Abstract
Rationale: A low respiratory arousal threshold is a physiological trait involved in obstructive sleep apnea (OSA) pathogenesis. Trazodone may increase arousal threshold without compromising upper airway muscles, which should improve OSA.
Objectives: We aimed to examine how trazodone alters OSA severity and arousal threshold. We hypothesized that trazodone would increase the arousal threshold and improve the apnea/hypopnea index (AHI) in selected patients with OSA.
Methods: Subjects were studied on two separate nights in a randomized crossover design. Fifteen unselected subjects with OSA (AHI ≥ 10/h) underwent a standard polysomnogram plus an epiglottic catheter to measure the arousal threshold. Subjects were studied after receiving trazodone (100 mg) and placebo, with 1 week between conditions. The arousal threshold was calculated as the nadir pressure before electrocortical arousal from approximately 20 spontaneous respiratory events selected randomly.
Measurements and Main Results: Compared with placebo, trazodone resulted in a significant reduction in AHI (38.7 vs. 28.5 events/h, P = 0.041), without worsening oxygen saturation or respiratory event duration. Trazodone was not associated with a significant change in the non-REM arousal threshold (−20.3 vs. −19.3 cm H2O, P = 0.51) compared with placebo. In subgroup analysis, responders to trazodone spent less time in N1 sleep (20.1% placebo vs. 9.0% trazodone, P = 0.052) and had an accompanying reduction in arousal index, whereas nonresponders were not observed to have a change in sleep parameters.
Conclusions: These findings suggest that trazodone could be effective therapy for patients with OSA without worsening hypoxemia. Future studies should focus on underlying mechanisms and combination therapies to eliminate OSA.
Clinical trial registered with www.clinicaltrials.gov (NCT 01817907).
Keywords: sleep-disordered breathing, obstructive sleep apnea pathogenesis, pharmacologic therapy, arousal, sedative
Obstructive sleep apnea (OSA) is a disorder characterized by repetitive pharyngeal collapse during sleep, resulting in sleep disruption (frequent arousals from sleep) and nocturnal desaturations. Patients with OSA experience comorbidities such as hypertension, diabetes, and cardiovascular disease (1–3). OSA continues to be undertreated in part because of inconsistent efficacy and variable adherence to existing therapies (4–6). As the physiological causes of OSA vary considerably between individuals (7, 8), finding alternative treatments using a more individualized therapeutic approach continues to be an important goal.
Although most patients with OSA have some anatomical predisposition to upper airway collapse, the respiratory “arousal threshold” has been identified as one of several nonanatomical factors involved in the pathogenesis of sleep apnea. During airway narrowing or closure, patients generate negative inspiratory pressure, eventually resulting in an arousal; this pressure can be measured to determine the arousal threshold. Patients with a “low” arousal threshold often wake up prematurely from respiratory events, a process that may perpetuate subsequent events (9). However, if patients were able to delay their arousal, they might have been able to restore airway patency through neuromuscular compensatory mechanisms. As such, a number of studies have attempted to manipulate pharmacologically the arousal threshold in patients with OSA using various sedatives/hypnotic agents. However, these studies have often demonstrated variable results in terms of improving OSA severity (10–14), which may be either attributed to the myorelaxant properties of some of the drugs used or that the arousal threshold was not a major factor contributing to OSA in these particular subjects (15–17) (e.g., they had highly collapsible airways). Thus, there is a need to determine the characteristics of those individuals who respond well to sedatives as well as identify which sedatives can increase the arousal threshold while preserving upper airway muscle activity.
Evidence suggests that trazodone, a commonly prescribed antidepressant with hypnotic properties, increases effectively the arousal threshold without altering upper airway muscle activity, making this medication a potentially useful candidate for treating OSA. In an animal model of OSA, trazodone (in combination with l-tryptophan) was shown to attenuate markedly the number of respiratory events in both non-REM (NREM) and REM sleep stages (18). In a group of unselected patients with OSA, Heinzer and colleagues demonstrated that trazodone increases the arousal threshold in response to hypercapnia, allowing those individuals to tolerate higher CO2 levels without arousal from sleep, but trazodone had no effect on the arousal threshold during incremental reductions in continuous positive airway pressure (CPAP) (19). Although the authors did not examine the impact of trazodone on OSA severity, the findings suggested that the effect of medication was dependent on the stimulus to arousal. More recently, in a small physiological study conducted in seven patients with OSA previously identified as having a low respiratory arousal threshold, the authors reported that the administration of trazodone increased the respiratory arousal threshold without major impairments in upper airway muscle activity (20). However, the increase in the arousal threshold did not translate into an improvement in OSA severity in this small group of patients. In contrast, a recent clinical study of eszopiclone (a nonmyorelaxant hypnotic) did show improvement in the apnea/hypopnea index (AHI) in selected patients with OSA as compared with placebo (21).
Controversy in the literature persists regarding hypnotic therapy in OSA, as prior studies have primarily used significant instrumentation and simulated respiratory events. Given this uncertainty, we sought to determine if trazodone would reduce the number of arousals and improve OSA severity via changes in arousal threshold in people with OSA under minimal instrumentation to mimic real-world conditions.
Methods
Patients
Fifteen people with OSA (9 men, 6 women) were recruited from our sleep clinic. Of the 15 recruited, 6 were currently treated with CPAP with usage documented by objective compliance meter and 5 were recruited from prior research protocols (19–21). Both CPAP-treated and -untreated subjects were recruited in an effort to make this study more clinically relevant, reflecting the usual patients who present looking for alternative therapies. All subjects had a history of OSA with an AHI greater than 10 events/h during supine NREM sleep, without known allergies to medications. Additionally, all subjects were otherwise healthy without any current smoking habits or taking any medications known to affect sleep. Written informed consent was given before participation in the study, which was approved by the Partners’ Human Research Committee.
Experimental Design and Protocol
We used a double-blinded randomized crossover study, whereby all subjects underwent two clinical polysomnograms (PSGs). Just before sleep, subjects were administered either trazodone (100 mg) or an indistinguishable placebo. Each participant was then instructed to sleep supine as much as possible throughout the duration of the night. A short 20-minute break to turn laterally was granted if necessary for patient comfort. Otherwise, they slept supine for the large majority of the night. The subjects returned for a second PSG, in which they received a placebo or trazodone 100 mg based on initial randomization.
Polysomnography
An in-laboratory clinical PSG was conducted using a standard clinical montage for evaluation of OSA, including EEGs, electrooculograms, submentalis and tibialis EMGs, thoracic and abdominal inductance plethysmography belts, finger pulse oximetry, airflow (both nasal pressure and thermocouple), snore sensor, and position. In addition, CO2 was continuously recorded from a catheter placed inside the nostril and measured with a capnograph (Vacumed, Ventura, CA). Subjects were additionally instrumented with an epiglottic pressure catheter (model MCP-500; Millar, Houston, TX) for the determination of the arousal threshold during sleep as described previously (21, 22). During this procedure, subjects were administered a nasal decongestant (0.05% oxymetazoline HCl) in each nostril. The more patent nostril was then anesthetized using 4% lidocaine HCl. The epiglottic catheter was inserted until the pressure transducer at the tip of the catheter could be seen in the back of the throat. Once confirmed that the pressure transducer was facing ventrally through the mouth, the epiglottic catheter was advanced an additional 1 to 2 cm so that it sat just caudal to the base of the tongue. To avoid displacement, the catheter was secured to the tip of the nose and cheek of the subject using tape. All signals were sampled at 125 Hz and were acquired using a 1401 digital-analog converter and Spike2 acquisition software (Cambridge Electronic Design Ltd, Cambridge, UK). A registered polysomnographic technician, blinded to the treatment condition, was responsible for the sleep staging using standard criteria and scoring respiratory events and arousals using Chicago criteria (23).
Data Analysis
The arousal threshold was defined as the average nadir epiglottic pressure just before an electrocortical arousal from 20 (or as many as were available randomly) selected arousals occurring in NREM sleep in conjunction with respiratory events (8, 21, 24, 25). Random arousals were selected by giving each arousal a sequential number, then using a random number generator to select the 20 to be included. Arousals were excluded from analysis in instances when they occurred spontaneously (i.e., not in conjunction with a respiratory event or where there was <2 cm H2O decrement in the epiglottic pressure catheter in the 30 s before arousal, as these were deemed to be spontaneous arousals) or when the accumulation of mucus/physiological interference caused artifacts on the epiglottic pressure catheter. Few arousals in stage REM sleep met these criteria; therefore, all summary data are reflective of all NREM stages of sleep.
Statistical Analysis
Either paired Student t tests or Wilcoxon signed rank tests were used to assess the effects of trazodone on the NREM arousal threshold and sleep indices. All statistics were performed using SigmaPlot (Systat Software, San Jose, CA). P ≤ 0.05 was considered significant. Normally distributed variables are presented as means ± SEM and nonnormally distributed variables are presented as median (interquartile range). Responders were defined a priori as the upper 50th percentile of subjects based on the percent change in AHI between placebo and trazodone conditions.
Results
Anthropometric Characteristics
Of the 15 participants recruited, two were excluded from the analysis because of poor/unreliable epiglottic pressure signals. The average age and body mass index for the remaining 13 subjects (6 women) was 52 ± 3 (range, 26–69) years and 31.8 ± 1.5 (range, 23.7–44.1) kg/m2.
Effect of Trazodone on Sleep Apnea Severity and Architecture
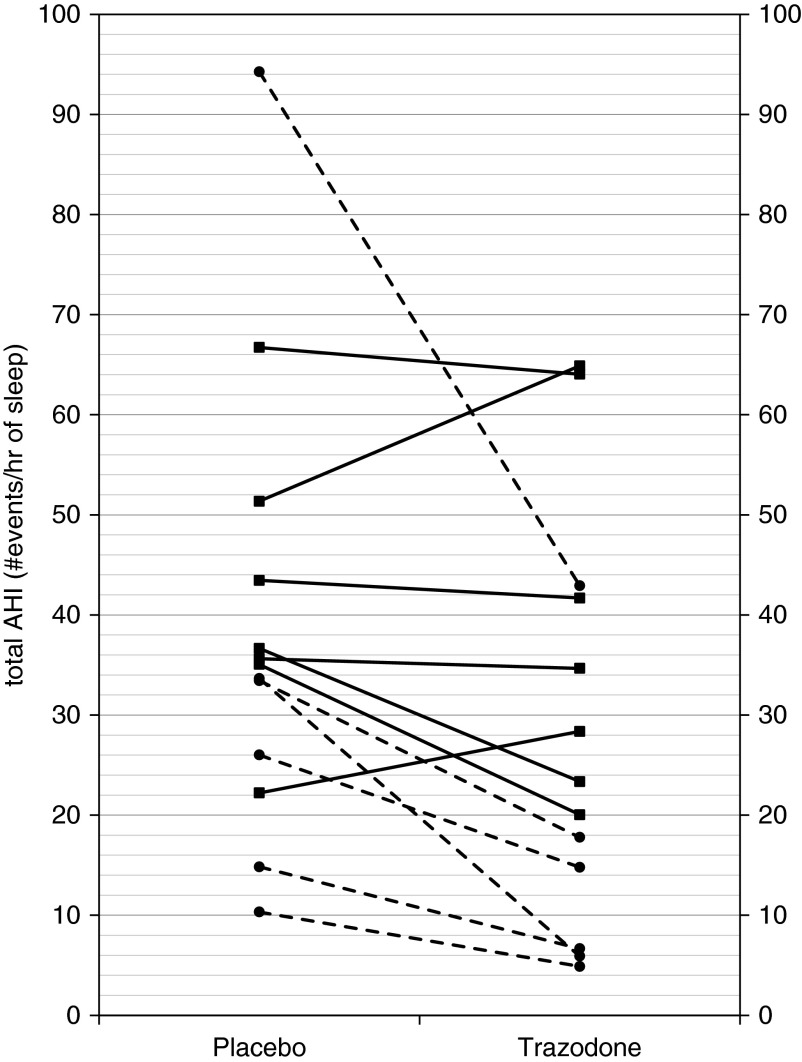
For the primary outcome, trazodone reduced the AHI when compared with placebo (38.7 ± 6.2 vs. 28.5 ± 5.6 events/h, P = 0.041; Table 1, Figure 1), without significantly worsening hypoxemia. There was one noticeable outlier when doing this analysis. This outlier was removed and the analysis repeated, showing similar findings (P = 0.05). No differences were observed in nadir oxygen saturation (78.8 ± 2.4% on placebo vs. 81.8 ± 1.8% on trazodone, P = 0.097) or time spent with saturation less than 90% (9.6 ± 3.1% vs. 8.3 ± 3.5%, P = 0.59). There was no change in the duration of respiratory events (31.9 ± 2 vs. 29.7 ± 2 s, P = 0.41). There were minimal central apneas or slow-wave sleep in either condition (<1% of total sleep time; Table 1). Compared with placebo, trazodone did not alter the total sleep time or the percent of time spent in N1, N2, or REM sleep (Table 1).
Table 1.
Sleep characteristics
| Parameter | Placebo | Trazodone |
|---|---|---|
| Total sleep time, min | 377.7 ± 17.2 | 379.4 ± 18.3 |
| Sleep efficiency, % total sleep time | 80.6 ± 3.8 | 81.4 ± 4.0 |
| Stage N1 sleep, % total sleep time | 20.5 ± 2.8 | 15.9 ± 3.2 |
| Stage N2 sleep, % total sleep time | 56.9 ± 2.2 | 61.4 ± 3.7 |
| Stage N3 sleep, % total sleep time | 0.3 (0, 8.5) | 0.9 (0, 9.8) |
| REM sleep, % total sleep time | 17.9 ± 2.7 | 17.5 ± 2.7 |
| Total AHI, events/h of sleep | 38.7 ± 6.2 | 28.5 ± 5.6* |
| NREM AHI, events/h of sleep | 39 ± 6.9 | 26.7 ± 6.6 |
| REM AHI, events/h of sleep | 40.9 ± 5.8 | 45 ± 5.8 |
| Central apnea index, events/h of sleep | 0 (0, 0.7) | 0.2 (0, 0.8) |
| Arousal index, arousals/h of sleep | 35.8 ± 4.6 | 29.2 ± 4.9 |
| Event durations, s | 31.9 ± 2 | 29.7 ± 2 |
| Sleep time with SpO2 < 90%, % | 9.6 ± 3.1 | 8.3 ± 3.5 |
| Average SaO2 nadir, % | 78.8 ± 2.4 | 81.8 ± 1.8 |
| Average SaO2, % | 93.2 ± 0.5 | 93.1 ± 0.5 |
| NREM arousal threshold, cm H2O | −20.3 ± 3.7 | −19.3 ± 3.8 |
Definition of abbreviations: AHI = apnea/hypopnea index; NREM = non-REM; SpO2 = oxygen saturation as measured by pulse oximetry.
Values are mean ± SEM or median (interquartile range).
Denotes a significant difference compared with the placebo condition (P ≤ 0.05).
Figure 1.
Apnea/hypopnea index (AHI) under conditions of placebo and trazodone for each subject. Trazadone significantly reduced total AHI (placebo, 38.7 ± 6.2 vs. trazodone, 28.5 ± 5.6; P = 0.04). Responders are indicated by a dashed line and circle marker (nonresponders are indicated by solid lines and squares).
Sleep stage–specific analysis of AHI shows that trazodone reduced the AHI when compared with placebo in NREM stage 1 sleep (Table 2; P = 0.002) but did not alter the AHI as much in NREM sleep stages 2 and 3.
Table 2.
Non-REM sleep stage–specific analysis
| AHI Placebo | AHI Trazodone | Arousal Threshold Placebo | Arousal Threshold Trazodone | ||
|---|---|---|---|---|---|
| NREM stage 1 | 66.7 ± 8.5 | 41.3 ± 9.4* | −19.3 ± 3.5 | −21.1 ± 4.0 | |
| NREM stage 2 | 29.2 ± 6.4 | 19.9 ± 5.6 | −18.2 (−32.0, −13.0) | −16.5 (−27.0, −14.0) | |
| NREM stage 3 | 5.5 (2.8, 10.0) | 1.8 (0.0, 6.8) | — | — |
Definition of abbreviations: AHI = apnea/hypopnea index; NREM = non-REM.
Values are mean ± SEM or median (interquartile range). Note: no arousal threshold measurements were obtained in NREM stage 3 because of one or a combination of the following factors: the epiglottis pressure signal did not meet measurable criteria, no respiratory events scored in NREM S3, no NREM S3 sleep was staged.
Denotes a significant difference compared with the placebo condition (P ≤ 0.05).
Effect of Trazodone on the Arousal Threshold
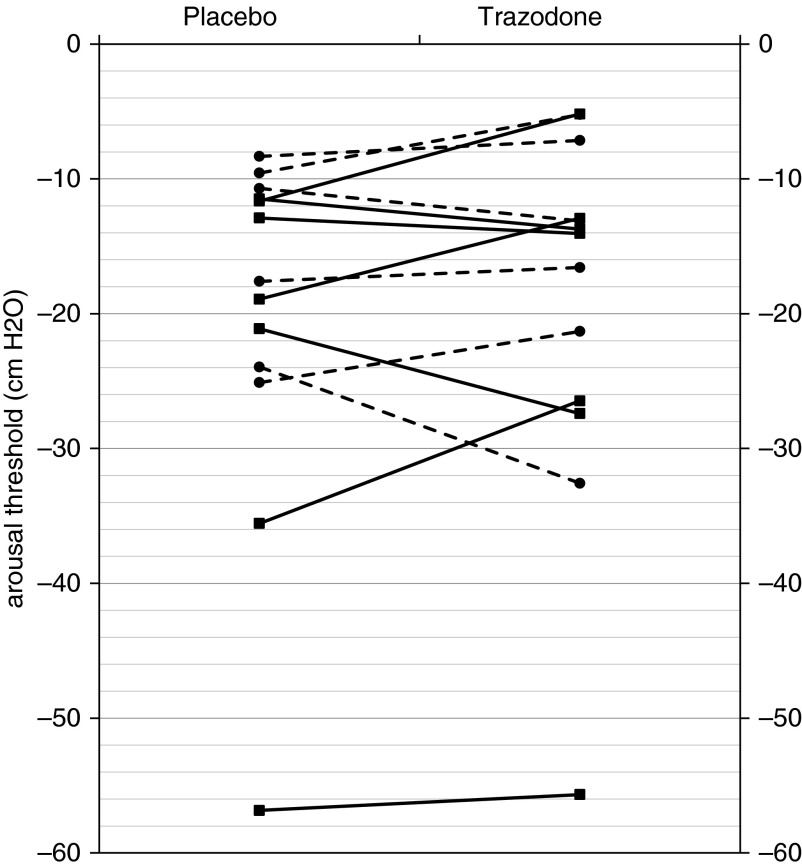
Despite the improvement in AHI, trazodone did not alter the NREM arousal threshold (−20.3 ± 3.7 vs. −19.3 ± 3.9 cm H2O, P = 0.52; Table 1). Within the placebo condition, there were a total of 94 arousal threshold measurements in N2 sleep, 101 in N1 sleep, 17 in REM sleep, and 0 in N3 sleep. Within the trazodone condition, there were a total of 68 arousal threshold measurements obtained in N2 sleep, 67 in N1 sleep, 14 in stage REM sleep, and 0 in N3 sleep.
Sleep stage–specific analysis (Table 2) shows a general increase in arousal threshold between the two conditions (placebo vs. trazodone); however, this alteration was not found to be statistically significant.
Responders to Therapy
When comparing the baseline sleep characteristics of those subjects who were classified as responders (n = 6) against nonresponders (Table 3), responders significantly reduced their time in N1 sleep on trazodone versus placebo (20.1 ± 4.3% vs. 9.01 ± 1.7%, P = 0.011; Table 3, Figure 2). The improvement in AHI was accompanied by an improvement in arousal index in responders. There was no significant difference observed in any sleep parameters in the nonresponder group between trazodone and placebo. Trazodone did not significantly alter the measured NREM arousal threshold in either the responder or nonresponder subgroups (Table 3).
Table 3.
Responder/nonresponder sleep characteristics
| Parameter | Responders (n = 6) |
Nonresponders (n = 7) |
||
|---|---|---|---|---|
| Placebo | Trazodone | Placebo | Trazodone | |
| Total sleep time, min | 346.7 ± 26.7 | 345.4 ± 32.0 | 404.3 ± 18.1 | 408.6 ± 14.4 |
| Sleep efficiency, % total sleep time | 75.1 ± 6.4 | 75.2 ± 7.4 | 85.4 ± 4.0 | 86.8 ± 3.0 |
| N1 sleep, % total sleep time | 20.1 ± 4.3 | 9.0 ± 1.7* | 20.7 ± 4.0 | 21.8 ± 4.9 |
| N2 sleep, % total sleep time | 58.9 ± 3.3 | 67.2 ± 6.4 | 55.1 ± 3.0 | 56.4 ± 3.7 |
| N3 sleep, % total sleep time | 4.4 ± 2.8 | 6.0 ± 4.3 | 5.2 ± 2.8 | 4.3 ± 2.2 |
| REM sleep, % total sleep time | 16.6 ± 4.9 | 17.7 ± 5.3 | 19.0 ± 3.2 | 17.4 ± 2.7 |
| Total AHI, events/h of sleep | 35.4 ± 12.4 | 15.5 ± 5.9* | 41.6 ± 5.4 | 39.6 ± 7.0 |
| Arousal index, arousals/h of sleep | 35.6 ± 8.5 | 19.0 ± 4.9* | 32.9 ± 3.4 | 34.3 ± 5.2 |
| Event durations, s | 33.6 ± 3.3 | 32.0 ± 3.0 | 30.4 ± 2.4 | 27.8 ± 2.7 |
| Time with SpO2 < 90%, % total sleep time | 3.1 ± 1.5 | 0.6 ± 0.5 | 15.2 ± 4.8 | 15.7 ± 4.3 |
| Average SpO2 nadir, % | 83.0 ± 2.5 | 84.3 ± 1.3 | 78 (66, 83) | 80 (73, 85) |
| Average SpO2, % | 94.0 ± 0.4 | 93.2 ± 0.3* | 92.5 ± 0.7 | 93.1 ± 1.0 |
| NREM arousal threshold, cm H2O | −15.9 ± 3.0 | −16.0 ± 4.1 | −24.1 ± 6.3 | −22.2 ± 6.3 |
Definition of abbreviations: AHI = apnea/hypopnea index; NREM = non-REM; SpO2 = oxygen saturation as measured by pulse oximetry.
Values are mean ± SEM or median (interquartile range).
Denotes a significant difference compared with the equivalent placebo condition (P < 0.05).
Figure 2.
Arousal threshold under conditions of placebo and trazodone for each subject. Trazodone did not alter the arousal threshold in non-REM sleep (−20.3 ± 3.7 vs. −19.3 ± 3.9 cm H2O; P = 0.52). Responders are indicated by a dashed line and circle marker (nonresponders are indicated by solid lines and squares).
Discussion
The major findings of our crossover physiological study suggest that 100 mg of trazodone administered just before sleep significantly improves AHI but was not found to cause significant alterations in the respiratory arousal threshold in an unselected group of patients with OSA. Trazodone appears to promote stable breathing primarily by mediating a decrease in N1 sleep. More research is warranted to determine conclusively the mechanisms by which trazodone improves OSA severity and identify better the physiological characteristics of people most likely to benefit from this pharmacological intervention.
The use of pharmacological interventions to treat OSA has been investigated to some extent; however, results have varied among people with OSA (10–14, 25–28). We believe that OSA improvement with some of these sedatives may vary due to the myorelaxant properties of these medications, which consequently resulted in increased upper airway resistance. In addition, the mechanism underlying apnea is known to vary across individuals, and thus results would be predicted to be different depending on the population studied (e.g., competing effects on upper airway muscles vs. arousal threshold). We chose to use trazodone because it is a commonly used generic antidepressant with hypnotic properties, is inexpensive, and lacks an effect on the pharyngeal dilator muscles in the dosage used (19, 20).
Studies have shown that breathing stability is related to airway patency in people with OSA, and several studies have been conducted investigating the role of the genioglossus muscle and other upper airway dilator muscles. Based on this research, increased activity of the pharyngeal dilator muscles is an important element to maintaining pharyngeal patency (29–31). If one could increase the arousal threshold with a medication like trazodone, it may be possible that the subject will stay asleep long enough to accumulate respiratory stimuli sufficient to recruit these upper airway dilator muscles and experience stable breathing with pharyngeal patency.
Because prior studies have shown an increase in arousal threshold with trazodone therapy, the lack of observed change in our study is worthy of discussion. The reason for this discrepancy is unclear but may reflect the samples studied (e.g., baseline arousal threshold and sleep stage distributions). Trazodone may be leading to changes in sleep architecture rather than affecting the arousal threshold importantly within a particular stage. This phenomenon of “stage migration” may account for prior benefits of eszopiclone and other agents in the literature (21). Another possibility is that the degree of instrumentation during the recordings might impact how much trazodone affects arousal threshold (e.g., perhaps heavily instrumented individuals with intramuscular genioglossus electrodes are prone to awakenings and thus more amenable to manipulation) (20). The type of stimulus provided to induce arousal may also have an impact, given that prior reports suggested differential effects of trazodone on arousal induced by chemoreceptive rather than mechanoreceptive stimuli (19). As the sample size in many of these studies is quite modest, we believe larger studies will be required to draw definitive conclusions.
Given that our results are not entirely as predicted a priori, we believe that some speculation is warranted regarding the impact of trazodone on OSA severity. First, trazodone may lead to reduced arousals, which themselves may be destabilizing for control of breathing (9, 32). The ventilatory response to arousal may lead to marked CO2 fluctuations, which can lead to obstructive or central apnea depending on the prevailing pharyngeal mechanics. Second, trazodone may be allowing the accumulation of respiratory stimuli not by changing the arousal threshold in N2 sleep per se but by reducing N1 sleep in which arousals commonly occur. Thus, the effects of trazodone could vary with the underlying sleep architecture of the individual but may still serve its intended purpose without changing the measured arousal threshold. Third, trazodone has complex neuropharmacology, including influences on histaminergic, noradrenergic, and serotonergic systems. In theory, trazodone could have central neurochemical effects on hypoglossal motor output, which could serve to stabilize upper airway mechanics independent of arousal pathways (18, 33). However, the complexity and variability of the physiology underlying OSA precludes our drawing definitive conclusions regarding which, if any, of the above mechanisms are predominating in the present study. Nonetheless, the present clinical study was designed to minimize instrumentation rather than assess mechanism, and thus our new findings likely justify further research in this area.
Limitations
The main limitations of this study include small sample size and lack of patient-oriented outcomes showing the magnitude of the observed AHI improvement had clinical relevance. Although prior studies have shown improvements in endothelial function and other markers of vascular risk with modest AHI improvements (34), we would view our findings as physiologically interesting but not clinically directive. Further work will be required to determine sources of heterogeneity such as prior CPAP therapy and mechanism underlying apnea (e.g., low versus high baseline arousal threshold). Nonetheless, trazodone may well be a viable therapeutic approach for a subset of individuals either in combination with other interventions (e.g., oxygen or positional therapy) or for patients in whom recurrent arousals are particularly problematic (e.g., those with insomnia plus OSA). Despite these limitations, we believe that our findings are of interest and bring us one step closer to pharmacotherapy for OSA.
Summary
In summary, trazodone modestly improved OSA without apparently prolonging events or worsening hypoxemia. The effect of trazodone on OSA might allow for more individualized therapies and new treatment opportunities for a particular group of patients with OSA who are not hypoxemic for large portions of the night. Future studies should focus on underlying mechanisms as well as determining which subset of patients with OSA might respond to pharmacotherapy with nonmyorelaxant hypnotic therapy, perhaps in combination with other therapeutic approaches.
Acknowledgments
Acknowledgment
The authors thank Ms. Lauren Hess and Ms. Alison Foster for their laboratory assistance.
Footnotes
Supported by the National Institutes of Health grants K24 HL 093,218 (A.M.) and 1 P01 HL 095,491 (A.M.), the Harvard Catalyst Clinical Research Center grant UL1 RR 025758–01, and the National Health and Medical Research Council of Australia’s CJ Martin Overseas Biomedical Fellowship 1035115 (B.A.E.).
Author Contributions: A.M., B.A.E., and A.W. participated in the experimental design of this project. E.T.S., B.A.E., D.G.M., and A.V. contributed to the data acquisition process. E.T.S., B.A.E., P.N.D., D.G.M., and A.M. analyzed and interpreted the data. E.T.S., B.A.E., R.O., J.E.O., and A.M. prepared the manuscript and were involved in the critical review and editing process.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 2.Terán-Santos J, Jiménez-Gómez A, Cordero-Guevara J Cooperative Group Burgos-Santander. The association between sleep apnea and the risk of traffic accidents. N Engl J Med. 1999;340:847–851. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 4.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 5.Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, Redline S, Henry JN, Getsy JE, Dinges DF. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 6.Engleman HM, McDonald JP, Graham D, Lello GE, Kingshott RN, Coleman EL, Mackay TW, Douglas NJ. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med. 2002;166:855–859. doi: 10.1164/rccm.2109023. [DOI] [PubMed] [Google Scholar]
- 7.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 8.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–153. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 10.Höijer U, Hedner J, Ejnell H, Grunstein R, Odelberg E, Elam M. Nitrazepam in patients with sleep apnoea: a double-blind placebo-controlled study. Eur Respir J. 1994;7:2011–2015. [PubMed] [Google Scholar]
- 11.Rosenberg R, Roach JM, Scharf M, Amato DA. A pilot study evaluating acute use of eszopiclone in patients with mild to moderate obstructive sleep apnea syndrome. Sleep Med. 2007;8:464–470. doi: 10.1016/j.sleep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Berry RB, Kouchi K, Bower J, Prosise G, Light RW. Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:450–454. doi: 10.1164/ajrccm.151.2.7842205. [DOI] [PubMed] [Google Scholar]
- 13.Lofaso F, Goldenberg F, Thebault C, Janus C, Harf A. Effect of zopiclone on sleep, night-time ventilation, and daytime vigilance in upper airway resistance syndrome. Eur Respir J. 1997;10:2573–2577. doi: 10.1183/09031936.97.10112572. [DOI] [PubMed] [Google Scholar]
- 14.Lu B, Budhiraja R, Parthasarathy S. Sedating medications and undiagnosed obstructive sleep apnea: physician determinants and patient consequences. J Clin Sleep Med. 2005;1:367–371. [PubMed] [Google Scholar]
- 15.Krol RC, Knuth SL, Bartlett D., Jr Selective reduction of genioglossal muscle activity by alcohol in normal human subjects. Am Rev Respir Dis. 1984;129:247–250. [PubMed] [Google Scholar]
- 16.Bonora M, St John WM, Bledsoe TA. Differential elevation by protriptyline and depression by diazepam of upper airway respiratory motor activity. Am Rev Respir Dis. 1985;131:41–45. doi: 10.1164/arrd.1985.131.1.41. [DOI] [PubMed] [Google Scholar]
- 17.Leiter JC, Knuth SL, Krol RC, Bartlett D., Jr The effect of diazepam on genioglossal muscle activity in normal human subjects. Am Rev Respir Dis. 1985;132:216–219. doi: 10.1164/arrd.1985.132.2.216. [DOI] [PubMed] [Google Scholar]
- 18.Veasey SC, Fenik P, Panckeri K, Pack AI, Hendricks JC. The effects of trazodone with L-tryptophan on sleep-disordered breathing in the English bulldog. Am J Respir Crit Care Med. 1999;160:1659–1667. doi: 10.1164/ajrccm.160.5.9812007. [DOI] [PubMed] [Google Scholar]
- 19.Heinzer RC, White DP, Jordan AS, Lo YL, Dover L, Stevenson K, Malhotra A. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J. 2008;31:1308–1312. doi: 10.1183/09031936.00067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckert DJ, Malhotra A, Wellman A, White DP. Trazodone increases the respiratory arousal threshold in obstructive sleep apnea patients with a low arousal threshold. Sleep. 2014;37:811–819. doi: 10.5665/sleep.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckert DJ, Owens RL, Kehlmann GB, Wellman A, Rahangdale S, Yim-Yeh S, White DP, Malhotra A. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120:505–514. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest. 2009;135:957–964. doi: 10.1378/chest.08-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep medicine; 2007. [Google Scholar]
- 24.Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis. 1990;142:295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- 25.Berry RB, McCasland CR, Light RW. The effect of triazolam on the arousal response to airway occlusion during sleep in normal subjects. Am Rev Respir Dis. 1992;146:1256–1260. doi: 10.1164/ajrccm/146.5_Pt_1.1256. [DOI] [PubMed] [Google Scholar]
- 26.Berry RB, Bonnet MH, Light RW. Effect of ethanol on the arousal response to airway occlusion during sleep in normal subjects. Am Rev Respir Dis. 1992;145:445–452. doi: 10.1164/ajrccm/145.2_Pt_1.445. [DOI] [PubMed] [Google Scholar]
- 27.Hedemark LL, Kronenberg RS. Flurazepam attenuates the arousal response to CO2 during sleep in normal subjects. Am Rev Respir Dis. 1983;128:980–983. doi: 10.1164/arrd.1983.128.6.980. [DOI] [PubMed] [Google Scholar]
- 28.Eikermann M, Eckert DJ, Chamberlin NL, Jordan AS, Zaremba S, Smith S, Rosow C, Malhotra A. Effects of pentobarbital on upper airway patency during sleep. Eur Respir J. 2010;36:569–576. doi: 10.1183/09031936.00153809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan AS, White DP, Lo YL, Wellman A, Eckert DJ, Yim-Yeh S, Eikermann M, Smith SA, Stevenson KE, Malhotra A. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009;32:361–368. doi: 10.1093/sleep/32.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordan AS, Wellman A, Heinzer RC, Lo YL, Schory K, Dover L, Gautam S, Malhotra A, White DP. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax. 2007;62:861–867. doi: 10.1136/thx.2006.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards BA, Connolly JG, Campana LM, Sands SA, Trinder JA, White DP, Wellman A, Malhotra A. Acetazolamide attenuates the ventilatory response to arousal in patients with obstructive sleep apnea. Sleep. 2013;36:281–285. doi: 10.5665/sleep.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horner RL, Hughes SW, Malhotra A. State-dependent and reflex drives to the upper airway: basic physiology with clinical implications. J Appl Physiol (1985) 2014;116:325–336. doi: 10.1152/japplphysiol.00531.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itzhaki S, Dorchin H, Clark G, Lavie L, Lavie P, Pillar G. The effects of 1-year treatment with a herbst mandibular advancement splint on obstructive sleep apnea, oxidative stress, and endothelial function. Chest. 2007;131:740–749. doi: 10.1378/chest.06-0965. [DOI] [PubMed] [Google Scholar]