physical examination did not reveal any abnormality other than pallor. Respiratory system, cardiovascular system, and preabdominal examination revealed no abnormality. In neurological examination there were grossly no deficits with bilateral, flexor plantar response. Due to speech impairment and uncooperativeness, his higher mental functions and some of the cranial nerve function, sensory function, motor function, as well as cerebellar functions could not be tested. The patient's behavior was observed in multiple settings. On the basis of clinical evaluation and ward behavior observation a diagnosis of “Psychosis not otherwise specified” is kept with differential diagnosis of “undifferentiated schizophrenia.” He was started on antipsychotic Tab. Risperidone and the dose was escalated to 6 mg per day over a period of 4 days along with anticholinergic Tab. Trihexyphenidyl 2 mg per day, but as the patient had developed extrapyramidal side effects in the form of tremor, rigidity, and excessive salivation. Tab. Lorazepam 2 mg per day was given for sedation. The dose of trihexyphenidyl was escalated up to 6 mg per day without much benefit in the extrapyramidal side effects. Risperidone was withdrawn and the patient was started with antipsychotic Tab. Olanzapine 5 mg per day and later escalated to 10 mg per day. Patient's clinical condition did not change and the extrapyramidal side effects were persisted. This warranted withdrawal of antipsychotics and review of diagnosis. To rule out any possible organicity, neuroimaging (initially CT scan of head and subsequently Magnetic Resonance Imaging of brain) was done that revealed bilateral chronic subdural hematoma with cerebral atrophy. Neurosurgical opinion was taken and the patient was operated in multiple settings. The Figures 1 and 2 shows the neuroimaging findings on preoperative MRI and postoperative CT scan of the brain, respectively. The hematoma was evacuated by burr-hole surgery. After evacuation of the hematoma in the first setting, the patient had improvement in his speech and emotional responsiveness. Perseveration was present in his speech. With subsequent evacuation of the hematoma, there was improvement in involvement in group activity, initiative as well as self-care. There was rebleed in the subdural space which was evacuated. The patient was managed with conservative treatment without psychotropic medications. His socialization had improved. He was involved in play activities like indoor games (Carom), which he was enjoying a lot.
Figure 1.
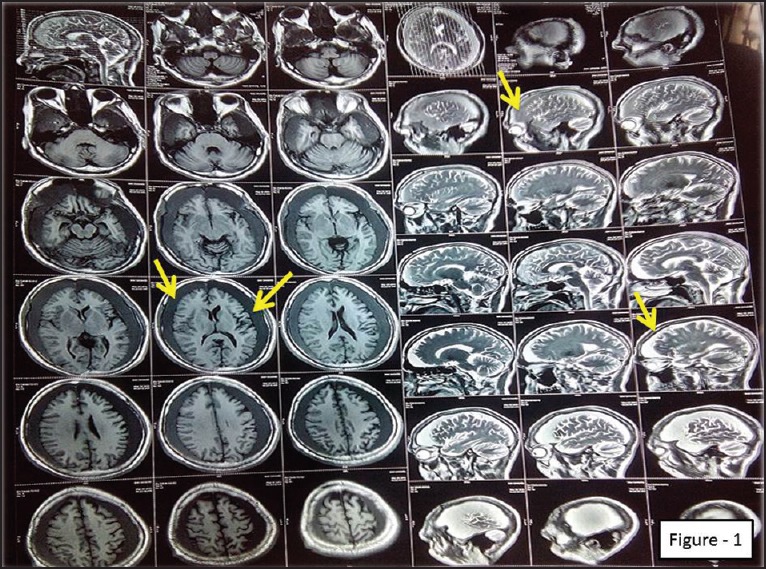
Magnetic Resonance Imaging of brain (pre-operative) showing bilateral subdural hematoma and diffuse cerebral atrophy
Figure 2.
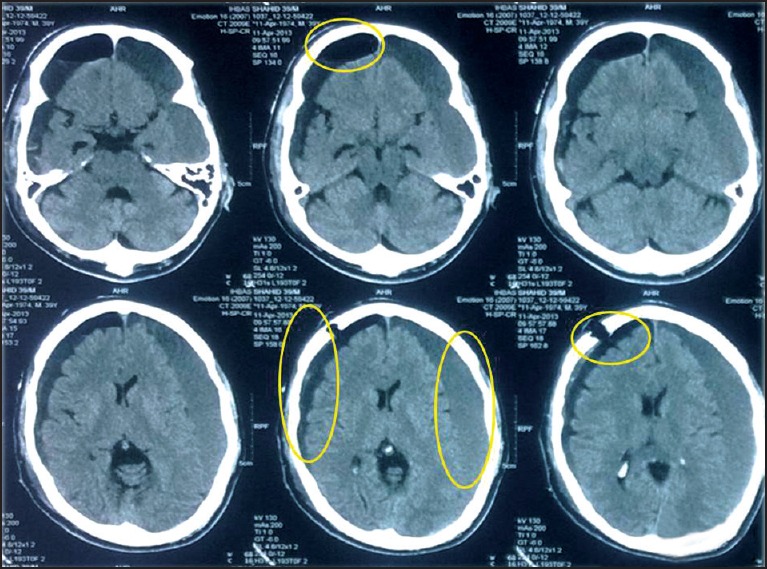
CT scan of head (post-operative) showing the burr-hole, postoperative collection of exudate in the subdural space, pneumocephalus changes, subdural hematoma on the opposite side (nonoperated side-left side), and diffuse cerebral atrophy
DISCUSSION
In this case, there was delay in detection of subdural hematoma. As the patient was not having any neurological deficit and his biological functions are little affected, the possibility of organicity was not considered initially. However, as the patient had developed neurological side effects to antipsychotics like Risperidoneand Olanzapine at lower doses and did not responded to the treatment, a possibility of organicity (central nervous system pathology) was suspected. After neuroimaging (noncontrast CT scan of head), bilateral subdural hematoma was detected. There was large bilateral subdural hematoma without any midline shift or compression of brain parenchyma. The architecture of sulci and gyri were intact despite of two large space occupying subdural hematomas on both sides. It suggests that there was some pre-existing potential space (resulting from diffuse cerebral atrophy) due to which, the hematoma had accommodated without any pressure effect. As craniocerebral disproportion is a major risk factor for chronic subdural hematoma[1] and in our patient there seems to be significant cranial–cerebral disproportion due to diffuse cerebral atrophy which might have been attributed to the subdural hematoma.
Patient's withdrawn behavior, absence of speech, apathy, blunted affect, and poor self-care was initially thought to be the manifestation of psychosis (possibly psychosis not otherwise specified or undifferentiated schizophrenia) and treatment had been started in that line, till the subdural hematomas are detected. All psychotropic medications were stopped immediately after neuroimaging. Patient's clinical presentation could be due to subdural hematoma or existing before subdural hematoma and might got distorted due to subdural hematoma.
The patient had diffuse cerebral atrophy at the age of 40 years which is an unusual phenomenon in an otherwise normal individual. So there is every possibility, that the patient might have psychiatric illness (long standing psychosis) or some nutritional or neurological illness before the development of hematoma which might have attributed to diffuse cerebral atrophy. The patient had shown clinical improvement, immediately following the evacuation of the subdural hematoma by burr-hole surgery. His speech, motor skills, and emotional responsiveness improved after surgery. Decompression of the brain by neurosurgical intervention resulted in the improvement. Chronic subdural hematoma may result in dementia like presentation and often needs evacuation by neurosurgical intervention which may reverse the dementia process.[5]
The subdural hematoma may have exclusively psychiatric manifestation and the treatment nonresponders or those who develop neurological adverse drug reaction in a low dose of psychotropic medications, needs to be evaluated rigorously. These groups of patients should undergo neuroimaging for ruling out possible brain pathology.[3]
In homeless patients, where no clinical history is available and mental status examination is not possible, reaching at a diagnosis is a big challenge. Due to lack of clinical information, misdiagnosis and wrong treatment may occur. But frequent review of diagnosis, utilization of clinical clues (adverse effects, nonresponse) and appropriate investigation (psychometric assessment or neuroimaging) may guide in reaching a diagnosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Tang J, Ai J, Macdonald RL. Developing a model of chronic subdural hematoma. ActaNeurochirSuppl. 2011;111:25–9. doi: 10.1007/978-3-7091-0693-8_5. [DOI] [PubMed] [Google Scholar]
- 2.Pencalet P. Clinical forms and prognostic factors of chronic subdural hematoma in the adult. Neurochirurgie. 2001;47:469–72. [PubMed] [Google Scholar]
- 3.Jomli R, Zgueb Y, Nacef F, Douki S. Chronic subdural hematoma and psychotic decompensation. Encephale. 2012;38:356–9. doi: 10.1016/j.encep.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Tsuboi K, Maki Y, Nose T, Matsuki T. Psychiatric symptoms of patients with chronic subdural hematoma. No ShinkeiGeka. 1984;12(Suppl3):275–9. [PubMed] [Google Scholar]
- 5.Schebesch KM, Woertgen C, Rothoerl RD, Ullrich OW, Brawanski AT. Cognitive decline as an important sign for an operable cause of dementia: Chronic subdural haematoma. ZentralblNeurochir. 2008;69:61–4. doi: 10.1055/s-2007-1004582. [DOI] [PubMed] [Google Scholar]
- 6.Hesselbrock R, Sawaya R, Means ED. Acute spontaneous subdural hematoma. SurgNeurol. 1984;21:363–6. doi: 10.1016/0090-3019(84)90115-0. [DOI] [PubMed] [Google Scholar]
- 7.Tokoro K, Nakajima F, Yamataki A. Acute spontaneous subdural hematoma of arterial origin. SurgNeurol. 1988;29:159–63. doi: 10.1016/0090-3019(88)90076-6. [DOI] [PubMed] [Google Scholar]
- 8.Wang HS, Kim SW, Kim SH. Spontaneous chronic subdural hematoma in an adolescent girl. J Korean NeurosurgSoc. 2013;53:201–3. doi: 10.3340/jkns.2013.53.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krupa M. Chronic subdural hematoma: A review of the literature. Part 1. Ann Acad Med Stetin. 2009;55:47–52. [PubMed] [Google Scholar]


