Abstract
Rationale: The 2011 combined Global Initiative for Chronic Obstructive Lung Disease (GOLD) assessment incorporates symptoms, exacerbation history, and spirometry in discriminating risk of exacerbations in patients with chronic obstructive pulmonary disease (COPD). Six-minute-walk distance (6MWD) and accelerometry also have been used to assess disease severity in COPD. The association between these measures and the risks of hospitalization and mortality in the context of GOLD 2011 is unknown.
Objectives: To describe changes in exercise tolerance and physical activity over time in patients with COPD and to test the hypothesis that lower baseline 6MWD or accelerometry step count is associated with increased risk of COPD-related hospitalization or all-cause mortality, independent of GOLD 2011 group.
Methods: Physical function and medical outcomes were prospectively assessed in 326 patients with moderate to severe COPD in INSPIRE-II, a randomized controlled trial of a coping skills training intervention. Cox models were used to determine if GOLD 2011 group, 6MWD, or accelerometry steps were associated with risk of COPD-related hospitalization or all-cause mortality.
Measurements and Main Results: Physical function declined over time in GOLD group D but remained stable in groups A, B, and C. GOLD classification was associated with time to death or first COPD-related hospitalization. Baseline 6MWD was more strongly associated with time to death or first COPD-related hospitalization (hazard ratio, 0.50 [95% confidence interval, 0.34, 0.73] per 150 m, P = 0.0003) than GOLD 2011 classification. A similar relationship was observed for accelerometry steps (hazard ratio, 0.80 [95% confidence interval, 0.70, 0.92] per 1,000 steps, P = 0.002).
Conclusions: Exercise tolerance and daily physical activity are important predictors of hospitalization and mortality in COPD, independent of GOLD 2011 classification. Physical function may represent a modifiable risk factor that warrants increased attention as a target for interventions to improve clinically meaningful outcomes in COPD.
Keywords: exercise, chronic obstructive pulmonary disease, accelerometry, mortality, hospitalization
Acute exacerbations of chronic obstructive pulmonary disease (COPD) are associated with substantial morbidity, mortality, and cost (1–4), but individual risk of these events is heterogeneous. In addition to age and FEV1, the most consistent predictor has been previous acute exacerbations (5). In light of the relatively weak power of spirometry alone to predict clinical outcomes, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) revised its clinical classification system beginning with its 2011 guidelines, incorporating both FEV1 and symptoms to create four risk categories labeled A through D (1). Several large studies have examined the value of the GOLD 2011 classification in predicting acute exacerbations, COPD-related hospitalizations, and mortality (6–11). Overall, these studies have shown clear separation of outcomes between groups A and D, whereas the risk associated with groups B and C may overlap. Outcomes vary substantially among subgroups of GOLD C and D in particular. In this context, there is a need to further refine the ability to stratify risk of acute events in COPD and especially to identify risk factors on which patients or providers can intervene.
Recently, there has been increasing interest in physical activity as a prognostic measure in COPD. Six-minute-walk distance (6MWD) has consistently been associated with survival in this population (12–17) and is a component of the multidimensional BODE (body mass index, airflow obstruction, dyspnea, and exercise capacity) index (18) grading system for COPD. Physical activity can be measured reliably using accelerometry in patients with COPD (19) and is lower in patients with COPD than in matched smoking control subjects (20). Accelerometry measures are associated with BODE index (21), quality of life (22), and acute exacerbations of COPD (23). Measures of physical activity based on both accelerometry steps and estimated energy expenditure have been associated with mortality in this population (24). To our knowledge, however, changes in 6MWD and measured daily physical activity over time have not been reported in a large population characterized by GOLD 2011 classification. Furthermore, the ability of 6MWD and accelerometry to predict COPD-related hospitalizations in the context of the GOLD 2011 classification has not been assessed.
The Investigational Study of Psychological Intervention in Recipients of Lung Transplantation-II (INSPIRE-II) study (25, 26) was designed to evaluate a telehealth coping skills training intervention in patients with moderate to severe COPD. Compared with a control group receiving COPD education only, this intervention was associated with no difference in the composite primary outcome of all-cause mortality and COPD-related hospitalizations, despite improvements in somatic and psychological quality-of-life outcomes. In the current analysis, we apply the GOLD 2011 classification to the INSPIRE-II cohort. We describe changes in 6MWD and daily accelerometry steps by GOLD 2011 group. We then test the hypothesis that physical function as measured by 6MWD or accelerometry is associated with a primary outcome of time to first COPD-related hospitalization or all-cause mortality, independent of GOLD 2011 group.
Some of the results of this study have been previously published in the form of an abstract (27).
Methods
Study Design and Subjects
Participants were enrolled in the INSPIRE-II randomized trial of coping skills training (25, 26). Briefly, INSPIRE-II was a dual-site (Duke University Medical Center and Ohio State University) randomized trial that included 326 participants with COPD. Inclusion criteria consisted of FEV1 25 to 80% predicted within 6 months of study enrollment, prebronchodilator FEV1/FVC < 0.7, and capacity to give informed consent and follow study procedures. Post-bronchodilator spirometry was not performed. Randomization was stratified by site (Duke or Ohio State), presence of a primary caregiver, sex, smoking status, and FEV1 (≤50% predicted or >50% predicted). The Duke and Ohio State Institutional Review Boards approved the protocol, and all subjects provided written informed consent. The first subject was randomized in January 2009, and the last follow-up was completed in June 2014.
GOLD 2011 Classification and Prior Exacerbations
The St. George’s Respiratory Questionnaire (SGRQ) (28) was used as a measure of COPD-related symptoms. The SGRQ has been used in previous analyses (7) as a surrogate for the COPD Assessment Test (CAT) or modified Medical Research Council dyspnea scale for determining GOLD 2011 group. An SGRQ cutoff score greater than or equal to 25 was used in place of the CAT cutoff of greater than or equal to 10, in keeping with prior studies (7) and the described correlation between the two instruments (29).
Prior acute exacerbations of COPD were assessed at the time of trial enrollment as either the number of courses of prednisone or the number of hospitalizations for COPD in the past year by self-report, whichever was greater. Both prior prednisone courses and prior hospitalizations were recorded as zero, one to two, three to five, or more than five; therefore, the cutoff of two or more prior exacerbations could not be used to define the higher-risk GOLD categories C and D. However, of the seven patients categorized as GOLD A or B on the basis of spirometry and SGRQ but with one to two prior exacerbations, all had had a prior hospitalization for COPD exacerbation and were therefore reassigned to GOLD C or D based on the 2014 revision of the GOLD classification (30). This left no patients at risk for misclassification of GOLD group.
Accelerometry and 6-Minute-Walk Test
Physical activity during daily life was quantified using the Kenz Lifecorder Plus accelerometer (Model NL-2160; Suzuken Co. Ltd., Nagoya, Japan). This device accurately counts more than 85% of manually observed steps in older adults at walking speeds as low as 54 m/min and greater than 95% of observed steps at speeds of at least 67 m/min (31). Patients wore the accelerometer for two successive days both before randomization (i.e., at baseline) and after 16 weeks of treatment. The average number of steps per day over the 2-day period at baseline was used for the current analysis.
Two subjects experienced technical problems with the accelerometer at baseline, and one subject had only 1 day of data; these three subjects were thus excluded from all analyses requiring accelerometry data. Thirty-five subjects did not have accelerometry data at 16 weeks and were thus excluded from analyses involving change in total steps over time; specifically, 20 dropped out, 3 died, and the remainder did not have interpretable data. The 6-minute-walk test (6MWT) was used as a marker of functional capacity (32). There were no missing 6MWT data at baseline. Twenty-five patients were missing 6MWT data at 16 weeks: 20 dropped out, 3 died, and 2 did not present for the in-person study visit.
Medical Events
Patients documented all medical encounters every 6 months. Medical records were reviewed by a physician assistant, and a consensus conference of study pulmonologists, blinded to treatment condition, adjudicated all medical events. A composite endpoint of time to first COPD-related hospitalization or all-cause mortality served as the primary outcome.
Statistical Analysis
Analyses were performed using SAS version 9.2 (Cary, NC). We elected to model GOLD groups categorically, using group A as the reference. Differences in baseline characteristics among GOLD groups were assessed using one-way analysis of variance or Chi-square test as appropriate. For analyses of changes in 6MWD accelerometry steps over time, we used analysis of covariance, using the post-treatment value of either 6MWD or total accelerometry steps as the response variable and the respective pretreatment value, age, Charlson comorbidity index, treatment site, and treatment group as predictors. Because the baseline value of the response variable is included in these models as a predictor, the parameter estimates can be interpreted as the association with residualized change in the response variable.
Thirty-five subjects were excluded from analyses of change in accelerometry steps due to missing values at 16 weeks, and 25 subjects were similarly excluded from analyses of change in 6MWD at 16 weeks. We evaluated the relationships between GOLD classification with and without physical function measures (accelerometry steps and 6MWD) and the primary outcome of time to first COPD-related hospitalization or all-cause mortality using Kaplan-Meier log-rank tests and Cox proportional hazards models. Patients with no events or who were lost to follow-up were censored at the time of last contact. For Kaplan-Meier plots, baseline 6MWD was stratified as less than 300 m, 300 to 449 m, and 450 or more m, and baseline accelerometry as less than 2,000, 2,000 to 3,999, and 4,000 or more steps, to approximate tertiles while also providing convenient numerical ranges to help facilitate interpretation.
Within the Cox models we controlled for age, Charlson comorbidity index, hospitalizations or use of prednisone for COPD exacerbation during the past year (0 = none; 1 = one; 2 = two or more), treatment site, and treatment arm. In these models, baseline 6MWD and accelerometry were modeled in their continuous form, scaled such that one unit represented 150 m and 1,000 steps, respectively. We first validated the prognostic value of the GOLD 2011 classification in our cohort, without physical function measures included in the Cox model. We then tested our primary hypotheses by adding baseline 6MWD or total daily accelerometry steps to separate models. We elected not to include both 6MWD and accelerometry steps in a single model due to the relatively high correlation between these measures (r = 0.59, P < 0.001). In separate models, we examined whether changes in 6MWD and total daily accelerometry steps from baseline to 16 weeks were associated with the same primary outcome (limited to events occurring after 16 wk). In these analyses, we adjusted for the same covariates as above, in addition to the baseline level of 6MWD or total daily accelerometry steps as appropriate.
Thirty-five subjects were excluded from the accelerometry change model due to missing data at 16 weeks, and 25 were excluded from the 6MWD change model for similar reasons. We evaluated the extent to which all models met assumptions, including additivity, linearity, and distribution of residuals. We found no evidence of significant violations of these assumptions.
Results
Baseline Characteristics
Baseline characteristics are shown in Table 1. Group D was the most common GOLD classification (n = 191 [59%]), followed by B (91 [28%]), A (27 [8%]), and C (17 [5%]). Baseline 6MWD (P < 0.001), duration of COPD (P < 0.001), total accelerometry steps (P < 0.001), and supplemental oxygen use (P < 0.001) all differed by GOLD 2011 group.
Table 1.
Baseline characteristics
| GOLD A (n = 27) | GOLD B (n = 91) | GOLD C (n = 17) | GOLD D (n = 191) | |
|---|---|---|---|---|
| Age, yr | 65.9 (7.5) | 66.6 (8.8) | 68.4 (10.2) | 65.7 (8.0) |
| Male, n (%) | 21 (78) | 58 (64) | 11 (65) | 109 (57) |
| White, n (%) | 26 (96) | 85 (93) | 15 (88) | 159 (83) |
| Smoking status, n (%) | ||||
| Current | 4 (15) | 16 (18) | 2 (12) | 36 (19) |
| Ever | 26 (96) | 88 (97) | 17 (100) | 177 (93) |
| Duration of COPD,* yr | 3.5 (3.3) | 5.0 (3.7) | 4.8 (3.2) | 6.8 (3.8) |
| FEV1, L | 2.03 (0.5) | 1.85 (0.5) | 1.19 (0.4) | 1.3 (0.4) |
| FEV1, % predicted | 64.7 (11.0) | 62.2 (8.8) | 40.1 (9.9) | 35.0 (10.9) |
| FEV1/FVC | 0.58 (0.10) | 0.60 (0.11) | 0.43 (0.12) | 0.45 (0.14) |
| 6MWD,* m | 472 (75) | 392 (105) | 435 (79) | 313 (102) |
| Accelerometry | ||||
| Total steps* | 6,791 (3,567) | 4,216 (2,729) | 5,456 (3,132) | 3,247 (2,634) |
| Supplemental oxygen use,* n (%) | 2 (7) | 14 (15) | 5 (29) | 98 (51) |
| Pulmonary rehab during study, n (%) | 2 (4) | 8 (9) | 2 (12) | 33 (17) |
| Exacerbations in prior yr, n | ||||
| 0 | 27 | 91 | 15 | 141 |
| 1–2 | 0 | 0 | 2 | 41 |
| ≥3 | 0 | 0 | 0 | 9 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Values given as mean (SD) unless otherwise noted.
P < 0.05 for differences across GOLD 2011 groups by one-way analysis of variance.
Exercise Tolerance and Physical Activity by GOLD 2011 Group
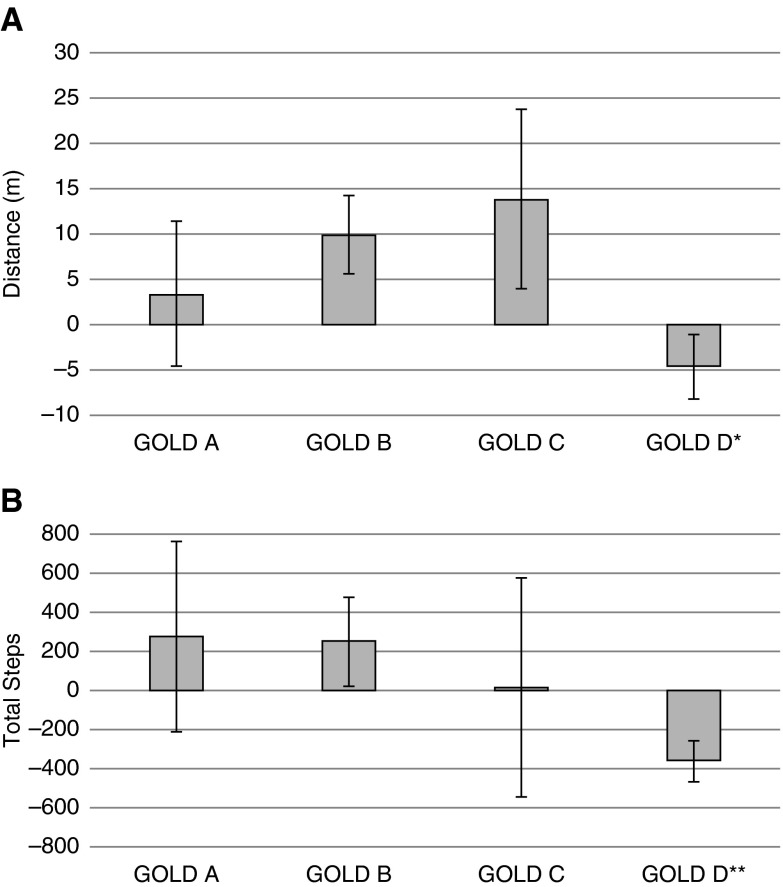
As shown in Figure 1, change in 6MWD over 16 weeks differed by GOLD 2011 group (P = 0.012 for one-way analysis of variance), with group D showing a decline that was significantly different from the relative increase in 6MWD in group B (P = 0.007). A similar pattern was observed for change in accelerometry steps over 16 weeks (P < 0.0001), with daily physical activity declining in group D compared with groups A (P = 0.0002), B (P = 0.0005), and C (P = 0.012).
Figure 1.
Changes in exercise tolerance and daily physical activity. Bars represent mean changes in (A) 6-minute-walk distance, and (B) total daily accelerometry steps over 16 weeks, by Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011 group. Vertical brackets represent SEM changes. *P < 0.05 for pairwise comparison of change in group D versus B. **P < 0.05 for pairwise comparisons of change in group D versus all other groups.
Relationships between GOLD 2011 Classification, Functional Status, and Outcomes
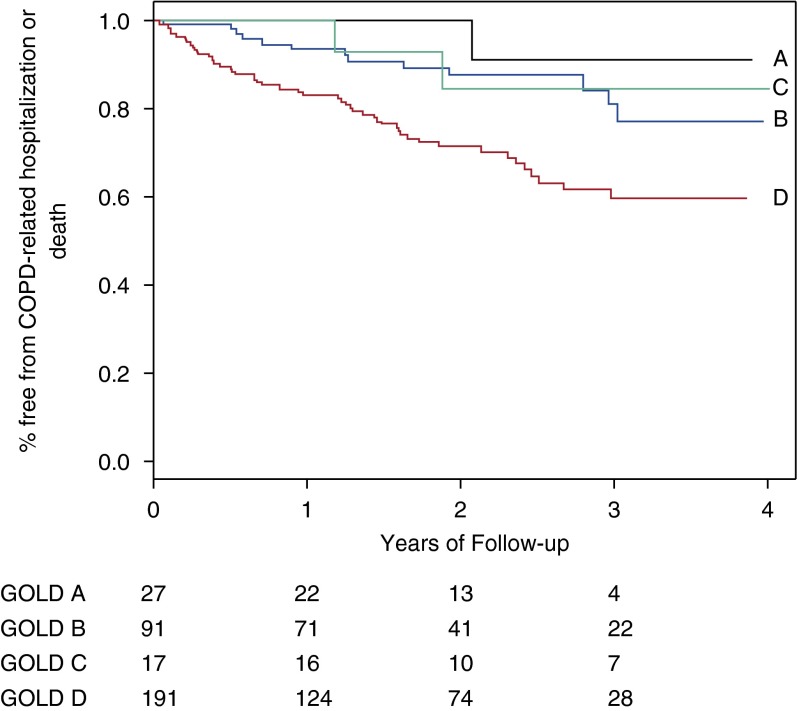
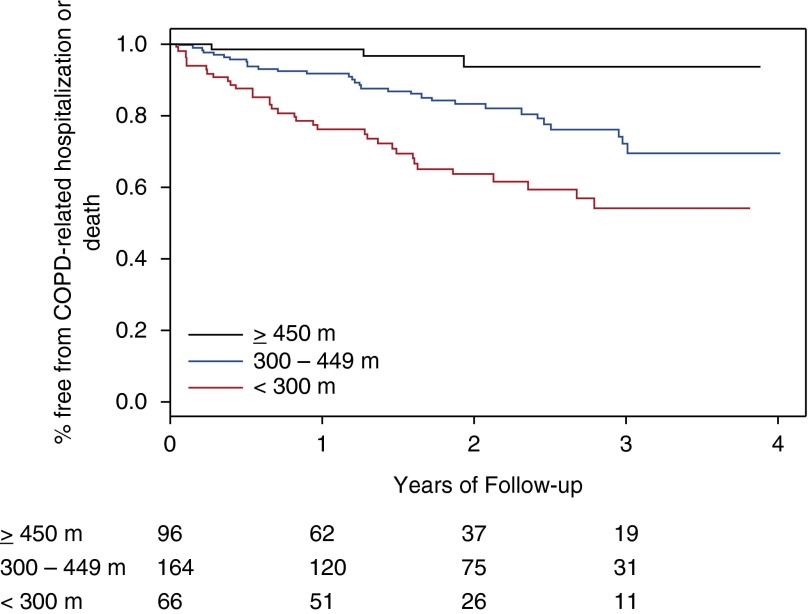
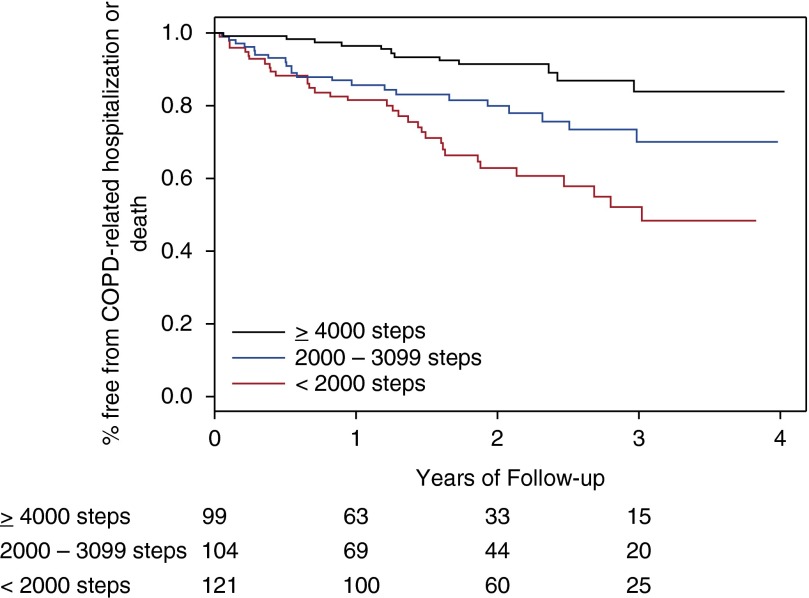
Sixty-nine individuals experienced the primary outcome event, including 12 deaths. In unadjusted Kaplan-Meier analyses, time to first COPD-related hospitalization or all-cause mortality was associated with GOLD 2011 group (P = 0.0007 for log-rank test, Figure 2) and strata of 6MWD (P < 0.0001, Figure 3) and total accelerometry steps (P < 0.0001, Figure 4).
Figure 2.
Risk of chronic obstructive pulmonary disease (COPD)-related hospitalization or all-cause mortality associated with Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011 classification. Curves represent GOLD 2011 group A (A, n = 27), group B (B, n = 91), group C (C, n = 17), and group D (D, n = 191). P = 0.0007 for Kaplan-Meier log-rank test. Subjects at risk are below the x axis.
Figure 3.
Impact of baseline 6-minute-walk distance on risk of chronic obstructive pulmonary disease (COPD)-related hospitalization or all-cause mortality. Stratified as less than 300 m (n = 96), 300 to 449 m (n = 164), and 450 m or more (n = 66). P < 0.0001 for Kaplan-Meier log-rank test. Subjects at risk are below the x axis.
Figure 4.
Impact of baseline daily accelerometry steps on risk of chronic obstructive pulmonary disease (COPD)-related hospitalization or all-cause mortality. Stratified as less than 2,000 steps (n = 99), 2,000 to 3,999 steps (n = 104), and 4,000 or more steps (n = 121). P < 0.0001 for Kaplan-Meier log-rank test. Subjects at risk are below the x axis.
In the multivariable Cox models (Table 2), we found that patients in GOLD group D were more likely to experience a COPD-related hospitalization or die during follow-up compared with patients in group A (hazard ratio [HR] = 7.34 [95% confidence interval (CI), 1.00, 53.61], P = 0.050), whereas groups B and C did not differ significantly from group A (P’s > 0.257). Within this model, greater number of prior exacerbations (P = 0.005) and older age (P = 0.004) were also associated with greater likelihood of COPD-related hospitalization or all-cause mortality. When baseline 6MWD was added to this model, the association between GOLD classification and the primary outcome was attenuated (P’s > 0.203). Higher 6MWD was associated with prolonged time to first COPD-related hospitalization or all-cause mortality (HR = 0.50 per 150-m difference in 6MWD [95% CI, 0.34, 0.73], P = 0.0003). Prior exacerbations (P = 0.011) continued to be predictive in this model. Similar associations were noted when total accelerometry steps were added to the model (without 6MWD): all associations between GOLD classification and outcome events were attenuated (P’s > 0.191), whereas greater accelerometry steps was significantly associated with prolonged time to the primary outcome event (HR = 0.80 per 1,000-step difference [95% CI, 0.70, 0.92], P = 0.002). Prior exacerbations (P = 0.002) and older age (P = 0.043) remained predictive in this model.
Table 2.
Impact of Global Initiative for Chronic Obstructive Lung Disease 2011 classification, 6-minute-walk distance, and accelerometry on risk of chronic obstructive pulmonary disease–related hospitalization or all-cause mortality
| Risk Factor | Model 1: GOLD 2011 |
Model 2: GOLD + 6-Min-Walk |
Model 3: GOLD + Accelerometry |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| GOLD group D vs. A | 7.34 (1.00, 53.61) | 0.050 | 3.73 (0.49, 28.39) | 0.203 | 3.85 (0.51, 29.04) | 0.191 |
| GOLD group C vs. A | 2.316 (0.20, 24.01) | 0.530 | 2.05 (0.19, 22.82) | 0.558 | 1.75 (0.16, 19.62) | 0.649 |
| GOLD group B vs. A | 3.26 (0.42, 25.07) | 0.257 | 2.16 (0.28, 16.90) | 0.464 | 2.14 (0.28, 16.75) | 0.467 |
| Prior exacerbations | 1.39 (1.10, 1.75) | 0.005 | 1.34 (1.07, 1.69) | 0.011 | 1.44 (1.14, 1.81) | 0.002 |
| Age, per 10 yr | 1.69 (1.19, 2.39) | 0.004 | 1.38 (0.96, 2.00) | 0.086 | 1.46 (1.01, 2.10) | 0.043 |
| Charlson comorbidity index | 0.94 (0.83, 1.07) | 0.343 | 0.92 (0.81, 1.05) | 0.226 | 0.93 (0.82, 1.06) | 0.254 |
| CST vs. health education | 1.24 (0.77, 2.00) | 0.386 | 1.23 (0.75, 1.99) | 0.413 | 1.24 (0.76, 2.01) | 0.392 |
| Duke vs. Ohio State site | 1.03 (0.56, 1.90) | 0.928 | 1.07 (0.57, 2.00) | 0.831 | 1.00 (0.54, 1.86) | 0.997 |
| 6MWD, per 150 m | — | — | 0.50 (0.34, 0.73) | 0.0003 | — | — |
| Total steps, per 1,000 | — | — | — | — | 0.80 (0.70, 0.92) | 0.002 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; CI = confidence interval; COPD = chronic obstructive pulmonary disease; CST = coping skills training; GOLD = Global Initiative for Chronic Obstructive Lung Disease; HR = hazard ratio.
In a separate model, accounting for the same covariates, increases in 6MWD during the 16-week intervention period were associated with a trend toward lower risk of COPD-related hospitalization or all-cause mortality (HR = 0.83 per 30-m increase [95% CI, 0.68, 1.02], P = 0.075). Similarly, increases in total accelerometry steps over 16 weeks were associated with a trend toward lower risk of COPD-related hospitalization or all-cause mortality (HR = 0.99 per 50-step increase [95% CI, 0.98, 1.00], P = 0.068). However, the HRs associated with GOLD 2011 groups could not be estimated in these models, thus not allowing for assessment of the independent effects of these physical activity measures.
Discussion
We describe short-term changes in exercise tolerance and measured physical activity during daily life among patients with COPD by GOLD 2011 classification and the impact of baseline physical activity measures on the risk of COPD-related hospitalization and all-cause mortality. At baseline, both 6MWD and total daily physical activity differed by GOLD 2011 group: patients in group A had the greatest exercise tolerance and activity level, patients in group D had the poorest exercise tolerance and activity level, and functional status of the intermediate-risk groups B and C varied according to the symptoms axis rather than airflow obstruction axis of the combined GOLD classification. Both 6MWD and total daily physical activity declined over a 16-week period in patients in group D, whereas these measures remained relatively stable over time in the other GOLD groups. In keeping with other published studies (6, 8, 10, 11), GOLD 2011 classification was associated with the risk of combined COPD-related hospitalization and all-cause mortality. In multivariable models, both 6MWD and accelerometry were more strongly associated than GOLD 2011 classification with time to first COPD-related hospitalization or all-cause mortality.
Several previous studies have examined physical function and clinical outcomes in the context of the GOLD 2011 classification. Differences in 6MWD across GOLD 2011 groups in our cohort were similar to both COPDGene (Genetic Epidemiology of COPD) (7) and ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints) study groups (6). Total daily accelerometry step counts were comparable to those reported across strata of spirometric severity by Jehn and colleagues (21). In a large study combining two Copenhagen-based epidemiologic cohorts, Lange and colleagues (10) reported a consistent risk pattern for both COPD-related hospitalizations and all-cause mortality at 1 and 3 years, with groups A and D having the lowest and highest risk, respectively. Risk was slightly higher in group B compared with group C for each outcome. In contrast, a stepwise increase in risk of acute exacerbation from group A to D was observed in the ECLIPSE (6) and Genetics of COPD (GenKOLS) (8) studies. Compared with these cohorts, patients in INSPIRE-II exhibited greater severity of COPD, with more than half of our population in GOLD group D and less than 10% in group A. Nonetheless, the pattern of risk across GOLD 2011 groups was generally similar to these prior reports.
Prior studies have found significant variability in risk of COPD exacerbation and related hospitalization within GOLD groups C and D (6, 7, 10). In particular, risk depends on the symptom scale used and whether patients are classified into these groups on the basis of prior exacerbations, severity of airflow obstruction, or both. This difference in outcomes within groups with uniformly poor lung function highlights the importance of identifying complementary means of stratifying risk within these GOLD 2011 groups. Our results suggest that differences in physical function may help explain this variability.
The association between 6MWD and outcomes in COPD was well-documented before the introduction of the GOLD 2011 classification. 6MWD has been consistently associated with survival, in some studies more strongly than FEV1 (12–17). In ECLIPSE, a threshold 6MWD was predictive of both overall survival and risk of COPD-related hospitalization (16), although 6MWD was not independently associated with acute exacerbation risk in multivariable models including FEV1 and SGRQ score (33). The multidimensional BODE index, which incorporates 6MWD, is predictive of both mortality (18) and COPD-related hospitalization (34). In our study, 6MWD had a similar impact on risk of COPD-related hospitalization or all-cause mortality, and this relationship was independent of GOLD 2011 classification, prior exacerbation history, and comorbidities.
In contrast to 6MWD, the relationship between daily physical activity and outcomes in COPD is a relatively new area of investigation. Newly diagnosed patients with COPD walk significantly fewer steps per day than matched smoking control patients with similar 6MWD (20). Daily accelerometry step counts are inversely associated with higher GOLD 2007 stage (21) and are predictive of quality of life independent of age and FEV1 (22). In INSPIRE-II, the pattern of total daily steps across GOLD 2011 groups is consistent with that of 6MWD, with patients in group C being slightly more physically active than those in group B in spite of objectively more severe airflow obstruction. In keeping with the longitudinal trajectory of 6MWD, total accelerometry steps decreased over 16 weeks in GOLD group D while remaining stable or increasing slightly in the other groups. Taken together, these data show not only that patients in group D represent the least physically functional and active patients with COPD but also that their disease status may be worsening at a higher rate compared with other groups. Decreasing physical activity over time may also lead to deconditioning, which compounds the exertional dyspnea associated with worsening disease severity.
Prior studies have shown that both self-reported (35) and accelerometry-measured (23) physical activity predict acute exacerbations of COPD. Recently, Moy and colleagues reported that an index of daily step count and C-reactive protein or IL-6 levels was associated with frequency of acute exacerbations of COPD (36). After hospitalizations for COPD exacerbations, lower physical activity levels as measured by accelerometry are associated with higher risk of 30-day readmission in a prospective study (37). Waschki and colleagues (24) compared both accelerometry step counts and a measure of physical activity level based on estimated energy expenditure to established predictors of mortality in COPD and found both measures to be more strongly associated with mortality than FEV1, SGRQ score, and 6MWD in individual pairwise comparisons. In contrast to these prior reports, the current study provides the opportunity to evaluate the association between measured physical activity and COPD-related hospitalizations in a larger, more advanced COPD population, in the context of the most current GOLD classification, and together with other known predictors of outcomes in COPD. Our data show that both baseline 6MWD and total daily physical activity are more strongly associated than GOLD 2011 group with combined COPD-related hospitalization or all-cause mortality. This association is robust to multivariable models incorporating Charlson comorbidity index, age, and previous exacerbations.
Our results highlight the importance of functional capacity in the assessment of patients with COPD. 6MWD can be assessed serially in the clinic or pulmonary rehabilitation setting, and accelerometry provides a relatively inexpensive, nonintrusive means of objectively assessing patients’ daily physical activity level outside of a controlled clinic setting. The degree to which risk of COPD-related hospitalization or mortality can be modified through changes in functional status remains uncertain. The effect of pulmonary rehabilitation specifically on long-term outcomes has been mixed; although there has been no significant mortality benefit in most large controlled studies, the effect on hospitalizations appears more favorable (38–42). In particular, Seymour and colleagues (40) showed decreased recurrent hospitalization in a randomized trial of pulmonary rehabilitation after acute exacerbation, emphasizing the need to focus on improving functional status in patients at highest risk for hospitalization.
Several limitations of our study should be considered. The study population was predominantly white; our results would benefit from validation in more racially diverse cohorts. INSPIRE-II was not designed to assess the risk of hospitalization or mortality on the basis of the current GOLD classification. Neither the modified Medical Research Council dyspnea scale nor CAT were used in the trial; however, the SGRQ correlates strongly with the CAT (29) and was the primary symptom assessment tool used to classify patients into GOLD 2011 groups in COPDGene (7). Although prior exacerbation history was assessed at the time of enrollment in INSPIRE-II, patients were categorized using the following ranges: zero, one to two, three to five, or more than five exacerbations in the past year. This categorization prevented us from using the threshold of two or more exacerbations to help define the higher-risk GOLD categories C and D (1). However, after assigning patients with one or more previous hospitalizations for COPD to categories C and D as per the 2014 revision of the GOLD guidelines (30), there were no remaining patients in categories A or B with one or two prior exacerbations, and thus all patients were classified appropriately. The cell sizes for the GOLD B and C categories were relatively small, which may have produced less reliable estimates for these groups in the Cox models (43). However, we believed it was most appropriate to model GOLD groups categorically, as not all studies have found a stepwise increase in risk from A to D. The accelerometer used in the study has not been previously validated in patients with COPD, and accuracy of the device was not formally assessed in INSPIRE-II. Finally, although we were able to perform exploratory analyses evaluating the effect of changes in physical activity measures on risk of COPD-related hospitalization or all-cause mortality, low event rates in these models prevented us from concomitantly evaluating the effect of GOLD 2011 group. Future studies should prospectively investigate the relationships between changes in physical activity, hospitalization, and mortality risk in COPD.
In summary, we have demonstrated that when adjusted for age, comorbidities, and prior exacerbation history, both baseline 6MWD and daily physical activity measured by accelerometry were more strongly associated than GOLD 2011 classification with time to first COPD-related hospitalization or all-cause mortality. Both 6MWD and daily physical activity declined over a relatively short period of time in GOLD group D as compared with other groups. These findings suggest that physical function is an important marker of risk in COPD and that interventions directed at reducing exacerbation risk through physical activity may warrant increased attention in this population.
Footnotes
Supported by National Institutes of Health grant HL065503 (J.A.B., primary investigator) and National Institutes of Health institutional training grant HL007538 (M.T.D.). The Ohio State Clinical Research Center was supported by National Center for Advancing Translational Sciences grant UL1TR001070.
Author Contributions: M.T.D. and P.J.S., manuscript preparation, data analysis; M.A.B., manuscript review, data analysis; S.K.M., K.E.W.-W., and C.F.E., subject recruitment and data collection, manuscript review; T.M., S.M.P., and J.A.B., manuscript review, concept design.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 3.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117:5S–9S. doi: 10.1378/chest.117.2_suppl.5s. [DOI] [PubMed] [Google Scholar]
- 5.Miravitlles M, Guerrero T, Mayordomo C, Sánchez-Agudo L, Nicolau F, Segu JL.Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory patients with COPD: a multiple logistic regression analysisThe EOLO Study GroupRespiration 200067495–501. [DOI] [PubMed] [Google Scholar]
- 6.Agusti A, Edwards LD, Celli B, Macnee W, Calverley PM, Mullerova H, Lomas DA, Wouters E, Bakke P, Rennard S, et al. Characteristics, stability and outcomes of the 2011 GOLD COPD groups in the ECLIPSE cohort. Eur Respir J. 2013;42:636–646. doi: 10.1183/09031936.00195212. [DOI] [PubMed] [Google Scholar]
- 7.Han MK, Muellerova H, Curran-Everett D, Dransfield MT, Washko GR, Regan EA, Bowler RP, Beaty TH, Hokanson JE, Lynch DA, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. The Lancet Respir Med. 2013;1:43–50. doi: 10.1016/S2213-2600(12)70044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johannessen A, Nilsen RM, Storebo M, Gulsvik A, Eagan T, Bakke P. Comparison of 2011 and 2007 Global Initiative for Chronic Obstructive Lung Disease guidelines for predicting mortality and hospitalization. Am J Respir Crit Care Med. 2013;188:51–59. doi: 10.1164/rccm.201212-2276OC. [DOI] [PubMed] [Google Scholar]
- 9.Jones PW, Nadeau G, Small M, Adamek L. Characteristics of a COPD population categorised using the GOLD framework by health status and exacerbations. Respir Med. 2014;108:129–135. doi: 10.1016/j.rmed.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Lange P, Marott JL, Vestbo J, Olsen KR, Ingebrigtsen TS, Dahl M, Nordestgaard BG. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186:975–981. doi: 10.1164/rccm.201207-1299OC. [DOI] [PubMed] [Google Scholar]
- 11.Soriano JB, Alfageme I, Almagro P, Casanova C, Esteban C, Soler-Cataluna JJ, de Torres JP, Martinez-Camblor P, Miravitlles M, Celli BR, et al. Distribution and prognostic validity of the new Global Initiative for Chronic Obstructive Lung Disease grading classification. Chest. 2013;143:694–702. doi: 10.1378/chest.12-1053. [DOI] [PubMed] [Google Scholar]
- 12.Casanova C, Cote C, Marin JM, Pinto-Plata V, de Torres JP, Aguirre-Jaime A, Vassaux C, Celli BR. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest. 2008;134:746–752. doi: 10.1378/chest.08-0520. [DOI] [PubMed] [Google Scholar]
- 13.Cote CG, Pinto-Plata V, Kasprzyk K, Dordelly LJ, Celli BR. The 6-min walk distance, peak oxygen uptake, and mortality in COPD. Chest. 2007;132:1778–1785. doi: 10.1378/chest.07-2050. [DOI] [PubMed] [Google Scholar]
- 14.Martinu T, Babyak MA, O’Connell CF, Carney RM, Trulock EP, Davis RD, Blumenthal JA, Palmer SM INSPIRE Investigators. Baseline 6-min walk distance predicts survival in lung transplant candidates. Am J Transplant. 2008;8:1498–1505. doi: 10.1111/j.1600-6143.2008.02264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto-Plata VM, Cote C, Cabral H, Taylor J, Celli BR. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J. 2004;23:28–33. doi: 10.1183/09031936.03.00034603. [DOI] [PubMed] [Google Scholar]
- 16.Spruit MA, Polkey MI, Celli B, Edwards LD, Watkins ML, Pinto-Plata V, Vestbo J, Calverley PM, Tal-Singer R, Agusti A, et al. Predicting outcomes from 6-minute walk distance in chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2012;13:291–297. doi: 10.1016/j.jamda.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Cote CG, Casanova C, Marin JM, Lopez MV, Pinto-Plata V, de Oca MM, Dordelly LJ, Nekach H, Celli BR. Validation and comparison of reference equations for the 6-min walk distance test. Eur Respir J. 2008;31:571–578. doi: 10.1183/09031936.00104507. [DOI] [PubMed] [Google Scholar]
- 18.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 19.Steele BG, Holt L, Belza B, Ferris S, Lakshminaryan S, Buchner DM. Quantitating physical activity in COPD using a triaxial accelerometer. Chest. 2000;117:1359–1367. doi: 10.1378/chest.117.5.1359. [DOI] [PubMed] [Google Scholar]
- 20.Van Remoortel H, Hornikx M, Demeyer H, Langer D, Burtin C, Decramer M, Gosselink R, Janssens W, Troosters T. Daily physical activity in subjects with newly diagnosed COPD. Thorax. 2013;68:962–963. doi: 10.1136/thoraxjnl-2013-203534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jehn M, Schmidt-Trucksass A, Meyer A, Schindler C, Tamm M, Stolz D. Association of daily physical activity volume and intensity with COPD severity. Respir Med. 2011;105:1846–1852. doi: 10.1016/j.rmed.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Jehn M, Schindler C, Meyer A, Tamm M, Schmidt-Trucksass A, Stolz D. Daily walking intensity as a predictor of quality of life in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2012;44:1212–1218. doi: 10.1249/MSS.0b013e318249d8d8. [DOI] [PubMed] [Google Scholar]
- 23.Moy ML, Teylan M, Weston NA, Gagnon DR, Garshick E. Daily step count predicts acute exacerbations in a US cohort with COPD. PLoS ONE. 2013;8:e60400. doi: 10.1371/journal.pone.0060400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waschki B, Kirsten A, Holz O, Muller KC, Meyer T, Watz H, Magnussen H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140:331–342. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- 25.Blumenthal JA, Emery CF, Smith PJ, Keefe FJ, Welty-Wolf K, Mabe S, Martinu T, Johnson JJ, Babyak MA, O’Hayer VF, et al. The effects of a telehealth coping skills intervention on outcomes in chronic obstructive pulmonary disease: primary results from the INSPIRE-II study. Psychosom Med. 2014;76:581–592. doi: 10.1097/PSY.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumenthal JA, Keefe FJ, Babyak MA, Fenwick CV, Johnson JM, Stott K, Funk RK, McAdams MJ, Palmer S, Martinu T, et al. Caregiver-assisted coping skills training for patients with COPD: background, design, and methodological issues for the INSPIRE-II study. Clin Trials. 2009;6:172–184. doi: 10.1177/1740774509102565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durheim MT, Smith PJ, Babyak MA, Mabe SK, Emery CF, Blumenthal JA, Palmer SM. Physical function as measured by 6-minute walk distance or accelerometry predicts clinical outcomes in COPD patients independent of GOLD 2011 [abstract] Am J Respir Crit Care Med. 2014;189:A6679. [Google Scholar]
- 28.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 29.Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, Perez T, Soler Cataluna JJ, van der Molen T, Adamek L, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J. 2011;38:29–35. doi: 10.1183/09031936.00177210. [DOI] [PubMed] [Google Scholar]
- 30.Global Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [updated 2014; accessed 2014 Dec 10]. Available from: http://www.goldcopd.com
- 31.Dondzila CJ, Swartz AM, Miller NE, Lenz EK, Strath SJ. Accuracy of uploadable pedometers in laboratory, overground, and free-living conditions in young and older adults. Int J Behav Nutr Phys Act. 2012;9:143. doi: 10.1186/1479-5868-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ATS statement: guidelines for the 6-minute-walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 33.Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 34.Marin JM, Carrizo SJ, Casanova C, Martinez-Camblor P, Soriano JB, Agusti AG, Celli BR. Prediction of risk of COPD exacerbations by the BODE index. Respir Med. 2009;103:373–378. doi: 10.1016/j.rmed.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61:772–778. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moy ML, Teylan M, Danilack VA, Gagnon DR, Garshick E. An index of daily step count and systemic inflammation predicts clinical outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:149–157. doi: 10.1513/AnnalsATS.201307-243OC. [DOI] [PubMed] [Google Scholar]
- 37.Chawla H, Bulathsinghala C, Tejada JP, Wakefield D, ZuWallack R. Physical activity as a predictor of thirty-day hospital readmission after a discharge for a clinical exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:1203–1209. doi: 10.1513/AnnalsATS.201405-198OC. [DOI] [PubMed] [Google Scholar]
- 38.Griffiths TL, Burr ML, Campbell IA, Lewis-Jenkins V, Mullins J, Shiels K, Turner-Lawlor PJ, Payne N, Newcombe RG, Ionescu AA, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet. 2000;355:362–368. doi: 10.1016/s0140-6736(99)07042-7. [DOI] [PubMed] [Google Scholar]
- 39.Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1995;122:823–832. doi: 10.7326/0003-4819-122-11-199506010-00003. [DOI] [PubMed] [Google Scholar]
- 40.Seymour JM, Moore L, Jolley CJ, Ward K, Creasey J, Steier JS, Yung B, Man WD, Hart N, Polkey MI, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax. 2010;65:423–428. doi: 10.1136/thx.2009.124164. [DOI] [PubMed] [Google Scholar]
- 41.Ries AL, Make BJ, Lee SM, Krasna MJ, Bartels M, Crouch R, Fishman AP. National Emphysema Treatment Trial Research Group. The effects of pulmonary rehabilitation in the national emphysema treatment trial. Chest. 2005;128:3799–3809. doi: 10.1378/chest.128.6.3799. [DOI] [PubMed] [Google Scholar]
- 42.Guell R, Casan P, Belda J, Sangenis M, Morante F, Guyatt GH, Sanchis J. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest. 2000;117:976–983. doi: 10.1378/chest.117.4.976. [DOI] [PubMed] [Google Scholar]
- 43.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–1510. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]