Abstract
Recent advances in surgical techniques and understanding of the pathophysiology of atrial fibrillation has led to the development of a less invasive thoracoscopic surgical treatment including video-assisted bilateral pulmonary vein isolation using bipolar radiofrequency ablation clamps. More recently, the same operation became possible via a totally thoracoscopic approach.
In this paper we describe technical aspects of the thoracoscopic approach to surgical treatment of AF and discuss its features, benefits and limitations. Furthermore, we present a new alternative technique of conduction testing using endoscopic multi-electrode recording catheters.
An alternative electrophysiological mapping strategy involves a multi-electrode recording catheter designed primarily for percutaneous endocardial electrophysiologic mapping procedure. According to our initial experience, the recordings obtained from the multi-electrode catheters positioned around the pulmonary veins are more accurate than the recordings obtained from the multifunctional ablation and pacing pen.
The totally thoracoscopic surgical ablation approach is a feasible and efficient treatment strategy for atrial fibrillation. The conduction testing can be easily and rapidly performed using a multifunctional pen or multi-electrode recording catheter.
Keywords: Arrhythmias, Cardiac; Cardiac Surgical Procedures; Endoscopy
Background
Atrial fibrillation (AF) is the most frequently occurring cardiac arrhythmia associated with a substantial morbidity and mortality. Incidence of AF is considerably high increasing with age and leading to significant complications, such as thrombo-embolism, heart failure, and disturbances in cognitive function. The lifetime risk to develop AF at the age of 55 years was shown to be 23.8% in men and 22.2% in women [1]. Additionally, cardiac surgical procedures may also trigger development of AF. The incidence of post-operative AF in patients undergoing cardiac surgery reported in previous studies varies between 20% and 50%, depending on age, comorbidities, procedure performed, and other perioperative factors [2].
A number of treatment approaches for AF have been developed and successfully implemented in the last decades. The Cox-Maze procedure evolved to the Cox-Maze III version, which was deemed the gold standard surgical open-chest treatment for AF. However, due to its invasiveness, it was superseded by catheter based techniques which were less invasive, had fewer complications but were associated with lower conversion rate to sinus rhythm (SR) [3]. Nevertheless, the combination of recent advances in surgical device technology and surgical techniques advances has led to the development of a minimally invasive surgical approach which can potentially combine the benefits of a minimally invasive strategy with the higher conversion rate to SR with better long-term results. This technique has been further improved in terms of its invasiveness, and a totally thoracoscopic treatment has become available. In this paper we describe, step-by-step, the totally thoracoscopic approach to surgical treatment of AF by means of video-assisted bilateral pulmonary vein isolation with bipolar radiofrequency (RF) ablation clamps. We also present a new alternative technique of conduction testing using multi-electrode recording catheters, which are usually utilized for electrophysiological mapping during catheter ablation procedures.
Surgical Technique and Management
After sedation and ventilation by a double lumen endotracheal tube and positioning a transesophageal ultrasound probe, the patient’s position on the operating table is adjusted by means of elevating the right side of thorax. Sterile drapes are placed as lateral as possible in order to facilitate access for thoracoscopic ports. After selective ventilation of the contralateral lung, a 10-mm port is placed into the 4th intercostal space (ICS) on the mid-axillary line and CO2 insufflation is started immediately. An additional 5 mm port is then inserted into the 3rd ICS on the mid-clavicular line. The final 10 mm port is placed into 7th ICS on the mid-axillary line (Figure 1). After substantial dissection of pleura including potential pleural adhesions using endoscissors and endograsper through the ports, a cranio-posterior pericardiotomy is performed anteriorly and parallel to the phrenic nerve, maintaining at least 4–5 cm distance from the phrenic nerve, to expose the superior (SVC) and inferior vena cava (IVC). In order to achieve better exposure of the pulmonary veins and the Waterston’s groove (interatrial groove) two pericardial retraction sutures should be placed using an endoscopic suture device carefully ensuring that excess tension is not applied, as this could inadvertently damage the phrenic nerve. Then, the space between the SVC and the right pulmonary artery is carefully dissected using the endoscopic suction device and endograsper in order to visualize the left atrial appendage. The Lumitip dissector (Lumitip®, ArtiCure, Inc., Ohio, USA), which offers blunt dissection facilitated by an illuminated tip in combination with an articulating distal dissection arm, can be used for creating sufficient space under the IVC between the right superior pulmonary vein and the right pulmonary artery and around the two right pulmonary veins (PVs). Both these features help the surgeon to precisely guide a tape (Glidepath Tape®, ArtiCure, Inc., Ohio, USA) through soft tissues and connect it with the ablation jaws (Isolator®, ArtiCure, Inc., Ohio, USA) of the clamp to guide it into place to create precisely positioned ablation lesions. During the ablation process, the bipolar jaws should be carefully placed upon the antrum to prevent ablation of the PVs and pulmonary vein stenosis. After successful placement, the jaws should be closed and ablation started and repeated 3–4 times at different positions in order to cover as much as possible of the ganglionic plexus situated on the antrum. Ganglionated plexus are potentially important ablation targets as they consist of sympathetic, parasympathetic, and mixed neurons. Excessive nerve activity from these sites can initiate and even sustain AF. The ganglionated plexus are usually found in the epicardial fat pad regions between the SVC and aorta ascendens, between the IVC, and the left atrium and over the PVs with close proximity to coronary sinus. After right-sided pulmonary vein isolation is completed, the remaining ganglionated plexus can be located using high-frequency stimulation with the AtriCure multifunctional pen (Isolator®, ArtiCure, Inc., Ohio, USA) and denervated by transpolar ablation.
Figure 1.

Three ports are placed in order to gain access and visualize the surgical ablation area. A 10-mm port is placed into the 4th intercostal space (ICS) on the mid-axillary line and CO2 insufflation is started immediately. An additional 5-mm port is then inserted into the 3rd ICS on the mid-clavicular line. The final 10-mm port is placed into 7th ICS on the mid-axillary line.
The next step is left-sided ablation, which is performed in a similar way, but with a few exceptions. First, the pericardiotomy is performed posteriorly to the phrenic nerve. Second, the ligament of Marshall, which is the remnant of the left common cardiac vein and might be a source of adrenergic atrial tachycardia, should be carefully dissected and ablated using the transpolar ablation pen. Third, the left atrial appendage (LAA), which is a major site for development of thrombi, can be amputated after left pulmonary vein isolation using, for example, an endostapler. Utmost care should be taken during this step as the LAA can be a source of significant bleeding, which is difficult to manage in the endoscopic setting. In our center, we now use a novel atraumatic device (AtriClip®, AtriCure, Inc., Ohio, USA) for LAA closure, which consists of 2 parallel rigid titanium tubes with elastic nitinol springs covered with a knit-braided polyester sheath. This device has been shown to be superior in terms of completeness of LAA exclusion and device-related adverse events and mortality [4].
After pericardium closure and chest drain insertion, the port access closure is performed in the usual manner. In order to relieve postoperative pain, a local anaesthetic (e.g., Lignocaine 1%) should be applied into the intercostal spaces.
There is no need for perioperative adjustment of antiarrhythmic drugs that patients may be taking before surgery. In cases of bradycardia, a reduction of the dose should be considered in order to suppress the negative chronotropic effect. Class III antiarrhythmics, such as amiodarone, should be continued post-operatively for at least 3 months even if a stable sinus rhythm is restored. To prevent potential embolic events, anticoagulation with warfarin is also recommended for at least 6 months.
Intraoperative testing of the conduction
One of the most crucial steps of the procedure in terms of achieving success is confirmation of conduction block. The conventional intraoperative testing is performed using a multifunctional ablation and pacing pen. The pen is placed sequentially on the superior and inferior pulmonary veins after removal of the bipolar clamp (Figure 2). During the ablation cycle, a graph of tissue conductance (current/voltage) versus time is displayed on the ablation and sensing unit (ASU) monitor (AtriCure, Inc., Ohio, USA) (Figure 3). This device produces and delivers RF energy in a bipolar mode, at a frequency of approximately 460 kHz, with a maximum output power range of 12–30 Watts. Using measurements of conductance, the ASU determines when transmurality has been achieved. The pen is then placed sequentially on the superior and inferior pulmonary veins after removal of the bipolar clamp to show entrance block. After that, the pen is used to pace the PV side of pulmonary vein isolation lesions to ensure the atrial body does not capture, which confirms exit block. For the success of the procedure it is essential to assess and confirm bi-directional conduction block of all ablation lines. If conduction block is not confirmed, ablation should be repeated and conduction block reassessed until bi-directional block is achieved.
Figure 2.
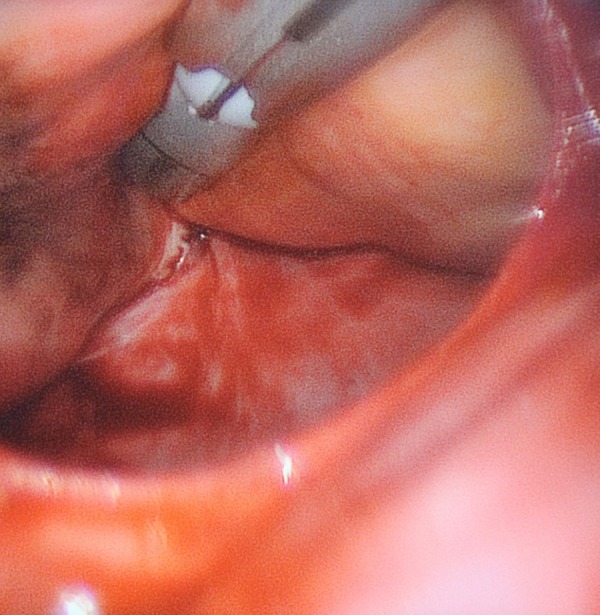
The pen is placed sequentially on the superior and inferior pulmonary veins.
Figure 3.
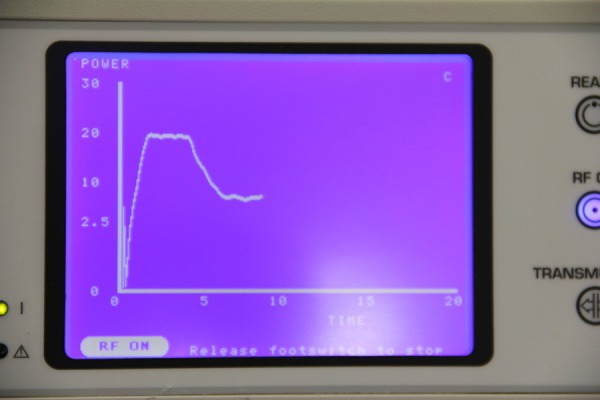
During the ablation cycle a graph of tissue conductance (current/voltage) versus time is displayed on the ablation and sensing unit (ASU) monitor (AtriCure, Inc., Ohio, USA).
One of the disadvantages of using conventional electrical testing, described previously, is that the pacing pen may miss the remaining regional electrical connection of the pulmonary vein. An alternative electrophysiological mapping strategy has been developed and successfully adopted in our center and involves using a 20-pole multi-electrode recording catheter (Bard RADIA™ Bidirectional Diagnostic Catheter) designed primarily for percutaneous endocardial electrophysiologic mapping procedure. The multi-electrode catheter is introduced through one of the ports, visualized by the camera and positioned using standard thoracoscopic instruments and is used for mapping the PVs at multiple locations to provide detailed confirmation of entrance block (Figure 4). According to our initial experience, the recordings obtained from the multi-electrode catheters positioned around the pulmonary veins are more accurate than the recordings obtained from the multifunctional ablation and pacing pen.
Figure 4.
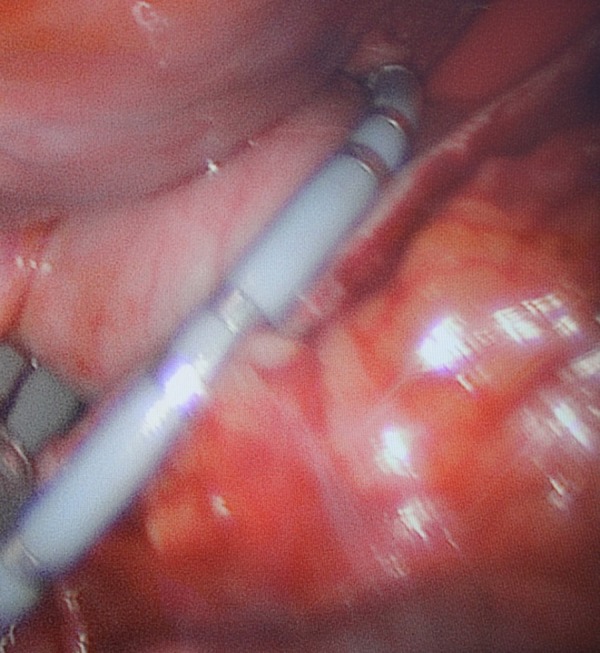
The multielectrode recording catheter is placed around the pulmonary veins for simultaneous conduction testing at multiple locations.
Selection criteria and limitations
The selection criteria for this treatment include patients who prefer the surgical approach, who failed 1 or more attempts of catheter ablation, patients with drug-refractory AF, inability to tolerate antiarrhythmic drugs or anticoagulation therapy, and with left ventricular ejection fraction of 25% or greater. Intolerance to single-lung ventilation and left atrial thrombi are absolute contraindications for the treatment. Further clinical conditions, such as previous cardiac and thoracic surgery, extensive pleural adhesions, and a large left atrium (>6 cm), might also be limiting factors in individual patients and should be considered as relative contraindications.
Discussion
Atrial fibrillation is the most common type of arrhythmia in humans. It is associated with a high incidence of stroke, congestive cardiac failure and significant mortality [5,6]. There is still a large scientific gap in terms of understanding the pathophysiology of AF. Previous research has suggested a multifactorial pathogenesis with ectopic foci from PV and non-PV sites. However, non-PV ectopic triggers may arise from SVC, posterior wall of LA, coronary sinus, Marshall ligament, and on the atrial septum. The main condition that contributes to initiation and maintenance of re-entry is associated with structural remodelling of atrial tissue, including myocyte sarcolemmal ion channels and intercellular communication among myocytes, and comprises microscopic and molecular changes related to protein synthesis. Particularly, connexins and N-cadherin – the group of proteins involved in intercellular transfer of molecules and ions among myocytes – were described in previous basic science research on AF as proteins involved in genesis and perpetuation of AF. Those mechanisms lead to shortening of repolarization and refractory periods, leading to a decrease in wavelength and re-entry circuits. Furthermore, myocyte degeneration-fibrosis, abnormalities of sinus node function as well as inflammation, and atrial myocarditis can also contribute to development of AF [7].
Previous research has shown that epicardial ablative strategies are associated with higher freedom from AF compared to catheter ablation, whereas there is no significant difference in terms of incidence of neurological complications and cardiac tamponade [3]. However, surgical ablation has been shown to be associated with significantly higher rates of other procedural adverse events, such as pneumothorax, major bleeding, and need for a pacemaker [8]. Freedom from AF at 1 year following isolated catheter ablation may reach up to 89% in cases of paroxysmal AF [9]. However, the results of this strategy in patients with persistent AF remain modest and often require multiple procedures [10].
The surgical treatment for AF was first described by James Cox in 1987 and has undergone numerous modifications, cumulating in the Cox-Maze III procedure with excellent results and freedom from symptomatic AF of up to 90% at 10 years [11]. Nevertheless, the need for median sternotomy, multiple atrial incisions, and use of cardio-pulmonary bypass (CPB) led to significant morbidity and mortality after this complex procedure [12]. For that reason, the Cox-Maze III operation was rarely used for lone AF and was used solely for those undergoing concomitant surgery. The totally thoracoscopic approach as described here, however, may combine the benefits of less invasiveness and higher efficacy, leading to a lower complication rate, lower morbidity, and longer freedom from AF.
Despite the novelty of this technique, the results after totally thoracoscopic approach for AF have been very promising. Oudeman et al. showed that 12 of 15 patients who underwent minimally invasive surgery were free from AF after 12 months [13]. A retrospective study by De Maat et al. revealed that after minimally invasive surgery, 69% of all patients were free from AF without the use of antiarrhythmic drugs and 83% with the use of antiarrhythmic drugs [14]. Pojar et al. reported that after thoracoscopic ablation, 93% of patients were in sinus rhythm at discharge, 76% of patients were free from AF without the need of antiarrhythmic drugs at 6 months after surgery, with a 1-year success rate of 73% [15].
Furthermore, our newly developed conduction testing technique has several advantages compared to the standard “point-by-point” testing. The multi-electrode recording catheter permits simultaneous recordings of intracardiac electrograms through multiple poles and is useful in assessing the circumferential ablation line that is conducted by the bipolar RF clamp pulmonary vein isolation. The flexibility of the catheter with regards to positioning in the totally thoracoscopic setting allows for an easier access to less accessible mapping areas using the surgical pen, such as the posterior wall of the left atrium.
Conclusions
To conclude, the totally thoracoscopic surgical ablation approach is a feasible and efficient treatment strategy for atrial fibrillation. This minimally invasive procedure might be associated with similar results to the open-chest surgical ablation. Due to no need for sternotomy, minimally invasive surgery can be associated with significantly reduced surgical morbidity. Furthermore, the conduction testing can be easily and rapidly performed using a multifunctional pen or multi-electrode recording catheter. However, prospective studies comparing the totally endoscopic and catheter ablation approaches, as well as both conduction testing methods combined, are needed to define whether the surgical strategy and our alternative mapping technique will translate into better outcomes, particularly in patients with persistent AF.
Footnotes
Source of support: Self financing
This paper was presented at the Annual Meeting of the German Society for Thoracic and Cardiovascular Surgery 2015, Freiburg, Germany
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Heeringa J, van der Kuip DA, Hofman A. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949–53. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 2.Echahidi N, Pibarot P, O’Hara G, Mathieu P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51(8):793–801. doi: 10.1016/j.jacc.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 3.Kearney K, Stephenson R, Phan K, et al. A systematic review of surgical ablation versus catheter ablation for atrial fibrillation. Ann Cardiothorac Surg. 2014;3(1):15–29. doi: 10.3978/j.issn.2225-319X.2014.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ailawadi G, Gerdisch MW, Harvey RL, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg. 2011;142(5):1002–9. 1009 e1001. doi: 10.1016/j.jtcvs.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 5.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeriouh M, Sabashnikov A, Choi YH, et al. A novel treatment strategy of new onset atrial fibrillation after cardiac surgery: an observational prospective study. J Cardiothorac Surg. 2014;9:83. doi: 10.1186/1749-8090-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-Quintana D, Lopez-Minguez JR, Pizarro G, et al. Triggers and anatomical substrates in the genesis and perpetuation of atrial fibrillation. Curr Cardiol Rev. 2012;8(4):310–26. doi: 10.2174/157340312803760721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation. 2012;125:23–30. doi: 10.1161/CIRCULATIONAHA.111.074047. [DOI] [PubMed] [Google Scholar]
- 9.Jais P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 10.Stabile G, Bertaglia E, Senatore G, et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation for the Cure of Atrial Fibrillation Study) Eur Heart J. 2006;27:216–21. doi: 10.1093/eurheartj/ehi583. [DOI] [PubMed] [Google Scholar]
- 11.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–28. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 12.Cox JL. Cardiac surgery for arrhythmias. J Cardiovasc Electrophysiol. 2004;15(2):250–62. doi: 10.1046/j.1540-8167.2004.03656.x. [DOI] [PubMed] [Google Scholar]
- 13.Oudeman M, Tjon A, Huijgen J, et al. A new approach to determine the results of minimally invasive pulmonary vein isolation using a continuous loop monitor: preliminary resultsdagger. Eur J Cardiothorac Surg. 2015 doi: 10.1093/ejcts/ezu552. pii: ezu552 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.De Maat GE, Pozzoli A, Scholten MF, et al. Long-term results of surgical minimally invasive pulmonary vein isolation for paroxysmal lone atrial fibrillation. Europace: Europace. 2015 doi: 10.1093/europace/euu287. pii: euu287 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Pojar M, Vojacek J, Haman L, et al. Thoracoscopic radiofrequency ablation for lone atrial fibrillation: box-lesion technique. J Card Surg. 2014;29(5):757–62. doi: 10.1111/jocs.12409. [DOI] [PubMed] [Google Scholar]


