Abstract
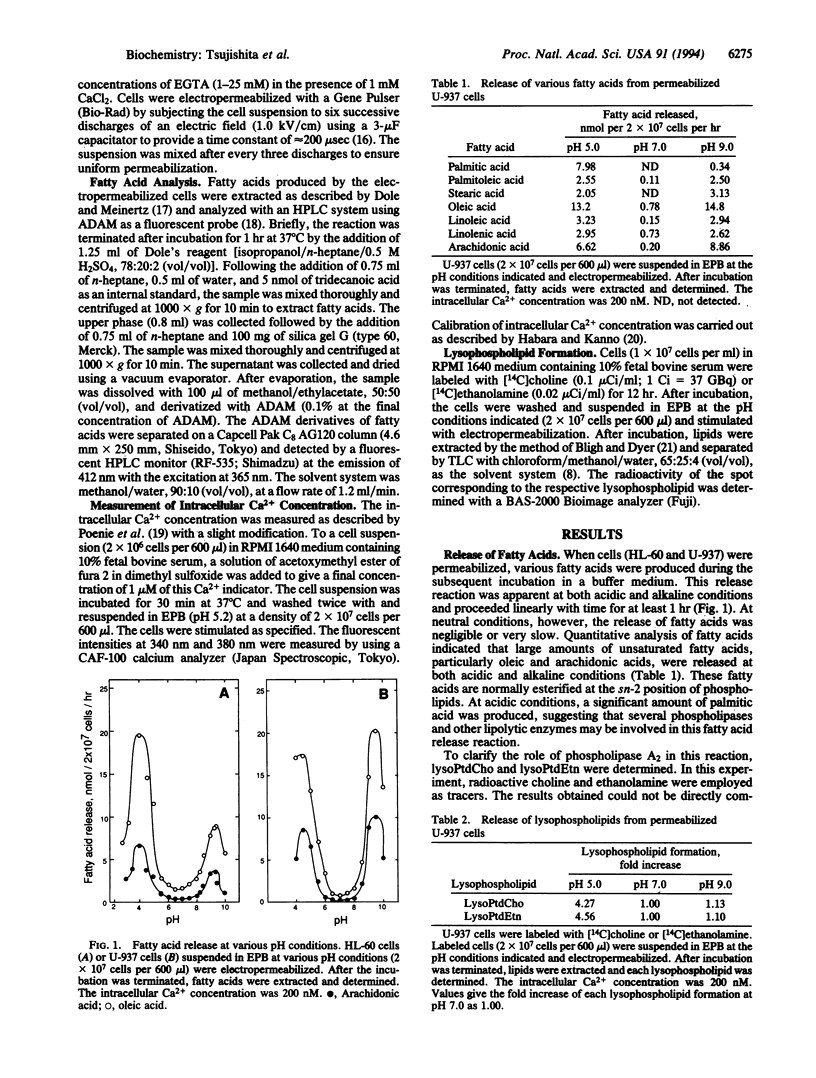
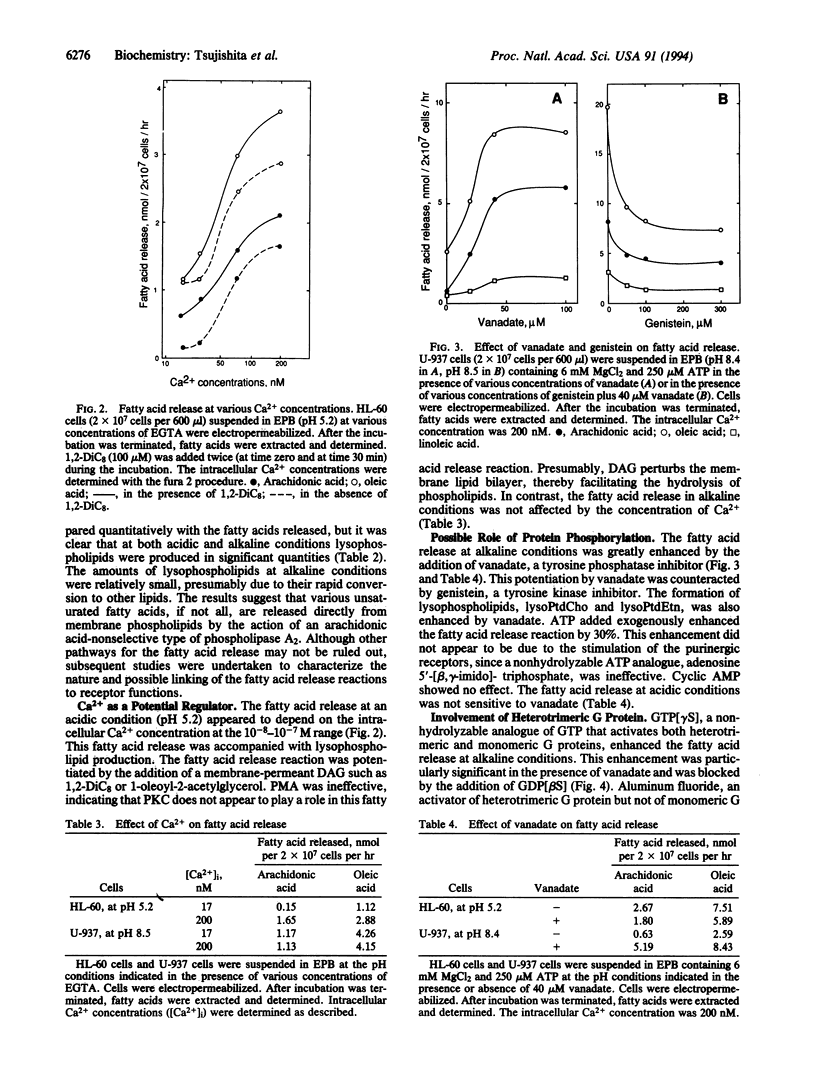
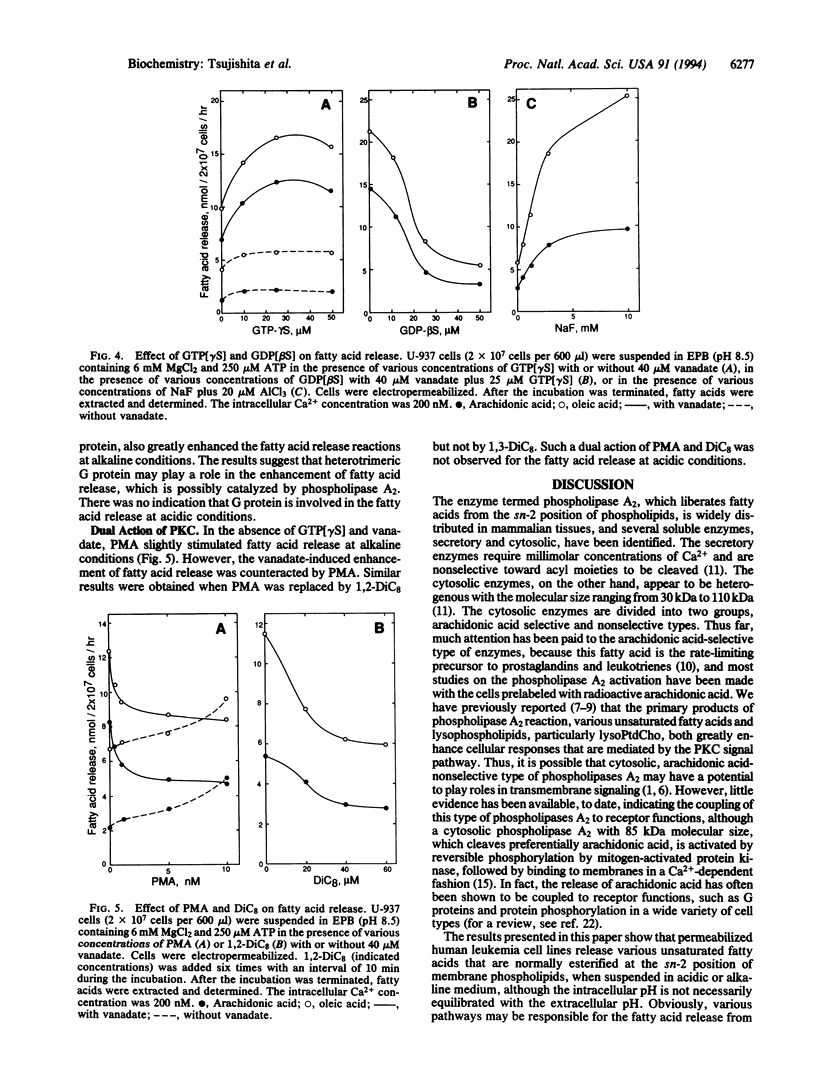
Permeabilized human leukemia HL-60 and U-937 cells suspended in an acidic or alkaline medium release various unsaturated fatty acids, most abundantly oleic and arachidonic acids. Concomitant production of lysophospholipids suggests that phospholipases A2 play a major role in this fatty acid release reaction. The fatty acid release at acidic conditions depends on the intracellular Ca2+ concentrations at the 10(-8)-10(-7) M range and is enhanced by membrane-permeant diacylglycerols, although this enhancement seems independent of protein kinase C activation. On the other hand, the fatty acid release at alkaline conditions is potentiated by vanadate, and this potentiation is counteracted by genistein, suggesting a role of tyrosine phosphorylation in this release reaction. GTP[gamma S], an activator of G proteins, greatly enhances the fatty acid release. Aluminum fluoride, another activator of heterotrimeric G proteins, also greatly potentiates this release reaction. Phorbol ester increases the fatty acid release at alkaline conditions, to some extent, whereas it counteracts the vanadate-induced potentiation of fatty acid release. The results imply that several phospholipases A2 are coupled to receptors for their activation, thereby functioning in the transmembrane control of cellular events.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asaoka Y., Nakamura S., Yoshida K., Nishizuka Y. Protein kinase C, calcium and phospholipid degradation. Trends Biochem Sci. 1992 Oct;17(10):414–417. doi: 10.1016/0968-0004(92)90011-w. [DOI] [PubMed] [Google Scholar]
- Asaoka Y., Oka M., Yoshida K., Sasaki Y., Nishizuka Y. Role of lysophosphatidylcholine in T-lymphocyte activation: involvement of phospholipase A2 in signal transduction through protein kinase C. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6447–6451. doi: 10.1073/pnas.89.14.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka Y., Yoshida K., Sasaki Y., Nishizuka Y. Potential role of phospholipase A2 in HL-60 cell differentiation to macrophages induced by protein kinase C activation. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4917–4921. doi: 10.1073/pnas.90.11.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J., Burch R. M., Jelsema C. L. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988 Mar;11(3):117–123. doi: 10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature. 1978 Dec 7;276(5688):620–622. doi: 10.1038/276620a0. [DOI] [PubMed] [Google Scholar]
- Barker S. A., Monti J. A., Christian S. T., Benington F., Morin R. D. 9-Diazomethylanthracene as a new fluorescence and ultraviolet label for the spectrometric detection of picomole quantities of fatty acids by high-pressure liquid chromatography. Anal Biochem. 1980 Sep 1;107(1):116–123. doi: 10.1016/0003-2697(80)90500-x. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. G-protein-regulated phospholipases C, D and A2-mediated signalling in neutrophils. Biochim Biophys Acta. 1992 Aug 14;1113(2):135–160. [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Dawson R. M., Hemington N. L., Irvine R. F. Diacylglycerol potentiates phospholipase attack upon phospholipid bilayers: possible connection with cell stimulation. Biochem Biophys Res Commun. 1983 Nov 30;117(1):196–201. doi: 10.1016/0006-291x(83)91560-7. [DOI] [PubMed] [Google Scholar]
- Dennis E. A., Rhee S. G., Billah M. M., Hannun Y. A. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991 Apr;5(7):2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Habara Y., Kanno T. Dose-dependency in spatial dynamics of [Ca2+]c in pancreatic acinar cells. Cell Calcium. 1991 Sep;12(8):533–542. doi: 10.1016/0143-4160(91)90073-n. [DOI] [PubMed] [Google Scholar]
- Kudo I., Murakami M., Hara S., Inoue K. Mammalian non-pancreatic phospholipases A2. Biochim Biophys Acta. 1993 Nov 3;1170(3):217–231. doi: 10.1016/0005-2760(93)90003-r. [DOI] [PubMed] [Google Scholar]
- Leslie C. C. Kinetic properties of a high molecular mass arachidonoyl-hydrolyzing phospholipase A2 that exhibits lysophospholipase activity. J Biol Chem. 1991 Jun 15;266(17):11366–11371. [PubMed] [Google Scholar]
- Lin L. L., Lin A. Y., Knopf J. L. Cytosolic phospholipase A2 is coupled to hormonally regulated release of arachidonic acid. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6147–6151. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. L., Wartmann M., Lin A. Y., Knopf J. L., Seth A., Davis R. J. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993 Jan 29;72(2):269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- Liscovitch M. Crosstalk among multiple signal-activated phospholipases. Trends Biochem Sci. 1992 Oct;17(10):393–399. doi: 10.1016/0968-0004(92)90007-v. [DOI] [PubMed] [Google Scholar]
- Macara I. G. Elevated phosphocholine concentration in ras-transformed NIH 3T3 cells arises from increased choline kinase activity, not from phosphatidylcholine breakdown. Mol Cell Biol. 1989 Jan;9(1):325–328. doi: 10.1128/mcb.9.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemenoff R. A., Winitz S., Qian N. X., Van Putten V., Johnson G. L., Heasley L. E. Phosphorylation and activation of a high molecular weight form of phospholipase A2 by p42 microtubule-associated protein 2 kinase and protein kinase C. J Biol Chem. 1993 Jan 25;268(3):1960–1964. [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Tsien R. Y., Steinhardt R. A. Changes of free calcium levels with stages of the cell division cycle. Nature. 1985 May 9;315(6015):147–149. doi: 10.1038/315147a0. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Asaoka Y., Nishizuka Y. Platelet activation by simultaneous actions of diacylglycerol and unsaturated fatty acids. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6443–6446. doi: 10.1073/pnas.89.14.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]



