Abstract
Rationale: Allergen sensitization is associated with asthma morbidity. A better understanding of allergen sensitization patterns among children hospitalized for asthma could help clinicians tailor care more effectively. To our knowledge, however, sensitization profiles among children hospitalized for asthma are unknown.
Objectives: We sought to describe allergen sensitization profiles and the distribution of self-reported in-home exposures among children hospitalized for asthma. We also sought to assess how sensitization profiles varied by sociodemographic and clinical factors.
Methods: This population-based cohort study includes data for 478 children, aged 4–16 years, hospitalized for an asthma exacerbation. Predictors included child age, race, sex, insurance status, reported income, salivary cotinine, exposure to traffic-related air pollution, asthma and atopic history, and season of admission. Outcomes included serum IgE specific to Alternaria alternata/A. tenuis, Aspergillus fumigatus, American cockroach, mouse epithelium, dust mite (Dermatophagoides pteronyssinus and farinae), cat dander, and dog dander (deemed sensitive if IgE ≥ 0.35). Self-reported adverse exposures included mold/mildew, water leaks, cockroaches, rodents, and cracks or holes in the walls or ceiling. Presence of carpeting and furry pets was also assessed.
Measurements and Main Results: More than 50% of included patients were sensitized to each of Alternaria, Aspergillus, dust mite, cat dander, and dog dander; 28% were sensitized to cockroach and 18% to mouse. Roughly 68% were sensitized to three or more allergens with evidence of clustering. African American children, compared with white children, were more likely to be sensitized to Alternaria, Aspergillus, cockroach, and dust mite (all P < 0.01). White children were more likely to be sensitized to mouse, cat, and dog (all P < 0.01). Lower income was associated with cockroach sensitization whereas higher income was associated with dog and cat sensitization (all P < 0.01). Atopic history was associated with sensitization to three or more allergens (P < 0.01). Although 42% reported exposure to at least one adverse in-home exposure (and 72% to carpet, 51% to furry pets), only weak relationships were seen between reported exposures and sensitizations.
Conclusions: Most children admitted to the hospital for asthma exacerbations are sensitized to multiple indoor allergens. Atopy on the inpatient unit serves as a potential target for improvement in chronic asthma management.
Keywords: asthma, allergen sensitization, environment, hospitalizations, pediatrics
Asthma, a leading cause of childhood morbidity, disproportionately impacts children of minority race and low socioeconomic status (1–6). One possible contributor to observed disparities is the link between asthma and the physical environment, mediated by allergen sensitization and exposure (7–11). Studies have demonstrated a strong connection between allergies and asthma-related health service use (12–14), symptom days (15), and suboptimal lung functioning (16). By understanding patterns of allergen sensitization, clinicians and public health officials may be more able to direct resources to those most likely to benefit.
Allergen sensitization is common among inner-city children with asthma, often as an extension or correlate of exposure to substandard housing (17–19). Skin prick testing for 14 common indoor and outdoor allergens, completed as part of the National Cooperative Inner-City Asthma Study with children aged 4–9 years recruited from emergency departments and inner-city clinics, demonstrated that 77% had at least one positive skin test and 47% had at least three (20). Others have shown that 45% of inner-city children with moderate to severe asthma are exposed to mold and 50% to cockroaches (15, 21). Routine allergy testing (22), medication changes or addition of allergen-specific immunotherapy (23, 24), and public health interventions to assess in-home allergen exposure and reduce or remediate exposures that are found all have the potential to reduce asthma morbidity among high-risk patients (25–27).
To our knowledge, however, there has been no characterization of allergen sensitization and family-reported exposures among children hospitalized with asthma. A broader understanding of allergen profiles within this high-morbidity population would be especially relevant as treatments, referrals, and postdischarge planning could be targeted to potentially remediable factors. Therefore, we sought to describe allergen sensitization profiles and self-reported indoor exposures among children hospitalized with asthma. We also sought to assess how these profiles varied by sociodemographic and clinical factors.
Methods
Study Design and Population
This work involved children hospitalized with asthma and enrolled in a population-based, prospective, observational cohort study conducted at the Cincinnati Children’s Hospital Medical Center (CCHMC, Cincinnati, OH), a large, urban, academic pediatric facility. Patients were recruited and enrolled in the Greater Cincinnati Asthma Risks Study (GCARS) between August 2010 and October 2011 according to previously described procedures (6). Briefly, patients aged 1–16 years were identified during a hospitalization by the admitting physician’s use of the evidence-based clinical pathway for acute asthma or bronchodilator-responsive wheezing. Children were excluded if they were removed from the pathway before discharge, had significant nonasthma respiratory or cardiovascular disease, resided outside of the CCHMC eight-county primary service area, or if their primary caregiver did not understand written or spoken English (approximately 2% of otherwise eligible).
Study recruitment took place, on average, 7 days/week and 12 hours/day; 774 children were successfully enrolled (62.9% of those eligible with staff available to recruit). Given that approximately 85% of child asthma hospitalizations within our primary service area occur at CCHMC facilities, the accrued sample was considered population-based (28, 29). For the study presented here, the GCARS sample was limited to children 4 years of age and older, resulting in a sample of 478 so as to allow for sensitization to inhaled allergens to occur and stabilize (30). The CCHMC Institutional Review Board approved this study.
Outcome and Predictors
The primary outcomes were sensitization to common indoor allergens, assessed using ImmunoCap (ARUP, Salt Lake City, UT), a measure of allergen-specific serum IgE. Although both serum and skin prick testing have similar diagnostic properties, serum was thought to be more appropriate to the acute, inpatient setting given skin prick testing’s potential side effect of wheeze. Also, patients had not been previously asked to withhold antihistamine use, which can interfere with skin prick results (22). Allergens tested included Alternaria alternata/A. tenuis, Aspergillus fumigatus, American cockroach, mouse epithelium, Dermatophagoides pteronyssinus, Dermatophagoides farinae, cat dander, and dog dander. Per convention, tests were considered positive when IgE was at least 0.35 kU/L.
Secondary outcomes included self-reported in-home environmental exposures. A face-to-face survey between study personnel and the patient’s caregiver included questions about household environmental conditions linked to both allergen sensitization and asthma morbidity. Caregivers were asked about adverse housing conditions: presence of mold or mildew, water leaks, cockroaches, rodents, and cracks or holes in the walls or ceiling. Caregivers were also asked about wall-to-wall carpeting and furry pets (31).
Predictors included child age, race, sex, insurance status, and reported household income. We also assessed salivary cotinine; exposure to carbon attributable to traffic (ECAT), which was used as a marker of traffic-related air pollution (32, 33); asthma and atopic history; and season of admission. Patient race was defined according to caregiver report: white/Caucasian; black/African American; Asian/Oriental; or Pacific Islander, American Indian, or Alaskan Native; or other. Ethnicity was characterized as Hispanic/non-Hispanic. For this study, racial categories were collapsed to African American, white, and multiracial/other. Children identified as Hispanic were included in the multiracial/other category. Reported income was collected as a categorical variable, ranging from less than $15,000 to $90,000 or more per year. Cotinine levels were collected via salivary sampling during the admission and defined as above or below the level of detection (34). ECAT was estimated with a land-use regression model using the home address at the time of admission (32, 33). It was dichotomized at the sample median. Caregivers were also asked whether the child was prescribed (or taking) any asthma-related medicines other than albuterol at the time of admission.
Asthma history was further characterized by caregiver report of asthma-related hospitalization, emergency or urgent care visit, or systemic steroid prescriptions in the previous year (each categorized as 0, 1, or ≥2). Asthma-related rehospitalization within 12 months of the index hospitalization was also identified. Severity of the index hospitalization was determined by the need for admission to the intensive care unit. History of atopy was assessed by asking whether the child had previously been diagnosed with allergic rhinitis or eczema. Season of index admission was also identified.
Statistical Analyses
We assessed the distribution of sociodemographic variables, salivary cotinine, ECAT, asthma history and rehospitalization, history of atopy, and season of index hospitalization. Next, we assessed the frequency with which children were sensitized to each of the eight included allergens and for the number sensitized to zero, one, two, and three or more allergens. For this aggregate measure, we treated dust mite as a single entity. Thus, if a child was sensitized to either D. pteronyssinus, D. farinae, or both, they were treated as having just one sensitization. We also assessed the frequency of self-reported adverse in-home exposures, focusing first on mold or mildew, water leaks, cockroaches, rodents, and cracks or holes, and the total number of reported adverse exposures (0, 1, 2, ≥3). Reported wall-to-wall carpet and furry pets were assessed as present or absent; however, they were not included in the aggregate measure of adverse exposures.
Bivariate analyses assessed the degree to which allergen sensitizations differed by potential predictors. Here, relationship significance was judged using either the chi square test or the Mantel–Haenszel test of trend. Analyses were performed with SAS software (Cary, NC).
We expected that sensitizations and exposures would aggregate in ways that were not random. Thus, in an attempt to more clearly depict allergen and exposure clustering, we performed a network analysis. To do this, we converted a binary (patient by variable) mode to a single mode (variable by variable) or 15 × 15 frequency matrix (including each of the 8 allergen and 7 exposure variables). In network analyses, there are multiple options for positioning the variables; here, multidimensional scaling was used to compute an x, y coordinate for each variable. The likelihood of co-occurrence was depicted by closer proximity of points. To determine how often variables co-occurred, frequency values were converted to quartiles (higher co-occurrence frequency = higher quartile). Frequency of co-occurrence was depicted by the thickness of ties between variables—thicker lines indicated higher quartile of co-occurrence. Multidimensional scaling analyses were performed with R statistical software (R Foundation for Statistical Computing, Vienna, Austria); network alyses were performed with UCINET (Analytic Technologies, Lexington, KY).
Results
A total of 478 children were included. This sample was 61% African American, 70% publicly insured, and 67% male with a median age of 8.0 years (Table 1). A total of 33% reported annual income less than $15,000. In addition, we found high rates of tobacco exposure with more than 75% having salivary cotinine above the level of detection. One-half reported being treated, at the time of hospitalization, with an asthma controller medication. Roughly 25% reported having been hospitalized in the preceding year for asthma, and nearly 18% were rehospitalized within 12 months of their index admission. Nearly 24% spent part of their index admission in the intensive care unit. A history of atopy was similarly common, with nearly 64% reporting a previous diagnosis of allergic rhinitis and 57% reporting eczema. A total of 55% had their index hospitalization in a fall season. Compared with enrolled children, those who were eligible but not enrolled did not differ with respect to age or sex. Enrolled children were, however, more likely to be African American and publicly insured. These differences were the same in the full GCARS cohort of children aged 1–16 years.
Table 1.
Sample characteristics of those enrolled in Greater Cincinnati Asthma Risks Study*
| n (or Median) | Percentage (or IQR) | |
|---|---|---|
| Age, yr | 8.0 | 4.9 |
| Race | ||
| African American | 292 | 61.2 |
| White | 149 | 31.2 |
| Other | 36 | 7.6 |
| Male sex | 321 | 67.2 |
| Insurance | ||
| Public | 334 | 70.0 |
| Private | 120 | 25.2 |
| Other | 23 | 4.8 |
| Income | ||
| <$15,000 | 158 | 33.4 |
| $15,000–$29,999 | 123 | 26.0 |
| $30,000–$59,999 | 105 | 22.2 |
| $60,000–$89,999 | 49 | 10.4 |
| ≥$90,000 | 38 | 8.0 |
| Salivary cotinine | ||
| Above level of detection | 340 | 76.6 |
| Below level of detection | 104 | 23.4 |
| Exposure to carbon attributable to traffic | ||
| Above sample median | 237 | 50.6 |
| Below sample median | 231 | 49.4 |
| Report being treated with asthma controller medication(s) | 237 | 49.6 |
| Report history of asthma-related hospitalization in last year | ||
| 0 | 355 | 74.6 |
| 1 | 74 | 15.6 |
| ≥2 | 47 | 9.9 |
| Report history of asthma-related emergency or urgent care visit in last year | ||
| 0 | 234 | 49.2 |
| 1 | 87 | 18.3 |
| ≥2 | 155 | 32.6 |
| Report history of asthma-related systemic steroid use in last year | ||
| 0 | 201 | 42.4 |
| 1 | 103 | 21.7 |
| ≥2 | 170 | 35.9 |
| Rehospitalized in 12 mo after index hospitalization | 85 | 17.8 |
| Index hospitalization included stay in intensive care unit | 112 | 23.6 |
| Atopic history | ||
| History of allergic rhinitis | 304 | 63.6 |
| History of eczema | 273 | 57.1 |
| Season of index hospitalization | ||
| Summer (2010, 2011) | 54 | 11.3 |
| Fall (2010, 2011) | 265 | 55.4 |
| Winter (2010–2011) | 56 | 11.7 |
| Spring (2011) | 103 | 21.6 |
Definition of abbreviation: IQR = interquartile range.
Enrolled children aged 4–16 years (n = 478).
Greater than 50% of children were sensitized to each of Alternaria, Aspergillus, D. pteronyssinus, D. farinae, cat dander, and dog dander (Table 2). Nearly 30% were sensitized to cockroaches while 18% were sensitized to mouse. Greater than 91% of children were sensitized to one or more of the tested allergens; 68% to three or more.
Table 2.
Allergen sensitization and reported in-home exposure frequencies for enrolled children*
| n | Percentage | |
|---|---|---|
| Allergen sensitization (IgE ≥ 0.35 kU/L) | ||
| Alternaria alternata/A. tenuis | 253 | 58.8 |
| Aspergillus fumigatus | 234 | 55.3 |
| American cockroach | 119 | 28.0 |
| Mouse epithelium | 78 | 18.2 |
| Dermatophagoides pteronyssinus | 246 | 57.5 |
| Dermatophagoides farinae | 258 | 60.0 |
| Cat dander | 233 | 54.4 |
| Dog dander | 279 | 64.7 |
| Number of allergen sensitizations† | ||
| 0 | 38 | 8.8 |
| 1 | 30 | 6.9 |
| 2 | 71 | 16.4 |
| ≥3 | 294 | 67.9 |
| Reported in-home exposures | ||
| Mold or mildew | 81 | 17.2 |
| Water leaks | 120 | 25.4 |
| Cockroaches | 58 | 12.2 |
| Rodents | 42 | 8.9 |
| Cracks or holes in the walls or ceiling | 115 | 24.5 |
| Number of reported in-home exposures | ||
| 0 | 274 | 57.7 |
| 1 | 75 | 15.8 |
| 2 | 67 | 14.1 |
| ≥3 | 59 | 12.4 |
| Reported in-home carpet | 340 | 71.7 |
| Reported in-home furry pets | 242 | 51.1 |
Enrolled children aged 4–16 years (n = 478).
For computation of cumulative number of allergen sensitizations, dust mite was considered a single entity (could be sensitized to either Dermatophagoides pteronyssinus or D. farinae and still be counted as one).
Table 2 also shows the frequency with which in-home exposures were reported. A total of 17% of respondents reported mold or mildew in their home; 12% reported the presence of cockroaches. Greater than 40% reported one or more adverse in-home exposures; 12% noted three or more. Greater than 70% reported the presence of in-home wall-to-wall carpet, and 51% furry pets.
Table 3 indicates that African American children were at significantly higher risk of sensitization to Alternaria, Aspergillus, cockroach, and dust mite (all P < 0.01). For example, 35% of African American children were sensitized to cockroach compared with 16% of white children. White children, on the other hand, were significantly more likely to be sensitized to mouse, cat, and dog (all P < 0.01). Race was not associated with likelihood of being sensitized to three or more allergens. Similar patterns were noted for insurance coverage, with those publicly insured more likely sensitized to Aspergillus and cockroach (both P < 0.05) and those privately insured more likely sensitized to cat and dog (both P < 0.05). Income was significantly associated with cockroach, cat, and dog sensitization. Children in households reporting income less than $15,000/year were much more likely to be sensitized to cockroach compared with those in households reporting income at or exceeding $90,000/year (35 vs. 9%; P < 0.01). The income gradient reversed for sensitization to cat and dog, with higher income children at significantly increased risk of sensitization (both P < 0.01).
Table 3.
Bivariate associations between allergen sensitization and selected patient characteristics
| Percentage Sensitized to Allergen as Determined by Serum
IgE ≥ 0.35 kU/L |
||||||||
|---|---|---|---|---|---|---|---|---|
| Alternaria | Aspergillus | American Cockroach | Mouse | Dust Mite (D. pter. or D. farinae) | Cat | Dog | Three or More Allergens | |
| All | 58.8 | 55.3 | 28.0 | 18.2 | 62.4 | 54.4 | 64.7 | 67.9 |
| Race | ||||||||
| African American | 66.8* | 67.8* | 34.5* | 13.4* | 68.8* | 44.3* | 59.3* | 69.8 |
| White | 45.2* | 31.1* | 16.0* | 26.9* | 51.1* | 73.1* | 75.6* | 65.2 |
| Other | 51.5* | 54.5* | 24.2* | 21.2* | 57.6* | 59.4* | 63.6* | 63.6 |
| Insurance | ||||||||
| Public | 60.4 | 59.3† | 33.7* | 16.9 | 65.8 | 49.2* | 60.9† | 68.6 |
| Private | 55.7 | 44.7† | 12.4* | 19.8 | 53.8 | 68.9* | 74.5† | 66.0 |
| Other | 52.4 | 50.0† | 25.0* | 28.6 | 57.1 | 57.1* | 71.4† | 66.7 |
| Income | ||||||||
| <$15,000 | 60.8 | 58.8 | 35.4* | 14.1 | 67.6 | 42.9* | 54.1* | 67.3 |
| $15,000–$29,999 | 58.7 | 57.8 | 30.8* | 16.8 | 60.9 | 49.1* | 64.6* | 66.4 |
| $30,000–$59,999 | 54.3 | 50.0 | 21.5* | 24.5 | 60.6 | 67.0* | 71.3* | 70.2 |
| $60,000–$89,999 | 61.9 | 50.0 | 22.0* | 19.1 | 59.5 | 71.4* | 78.6* | 71.4 |
| ≥$90,000 | 60.6 | 53.1 | 9.1* | 21.2 | 54.6 | 69.7* | 78.8* | 66.7 |
| Salivary cotinine | ||||||||
| Above LOD | 56.0 | 54.9 | 29.7 | 20.1 | 62.9 | 51.0* | 62.6 | 67.0 |
| Below LOD | 64.2 | 52.7 | 20.2 | 14.9 | 60.0 | 68.1* | 71.6 | 70.5 |
| Exposure to carbon attributable to traffic | ||||||||
| Above median | 60.6 | 56.6 | 30.9 | 20.2 | 63.0 | 52.7 | 66.4 | 70.5 |
| Below median | 56.6 | 54.3 | 26.0 | 16.6 | 62.4 | 56.4 | 63.4 | 65.7 |
| Report being on asthma controller medication(s) | ||||||||
| Yes | 64.8† | 60.1† | 32.7† | 19.9 | 66.2 | 54.3 | 64.8 | 72.0 |
| No | 52.8† | 50.5† | 23.5† | 16.6 | 59.0 | 54.9 | 65.0 | 64.2 |
| Report history of asthma-related hospitalization in last year | ||||||||
| 0 | 54.4* | 51.1* | 32.4† | 19.1 | 58.3* | 54.5 | 63.9 | 65.2 |
| 1 | 69.6* | 64.7* | 30.4† | 17.4 | 65.2* | 52.5 | 63.8 | 73.9 |
| ≥2 | 77.5* | 73.2* | 55.0† | 12.8 | 82.5* | 53.8 | 70.0 | 80.5 |
| Report history of asthma-related emergency or urgent care visit in last year | ||||||||
| 0 | 57.7 | 51.0 | 31.1 | 16.9 | 60.1 | 53.1 | 62.9 | 68.1 |
| 1 | 60.8 | 57.0 | 34.2 | 16.5 | 61.3 | 58.8 | 60.0 | 61.7 |
| ≥2 | 59.9 | 61.7 | 39.0 | 21.3 | 64.2 | 52.9 | 69.3 | 71.7 |
| Report history of asthma-related systemic steroid use in last year | ||||||||
| 0 | 55.9† | 50.8 | 29.2 | 14.0 | 58.6 | 49.5 | 59.7† | 64.5 |
| 1 | 52.1† | 52.2 | 34.0 | 20.2 | 60.6 | 53.2 | 61.7† | 64.9 |
| ≥2 | 67.4† | 63.6 | 39.7 | 22.6 | 65.5 | 60.5 | 72.3† | 74.0 |
| Rehospitalized in 12 mo after index hospitalization | ||||||||
| Yes | 64.5 | 61.8 | 42.1 | 21.3 | 64.9 | 50.7 | 62.3 | 70.5 |
| No | 57.6 | 53.9 | 32.4 | 17.5 | 61.0 | 55.0 | 65.0 | 67.6 |
| Index hospitalization included stay in intensive care unit | ||||||||
| Yes | 65.0 | 63.6 | 38.0 | 19.2 | 72.0† | 50.5 | 65.0 | 70.3 |
| No | 56.9 | 52.7 | 33.2 | 18.0 | 58.5† | 55.2 | 64.3 | 67.5 |
| History of allergic rhinitis | ||||||||
| Yes | 64.6* | 61.7* | 32.5* | 20.9† | 68.3* | 62.6* | 73.5* | 77.0* |
| No | 49.7* | 46.9* | 17.7* | 11.9† | 52.8* | 39.4* | 49.3* | 52.4* |
| History of eczema | ||||||||
| Yes | 61.6 | 62.7* | 32.8† | 19.1 | 66.3† | 54.6 | 66.7 | 71.3 |
| No | 54.6 | 46.2* | 21.8† | 16.9 | 56.8† | 54.1 | 62.8 | 63.0 |
| Season of index hospitalization | ||||||||
| Summer (2010, 2011) | 53.2* | 35.7* | 32.6 | 23.4 | 53.2 | 53.2 | 68.1 | 60.4 |
| Fall (2010, 2011) | 67.4* | 64.6* | 26.7 | 15.3 | 65.4 | 53.5 | 63.4 | 70.5 |
| Winter (2010–2011) | 46.9* | 40.8* | 31.3 | 29.2 | 67.4 | 61.2 | 77.6 | 73.5 |
| Spring (2011) | 45.7* | 47.8* | 27.5 | 17.4 | 56.5 | 53.9 | 59.8 | 62.0 |
Definition of abbreviations: D. farinae = Dermatophagoides farinae; D. pter. = Dermatophagoides pteronyssinus; LOD = limit of detection.
Note: Significance indicated by boldface.
Two-sided chi square or Mantel–Haenszel chi square (P < 0.01).
Two-sided chi square or Mantel–Haenszel chi square (P < 0.05).
Reported history of hospitalization was significantly associated with sensitization to Alternaria, Aspergillus, cockroach, and dust mites (each P < 0.05). Associations with rehospitalization trended toward but did not reach statistical significance. Similar patterns were noted for severity of hospitalization; however, just an association with sensitization to dust mite reached significance (P < 0.05). Those reporting a previous diagnosis of allergic rhinitis were more likely to be sensitized to each tested allergen (all P < 0.05); 77% of those with a history of allergic rhinitis were sensitized to three or more allergens compared with 52% without such a history (P < 0.01). Season seemed to show relevance only for Alternaria and Aspergillus, with those being hospitalized in the fall having the highest sensitization rates (both P < 0.01).
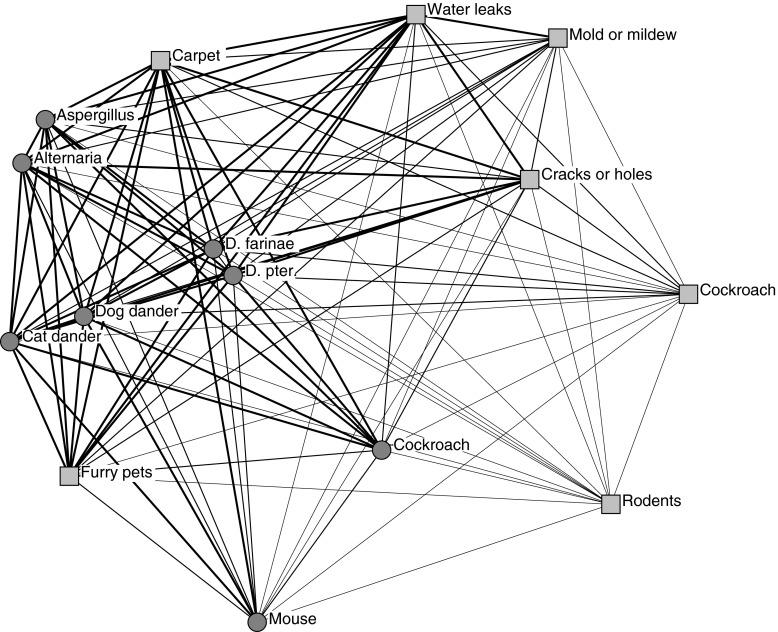
Figure 1 illustrates patterns of co-occurrence between allergen sensitizations and reported exposures. Variables (e.g., specific sensitizations) that are closer in space are more likely to have co-occurred. For example, sensitizations to Alternaria and Aspergillus are close together, as are sensitizations to D. pteronyssinus and D. farinae. This indicates that patients sensitized to one mold (or one dust mite) were more likely to also be sensitized to the other tested mold (or the other dust mite). The thickness of lines connecting different variables illustrates the frequency of co-occurrence—thicker lines illustrate relationships in higher quartiles (i.e., more frequent co-occurrence). For example, the close and thick linkages between the two molds and the two dust mites suggest that cosensitization to both occurred more frequently. Thus, the tested molds, and the two tested dust mites, had both an increased likelihood of and more frequent cosensitization. The network analysis also demonstrates that reported adverse environmental exposures (e.g., mold, cockroach) are only weakly linked to allergen sensitizations. Although reported exposure to carpet is depicted in relatively close proximity to molds and dust mites, and reported furry pets in relatively close proximity to cat and dog, other exposures all appear further away and with thinner connections to allergen sensitizations.
Figure 1.
Relationships between allergen sensitizations and reported in-home exposures depicted using network analysis. Circles represent allergen sensitizations; squares represent reported in-home exposures. Multidimensional scaling was achieved in 89 iterations and the resulting stress of 0.067 indicated a good fit. Proximity between variables illustrates likelihood of co-occurrence. Thickness of ties between variables illustrates frequency of co-occurrence. D. farinae = Dermatophagoides farinae; D. pter. = Dermatophagoides pteronyssinus.
Discussion
Indoor allergens are highly relevant to the expression of asthma morbidity. This study is the first, to our knowledge, to investigate allergen sensitization profiles and reported in-home environmental exposures in a population-based sample of children hospitalized for asthma. We found that nearly 70% of children hospitalized with asthma were sensitized to three or more common indoor allergens and that greater than 40% reported at least one adverse in-home exposure. Allergen sensitization varied significantly, and in different ways, across sociodemographic and clinical factors. However, when allergens were examined in aggregate (i.e., children with ≥3 sensitizations), such gradients were lost. A network analysis provided the reason—certain sensitizations often co-occurred, and clustering was not random. Such patterns, however, were only weakly related to reported in-home exposures. These findings suggest that in-hospital allergen testing and medical management may be warranted and that the clustering of allergens might offer insights into future tailored interventions.
We found allergen sensitization to be common among children hospitalized with asthma—91% were sensitized to one or more allergens, 68% to three or more. In a study using National Health and Nutrition Examination Survey (NHANES) data, Stevenson and colleagues demonstrated sensitization rates, measured via skin prick, lower than what we found in our inpatient asthmatic sample (17). NHANES was designed as a nationally representative sample; however, it differs from an inpatient population. The Inner City Asthma Study (ICAS) was a sample likely more similar to our own, an outpatient cohort of urban children, aged 5–11 years, with moderate to severe asthma. To be eligible for the ICAS, children needed to have had an emergency department visit or hospitalization in the preceding 6 months. These children were found to be highly sensitized to allergens such as mold, cockroach, rodent, dust mites, cat, and dog—94% had a positive skin test result to at least one of these allergens (15). Although we used serum-specific IgE instead of skin testing (22), we tested for similar allergens. Like the ICAS, our sample represented a high-risk group of patients. Unlike the ICAS, our sample included hospitalized patients, in the midst of exacerbation. The inpatient setting may be a good opportunity to pursue serum-specific IgE testing given that outpatient follow-up does not always occur.
There are verified links between allergen sensitization and exposure and both diagnosis of asthma (35) and asthma morbidity (12, 15). Although we saw trends toward associations between allergen sensitization and risk of rehospitalization, and severity of hospital course, relationships mostly did not meet statistical significance. This likely indicates that these analyses were underpowered. We did, however, find significant relationships between sensitization to molds, cockroach, and dust mites and reported history of hospitalization in the previous year. Thus, sensitization in our inpatient population may have contributed to their hospitalization. Wang and colleagues identified that children with asthma who are sensitized to environmental allergens are more likely to have increased health care and medication use, and that serum IgE and skin prick testing can serve as “markers of severe asthma for inner-city children” (12). Similarly, Matsui and colleagues showed that higher serum IgE levels corresponding to cockroach, mouse, dust mite, and cat were associated with poorer lung functioning and higher rates of exacerbations among outpatient children (36). Such findings complement our own, highlighting the potential relevance of allergen sensitization to the acute, inpatient setting.
We found significant relationships between certain allergens and sociodemographic factors; however, relationships often went in differing directions. For example, sensitization to allergens commonly associated with substandard housing (e.g., cockroaches) was more likely present for lower income patients. Alternatively, higher income individuals were more likely sensitized to cats and dogs. Similar gradients have been noted for reported environmental exposures in a cohort of asthmatic outpatients sampled from a managed care organization (37). Interestingly, we also found that mouse sensitization was more common among white children and trended toward being more common among middle and higher income individuals. This stands in potential contrast to evidence demonstrating a strong relationship between mouse sensitization and asthma morbidity among lower income, inner-city patients (38). In Baltimore, mouse sensitization and exposure have been shown to be common and consistently linked to adverse outcomes (16). It is unclear, from our data, if mouse allergy reaches the same level of significance in greater Cincinnati.
The high rate of atopy on the inpatient asthma unit warrants a reevaluation of how chronic management could be initiated or modified before discharge. Identification of allergies may impact clinical decision making. For example, allergy testing (22) may allow for more sophisticated asthma phenotyping. Studies have identified asthma phenotypes, or clusters, that are potentially amenable to more tailored medical care (39, 40). Indeed, medication changes or optimization, such as targeted addition of antihistamines, allergen-specific immunotherapy (23, 24), or omalizumab; or referrals for in-home exposure assessment and remediation have the potential to reduce future exacerbations and improve asthma control (25–27). Initiation of in-home assessments may be particularly relevant given that reported exposures often differ from what is truly found in the home (21). The inpatient population could be an apt starting point for initiation of such assessments and interventions. Such actions—both allergy testing and home remediation—warrant further cost-effectiveness analyses to determine whether they should be adopted more broadly.
The network analysis provides a novel tool with which to conceptualize and visualize relationships between allergens and may impact how clinical decisions are made. Not surprisingly, the network analysis highlighted close linkages between the two molds and between the two dust mites. We also found that sensitization to molds was more likely to co-occur, and frequently co-occurred, with sensitization to dust mites. This is consistent with demonstrated associations between the verified presence of in-home mold and dust (41). Reported exposures were only weakly related to sensitizations—again, consistent with previous work highlighting the disparity between reported and true exposure (21). For example, although the network analysis depicts reported cockroach presence in relatively close proximity to cockroach sensitization, the relationship remains weak. Reporting adverse in-home exposures such as cockroaches may be prone to social desirability bias, with respondents potentially reluctant to divulge substandard housing conditions. It is also possible that children may be exposed in more than one location (e.g., grandparent’s home, school) (42). Still, the link between sensitization and exposure is well established, and we expect our data represent an underreporting or underrecognition of true exposure. This may represent an argument for more directed allergen testing or, potentially, in-home assessments for those individuals with asthma at highest risk.
In-home assessments, or interventions directed at improving housing stock, may be an effective way to screen for and mitigate allergen exposures. Many have shown the impact environmental exposures have on asthma morbidity and the ubiquity of potentially harmful allergens (7, 43). Still, some have shown that reported exposures are rarely acted on, that interventions aimed at more detailed assessment or remediation are uncommon even though such action may improve symptoms and reduce disparities (37, 44). For example, in Lowell, Massachusetts, an in-home environmental assessment and subsequent intervention to decrease triggers led to health improvements and cost savings for a population of low-income children with asthma (45). Locally, we have found success through relationships with health department housing inspectors (27) and a medical–legal partnership (46, 47). Such interventions could play a significant role in the promotion of chronic asthma control for high-risk children.
There were limitations to this study. First, the case definition for asthma used in this study was based on physician evaluation. This leaves open the possibility that some children, particularly younger children, could have virally induced wheezing and not chronic asthma. Second, allergens tested as part of this analysis are primarily found indoors, although the tested molds may also be found outdoors. Clearly, outdoor allergens are relevant, especially during spring and fall, which were the seasons with the highest admission rates. In the future, we plan to look more specifically at outdoor allergens, such as tree and grass pollens. Third, our sample was composed of children from greater Cincinnati, thereby limiting generalizability. Sensitization and exposure patterns may, indeed, differ across geographic regions. Fourth, exposures were measured by self-report; there was no in-home inspection. This may have contributed to unreliable exposure data. Still, although environmental histories are frequently uncommon on the inpatient asthma units, in-home environmental assessments as a part of clinical care are even less common (48).
Conclusions
Nearly 70% of patients in a population-based cohort of children hospitalized with asthma were sensitized to three or more indoor allergens. Future studies will assess the impact of both indoor and outdoor allergen sensitization on asthma morbidity after discharge from the hospital, and how allergens contribute to asthma-related disparities. We also plan to explore how knowledge of inpatient allergen sensitization profiles could be translated into testable clinical and environmental interventions aimed at reducing both morbidity and disparities.
Acknowledgments
Acknowledgment
The authors thank clinical research coordinators Hadley Sauers, Emily Greenberg, Angela Howald, Elizabeth Monti, Stacey Rieck, and Heather Strong for their support and dedication.
Footnotes
Supported by NIH 1R01AI88116 (as part of the Greater Cincinnati Asthma Risks Study) (principal investigator, R.S.K.; coinvestigators, A.F.B., B.H., C.M.K., T.W.G.). Use of REDCap was supported by the Center for Clinical and Translational Science and Training (NCRR/NIH UL1-RR026314-01). A.F.B. also received funding through the Cincinnati Children’s Research Foundation Procter Scholar Award and NIH 1K23AI112916. Funders played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. This paper is subject to the NIH Public Access Policy (http://publicaccess.nih.gov).
Author Contributions: A.F.B. conceptualized and designed the study, performed the initial analysis, drafted the initial manuscript, revised the manuscripts, and approved the final manuscript as submitted. B.H. advised the initial study analysis, performed subsequent analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted. C.M.K., T.W.G., and M.B.L. advised the initial study analysis, reviewed and revised the manuscript, and approved the final manuscript as submitted. D.J.M. performed study analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted. R.S.K. conceptualized and designed the study, supervised data collection, critically reviewed and revised the manuscript, and approved the final manuscript as submitted.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123:S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 2.Hill TD, Graham LM, Divgi V. Racial disparities in pediatric asthma: a review of the literature. Curr Allergy Asthma Rep. 2011;11:85–90. doi: 10.1007/s11882-010-0159-2. [DOI] [PubMed] [Google Scholar]
- 3.Williams DR, Sternthal M, Wright RJ. Social determinants: taking the social context of asthma seriously. Pediatrics. 2009;123:S174–S184. doi: 10.1542/peds.2008-2233H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright RJ, Subramanian SV. Advancing a multilevel framework for epidemiologic research on asthma disparities. Chest. 2007;132(5, Suppl):757S–769S. doi: 10.1378/chest.07-1904. [DOI] [PubMed] [Google Scholar]
- 5.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Rep. 2011;32:1–14. [PubMed] [Google Scholar]
- 6.Beck AF, Huang B, Simmons JM, Moncrief T, Sauers HS, Chen C, Ryan PH, Newman NC, Kahn RS. Role of financial and social hardships in asthma racial disparities. Pediatrics. 2014;133:431–439. doi: 10.1542/peds.2013-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanphear BP, Aligne CA, Auinger P, Weitzman M, Byrd RS. Residential exposures associated with asthma in US children. Pediatrics. 2001;107:505–511. doi: 10.1542/peds.107.3.505. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld L, Chew GL, Rudd R, Emmons K, Acosta L, Perzanowski M, Acevedo-García D. Are building-level characteristics associated with indoor allergens in the household? J Urban Health. 2011;88:14–29. doi: 10.1007/s11524-010-9527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olmedo O, Goldstein IF, Acosta L, Divjan A, Rundle AG, Chew GL, Mellins RB, Hoepner L, Andrews H, Lopez-Pintado S, et al. Neighborhood differences in exposure and sensitization to cockroach, mouse, dust mite, cat, and dog allergens in New York City. J Allergy Clin Immunol. 2011;128:284–292.e7. doi: 10.1016/j.jaci.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rauh VA, Landrigan PJ, Claudio L. Housing and health: intersection of poverty and environmental exposures. Ann N Y Acad Sci. 2008;1136:276–288. doi: 10.1196/annals.1425.032. [DOI] [PubMed] [Google Scholar]
- 11.Kopel LS, Phipatanakul W, Gaffin JM. Social disadvantage and asthma control in children. Paediatr Respir Rev. 2014;15:256–262, quiz 262–263. doi: 10.1016/j.prrv.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Visness CM, Calatroni A, Gergen PJ, Mitchell HE, Sampson HA. Effect of environmental allergen sensitization on asthma morbidity in inner-city asthmatic children. Clin Exp Allergy. 2009;39:1381–1389. doi: 10.1111/j.1365-2222.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry T, Matsui E, Merriman B, Duong T, Eggleston P. The prevalence of rat allergen in inner-city homes and its relationship to sensitization and asthma morbidity. J Allergy Clin Immunol. 2003;112:346–352. doi: 10.1067/mai.2003.1640. [DOI] [PubMed] [Google Scholar]
- 14.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 15.Gruchalla RS, Pongracic J, Plaut M, Evans R, III, Visness CM, Walter M, Crain EF, Kattan M, Morgan WJ, Steinbach S, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Ahluwalia SK, Peng RD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, Matsui EC. Mouse allergen is the major allergen of public health relevance in Baltimore City. J Allergy Clin Immunol. 2013;132:830–835.e1–2. doi: 10.1016/j.jaci.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevenson LA, Gergen PJ, Hoover DR, Rosenstreich D, Mannino DM, Matte TD. Sociodemographic correlates of indoor allergen sensitivity among United States children. J Allergy Clin Immunol. 2001;108:747–752. doi: 10.1067/mai.2001.119410. [DOI] [PubMed] [Google Scholar]
- 18.Krieger JW, Song L, Takaro TK, Stout J. Asthma and the home environment of low-income urban children: preliminary findings from the Seattle-King County Healthy Homes Project. J Urban Health. 2000;77:50–67. doi: 10.1007/BF02350962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rauh VA, Chew GR, Garfinkel RS. Deteriorated housing contributes to high cockroach allergen levels in inner-city households. Environ Health Perspect. 2002;110:323–327. doi: 10.1289/ehp.02110s2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kattan M, Mitchell H, Eggleston P, Gergen P, Crain E, Redline S, Weiss K, Evans R, III, Kaslow R, Kercsmar C, et al. Characteristics of inner-city children with asthma: the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24:253–262. doi: 10.1002/(sici)1099-0496(199710)24:4<253::aid-ppul4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Crain EF, Walter M, O’Connor GT, Mitchell H, Gruchalla RS, Kattan M, Malindzak GS, Enright P, Evans R, III, Morgan W, et al. Home and allergic characteristics of children with asthma in seven U.S. urban communities and design of an environmental intervention: the Inner-City Asthma Study. Environ Health Perspect. 2002;110:939–945. doi: 10.1289/ehp.02110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sicherer SH, Wood RA American Academy of Pediatrics Section on Allergy and Immunology. Allergy testing in childhood: using allergen-specific IgE tests. Pediatrics. 2012;129:193–197. doi: 10.1542/peds.2011-2382. [DOI] [PubMed] [Google Scholar]
- 23.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, Gruchalla RS, Kattan M, Teach SJ, Pongracic JA, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aryan Z, Compalati E, Canonica GW, Rezaei N.Allergen-specific immunotherapy in asthmatic children: from the basis to clinical applications Expert Rev Vaccines 201312639–659.[Published erratum appears in Expert Rev Vaccines 12:972.] [DOI] [PubMed] [Google Scholar]
- 25.Labre MP, Herman EJ, Dumitru GG, Valenzuela KA, Cechman CL. Public health interventions for asthma: an umbrella review, 1990–2010. Am J Prev Med. 2012;42:403–410. doi: 10.1016/j.amepre.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Crocker DD, Kinyota S, Dumitru GG, Ligon CB, Herman EJ, Ferdinands JM, Hopkins DP, Lawrence BM, Sipe TA Task Force on Community Preventive Services. Effectiveness of home-based, multi-trigger, multicomponent interventions with an environmental focus for reducing asthma morbidity: a community guide systematic review. Am J Prev Med. 2011;41:S5–S32. doi: 10.1016/j.amepre.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Beck AF, Simmons JM, Sauers HS, Sharkey K, Alam M, Jones C, Kahn RS. Connecting at-risk inpatient asthmatics to a community-based program to reduce home environmental risks: care system redesign using quality improvement methods. Hosp Pediatr. 2013;3:326–334. doi: 10.1542/hpeds.2013-0047. [DOI] [PubMed] [Google Scholar]
- 28.Bosnjakovic E. Columbus, OH: Ohio Hospital Association; 2009. Insight database. [Google Scholar]
- 29.Szklo M. Population-based cohort studies. Epidemiol Rev. 1998;20:81–90. doi: 10.1093/oxfordjournals.epirev.a017974. [DOI] [PubMed] [Google Scholar]
- 30.Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics. 2001;108:E33. doi: 10.1542/peds.108.2.e33. [DOI] [PubMed] [Google Scholar]
- 31.Wilson J, Dixon SL, Breysse P, Jacobs D, Adamkiewicz G, Chew GL, Dearborn D, Krieger J, Sandel M, Spanier A. Housing and allergens: a pooled analysis of nine US studies. Environ Res. 2010;110:189–198. doi: 10.1016/j.envres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Newman NC, Ryan PH, Huang B, Beck AF, Sauers HS, Kahn RS. Traffic-related air pollution and asthma hospital readmission in children: a longitudinal cohort study. J Pediatr. 2014;164:1396–1402.e1. doi: 10.1016/j.jpeds.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan PH, Lemasters GK, Levin L, Burkle J, Biswas P, Hu S, Grinshpun S, Reponen T. A land-use regression model for estimating microenvironmental diesel exposure given multiple addresses from birth through childhood. Sci Total Environ. 2008;404:139–147. doi: 10.1016/j.scitotenv.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 34.Howrylak JA, Spanier AJ, Huang B, Peake RW, Kellogg MD, Sauers H, Kahn RS. Cotinine in children admitted for asthma and readmission. Pediatrics. 2014;133:e355–e362. doi: 10.1542/peds.2013-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanphear BP, Kahn RS, Berger O, Auinger P, Bortnick SM, Nahhas RW. Contribution of residential exposures to asthma in US children and adolescents. Pediatrics. 2001;107:E98. doi: 10.1542/peds.107.6.e98. [DOI] [PubMed] [Google Scholar]
- 36.Matsui EC, Sampson HA, Bahnson HT, Gruchalla RS, Pongracic JA, Teach SJ, Gergen PJ, Bloomberg GR, Chmiel JF, Liu AH, et al. Inner-City Asthma Consortium. Allergen-specific IgE as a biomarker of exposure plus sensitization in inner-city adolescents with asthma. Allergy. 2010;65:1414–1422. doi: 10.1111/j.1398-9995.2010.02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finkelstein JA, Fuhlbrigge A, Lozano P, Grant EN, Shulruff R, Arduino KE, Weiss KB. Parent-reported environmental exposures and environmental control measures for children with asthma. Arch Pediatr Adolesc Med. 2002;156:258–264. doi: 10.1001/archpedi.156.3.258. [DOI] [PubMed] [Google Scholar]
- 38.Moncrief T, Kahn R, Assa’ad A. Mouse sensitization as an independent risk factor for asthma morbidity. Ann Allergy Asthma Immunol. 2012;108:135–140. doi: 10.1016/j.anai.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Just J, Saint-Pierre P, Gouvis-Echraghi R, Laoudi Y, Roufai L, Momas I, Annesi Maesano I. Childhood allergic asthma is not a single phenotype. J Pediatr. 2014;164:815–820. doi: 10.1016/j.jpeds.2013.11.037. [DOI] [PubMed] [Google Scholar]
- 40.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, Haselkorn T. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2014;133:1549–1556. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Reponen T, Levin L, Zheng S, Vesper S, Ryan P, Grinshpun SA, LeMasters G. Family and home characteristics correlate with mold in homes. Environ Res. 2013;124:67–70. doi: 10.1016/j.envres.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moncrief T, Beck AF, Olano K, Huang B, Kahn RS. Routinely sleeping away from home and the association with child asthma readmission. J Community Health. 2014;39:1209–1215. doi: 10.1007/s10900-014-9880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leaderer BP, Belanger K, Triche E, Holford T, Gold DR, Kim Y, Jankun T, Ren P, McSharry JE, Platts-Mills TA, et al. Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in the northeastern United States: impact of socioeconomic factors and population density. Environ Health Perspect. 2002;110:419–425. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R, III, Stout J, Malindzak G, Smartt E, Plaut M, et al. Inner-City Asthma Study Group. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 45.Turcotte DA, Alker H, Chaves E, Gore R, Woskie S. Healthy homes: in-home environmental asthma intervention in a diverse urban community. Am J Public Health. 2014;104:665–671. doi: 10.2105/AJPH.2013.301695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein MD, Beck AF, Henize AW, Parrish DS, Fink EE, Kahn RS. Doctors and lawyers collaborating to HeLP children—outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24:1063–1073. doi: 10.1353/hpu.2013.0147. [DOI] [PubMed] [Google Scholar]
- 47.Beck AF, Klein MD, Schaffzin JK, Tallent V, Gillam M, Kahn RS. Identifying and treating a substandard housing cluster using a medical–legal partnership. Pediatrics. 2012;130:831–838. doi: 10.1542/peds.2012-0769. [DOI] [PubMed] [Google Scholar]
- 48.Beck AF, Sauers HS, Kahn RS, Yau C, Weiser J, Simmons JM. Improved documentation and care planning with an asthma-specific history and physical. Hosp Pediatr. 2012;2:194–201. doi: 10.1542/hpeds.2012-0016. [DOI] [PubMed] [Google Scholar]