Abstract
Rationale: Pulmonary nontuberculous mycobacteria (NTM) disease is a chronic, nonreportable illness, making it difficult to monitor. Although recent studies suggest an increasing prevalence of NTM disease in the United States, the incidence and temporal trends are unknown.
Objectives: To describe incident cases and calculate the incidence and temporal trends of pulmonary NTM disease in Oregon.
Methods: We contacted all laboratories performing mycobacterial cultures on Oregon residents and collected demographic and specimen information for patients with NTM isolated during 2007 to 2012. We defined a case of pulmonary NTM disease using the 2007 American Thoracic Society/Infectious Disease Society of America microbiologic criteria. We used similar state-wide mycobacterial laboratory data from 2005 to 2006 to exclude prevalent cases from our calculations. We calculated annual pulmonary NTM disease incidence within Oregon during 2007 to 2012, described cases demographically and microbiologically, and evaluated incidence trends over time using a Poisson model.
Measurements and Main Results: We identified 1,146 incident pulmonary NTM cases in Oregon residents from 2007 to 2012. The median age was 69 years (range, 0.9–97 yr). Cases were more likely female (56%), but among patients less than 60 years old, disease was more common in male subjects (54%). Most (86%) were Mycobacterium avium/intracellulare cases; 68 (6%) were Mycobacterium abscessus/chelonae cases. Although not statistically significant, incidence increased from 4.8/100,000 in 2007 to 5.6/100,000 in 2012 (P for trend, 0.21). Incidence increased with age, to more than 25/100,000 in patients 80 years of age or older.
Conclusions: This is the first population-based estimate of pulmonary NTM disease incidence in a region within the United States. In Oregon, disease incidence rose slightly during 2007 to 2012, and although more common in female individuals overall, disease was more common among male individuals less than 60 years of age.
Keywords: nontuberculous mycobacteria, Mycobacterium avium complex, Mycobacterium chelonae, mycobacterial infections, nontuberculous
Nontuberculous mycobacteria (NTM) are ubiquitous environmental organisms that cause chronic lung infection. To date, pulmonary NTM disease has not been reportable in the United States, making estimates of disease incidence and trends in incidence difficult to determine. Our previous statewide laboratory surveillance project in Oregon showed that 75% of all NTM disease is pulmonary and found the annualized prevalence of pulmonary NTM disease to be 5.6/100,000 in 2005 to 2006 (1). Other investigators examining long-term disease prevalence trends concluded that the prevalence increased 2 to 6% per year in Ontario, Canada from 1998 to 2010 and the Western United States from 1994 to 2006 (2, 3). Pulmonary NTM disease is chronic, so prevalence estimates will overestimate the incidence of new cases by an unknown margin. To date, no studies have reported the population-based incidence of NTM disease.
The current American Thoracic Society (ATS)/Infectious Disease Society of America (IDSA) clinical case definition for pulmonary NTM disease includes a combination of symptoms, characteristic radiographic findings, and microbiological criteria (4). Because NTM diagnosis requires a positive laboratory culture for diagnosis, laboratory-based surveillance is a valuable tool for measuring the incidence of this increasingly important disease. Our prior work validated the microbiologic criteria alone as having high positive predictive value (86%) for patients with pulmonary disease, suggesting that laboratory culture data could be used for surveillance purposes (5). Accordingly, within a special public health surveillance project, we used Oregon statewide laboratory data from 2007 to 2012 to estimate pulmonary disease incidence and its trends over time as well as to describe the demographic and microbiologic characteristics of incident disease in population-based setting. Preliminary results from this study were previously reported in the form of an abstract (6).
Methods
We contacted all laboratories processing mycobacterial specimens from Oregon residents (N = 17): 13 labs performing acid-fast bacillus culture in Oregon and 4 out-of-state reference microbiology laboratories that process Oregon patient samples. For positive NTM culture results from January 1, 2007 through December 31, 2012 we requested: patient name, date of birth or age, address or county and zip code of residence, species isolated, date of collection, and specimen site of isolation from these laboratories. We considered Mycobacterium gordonae species to be a contaminant and excluded these reports from analysis. Isolates from an unknown source were also excluded.
NTM Case Definition
We defined pulmonary NTM cases using the microbiological criteria of the 2007 ATS/IDSA guidelines (4). Pulmonary cases had a single NTM isolate from bronchoscopy, bronchoalveolar lavage, lung tissue, or pleural fluid, or two positive sputum or unspecified respiratory cultures. Noncases had a single positive sputum culture for a distinct species. We reported Mycobacterium chelonae when identified; otherwise we combined Mycobacterium abscessus/chelonae and M. abscessus into M. abscessus/chelonae complex. Mycobacterium fortuitum complex includes all M. fortuitum and M. fortuitum/chelonae. Cases were considered incident in the year they met the case definition. Patients meeting case criteria within 2005 to 2006 were excluded from incidence calculations using 2007 to 2012 data.
Statistical Analyses
We described the age, sex, and species characteristics of incident cases and noncases. We calculated the overall annual incidence (number of new cases in a given year divided by the midyear population) for 2007 to 2012, using all reports from 2005 to 2012. The overall and age-, sex-, and county-specific population of Oregon from 2007 to 2012 were downloaded from the Portland State University Population Research Center (7). We evaluated trends and calculated age- and sex-specific incidence. We also calculated an incidence rate age-adjusted to the 2000 Standard U.S. population (8). To directly compare with our results from 2005 to 2006, where we were only able to calculate a prevalence measure based on 2 years of data, we calculated a similar measure using data from 2011 to 2012 only and ignored all data before the time period. For geographical incidence estimates, patients with zip codes were assigned to a county, and those missing county or zip code were assigned the county of the clinic where they were cultured. One laboratory in Central Oregon was unable to provide an address, county, or state of residence for patient isolates, but this laboratory is located more than 120 miles from any state border, and we assumed that all such isolates came from Oregon residents and assigned them the zip code of the laboratory. The state is divided into two geographically distinct regions, with the Cascade Mountains running north to south and separating a more humid western half of the state with a high and arid eastern half. Incidence rates were calculated for each region, for comparison to our previous results (1, 9).
All data were imported into SAS 9.3 (SAS Institute Inc., Cary, NC) for analysis. We compared demographics between cases and noncases using the Wilcoxon rank sum test (age) and Chi-square test (sex). Standard 95% confidence intervals (CIs) were calculated for incidence, and a Poisson model was fit using a log link for the temporal trend analysis. The study was considered public health practice by the Oregon Health Authority and conducted under OAR 333-019-0005.
Results
Laboratory Reporting
All 17 laboratories were able to provide results from 2007 to 2012. We identified 2,344 patients with 3,962 positive NTM cultures over the 6-year period. Of the 3,962 results, 100 (2.5%) had an unknown or excluded specimen source, and 507 (12.8%) were extrapulmonary, leaving 3,355 (84.7%) pulmonary culture results to evaluate. We received up to 25 positive culture results per patient, although 1,611 (68%) had only one positive. Two laboratories were missing data from 2007 and 2008. One reported a single positive result during 2010 to 2012 (corresponding to 0 cases), and the other laboratory accounted for only 2.8 to 3.9% of samples and 3.7 to 4.5% of total pulmonary cases 2010 to 2012.
Case Identification and Demographics
We identified 1,146 incident pulmonary cases. The median age of cases was 69 years (range 0.9–97 yr), and overall female cases accounted for 56% of all cases (Table 1). Among the 1,146 cases, 660 (57.6%) were identified from a pulmonary bronchoalveolar lavage sample; 486 (42.4%) cases were identified from two sputum samples. The two sputum samples used to meet case criteria were taken a median of 8 days apart (range, 0–1,647 d), and 21 cases (1.8%) had positive isolates collected more than 2 years apart. Eight patients did not meet the pulmonary case definition despite two positive sputum cultures because they had two different species identified. An additional 798 patients had 806 species-specific single positive sputum cultures during the study period and did not meet the case definition. These patients were younger (median age, 66 yr) and less likely to be female (50%) compared with cases (Table 1). We had no access to information regarding negative cultures, and it is unknown whether such patients had further microbiologic evaluation.
Table 1.
Patient characteristics and species of mycobacteria associated with incident pulmonary nontuberculous Mycobacterium cases (N = 1,146) and noncases (N = 806), Oregon 2007 to 2012
| Incident Cases* (N = 1,146) | Noncases* (N = 806) | P Value | |
|---|---|---|---|
| Age, median (range), yr | 69 (0–97) | 66 (4–95) | 0.002 |
| Female sex, % | 55.7 | 49.6 | 0.02 |
| Mycobacterium species | |||
| Mycobacterium avium/intracellulare complex | 982 (85.7) | 667 (82.8) | |
| Mycobacterium abscessus chelonae complex† | 68 (5.9) | 33 (4.1) | |
| Mycobacterium chelonae | 14 (1.2) | 6 (0.7) | |
| Mycobacterium kansasii | 14 (1.2) | 6 (0.7) | |
| Mycobacterium lentiflavum | 12 (1.0) | 8 (1.0) | |
| Mycobacterium fortuitum complex† | 10 (0.9) | 23 (2.9) | |
| Mycobacterium xenopi | 3 (0.3) | 2 (0.2) | |
| Mycobacterium immunogenum | 2 (0.2) | 3 (0.4) | |
| Mycobacterium kumamotonense | 2 (0.2) | 1 (0.1) | |
| Mycobacterium paraffinicum | 2 (0.2) | 1 (0.1) | |
| Mycobacterium nebraskense | 1 (0.1) | 4 (0.5) | |
| Mycobacterium arupense | 1 (0.1) | 2 (0.2) | |
| Mycobacterium mucogenicum/phocaicum | 1 (0.1) | 2 (0.2) | |
| Mycobacterium porcinum complex | — | 2 (0.2) | |
| Other‡ | 35 (3.1) | 46 (5.7) |
Definition of abbreviations: ATS = American Thoracic Society; IDSA = Infectious Disease Society of America; NTM = nontuberculous mycobacteria.
Results are no. (%) unless otherwise noted. P value for age calculate using Wilcoxon rank sum test and sex using Chi-square test.
Cases meeting 2007 ATS/IDSA laboratory case definition: NTM detected in two sputum samples (same species) or one of any other lung or pleural fluid; noncases had a single positive sputum culture. Patients may contribute multiple case/noncase episodes for different species.
M. chelonae/abscessus complex is all M. abscessus and M. chelonae/abscessus; M. fortuitum complex is all M. fortuitum and M. fortuitum/chelonae.
Other species associated with NTM cases: 26 “Mycobacterium species” not identified and one case each of Mycobacterium chlorophenolicum, Mycobacterium flavescens, Mycobacterium frederiksbergense, Mycobacterium holsaticum, Mycobacterium kubicae, Mycobacterium nonchromogenicum, Mycobacterium parascrofulaceum, Mycobacterium terrae complex, Mycobacterium timonense; among noncases other species include 38 “Mycobacterium species” and one each of Mycobacterium alvei, Mycobacterium bohemicum, Mycobacterium heckeshornense, Mycobacterium interjectum, Mycobacterium mageritense, Mycobacterium malmoense, Mycobacterium septicum, and M. terrae complex.
Mycobacterial Species
M. avium/intracellulare complex (MAI) species were the most common isolates among patients who met NTM case criteria (Table 1) and were associated with 85.7% of cases. Rapid growers (M. abscessus/chelonae complex, M. fortuitum complex, M. chelonae) caused 8.1% of pulmonary cases. Twenty-four (2.1%) patients met the case definition for two NTM species. Of these 24 dual-infected cases, 22 had MAI, with 15 (62.5%) also infected with M. abscessus/chelonae complex, 3 (12.5%) also infected with M. chelonae, and 4 also infected with other species (16.7%): Mycobacterium lentiflavum, Mycobacterium timonense, M. fortuitum, and Mycobacterium xenopi. The other two were dually infected with Mycobacterium nonchromogenicum/M. fortuitum and M. abscessus/M. lentiflavum. The diversity of species identified included 10 species associated with at least two cases and 11 additional species associated with one case each. Among the noncases with a single sputum culture (Table 1), 82.8% were MAI, 7.7% were rapid growers, and the following additional species were each isolated from a single patient: Mycobacterium bohemicum, Mycobacterium mageritense, Mycobacterium malmoense, Mycobacterium porcinum complex, and Mycobacterium septicum.
Incidence over Time
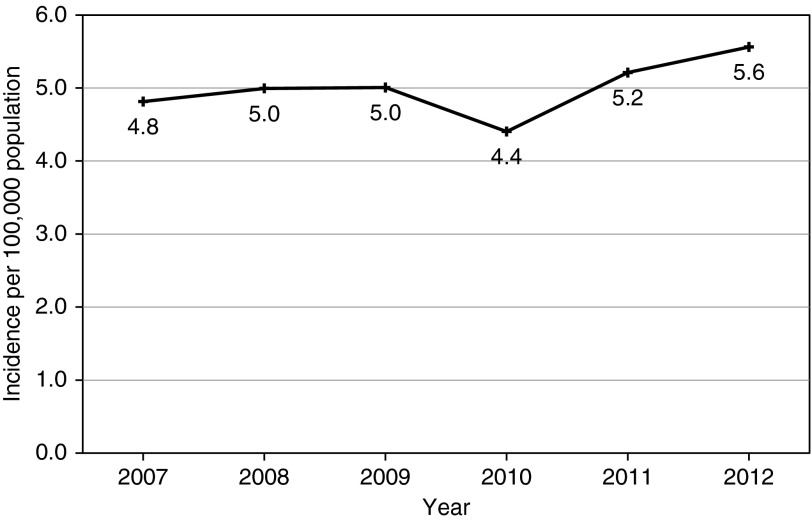
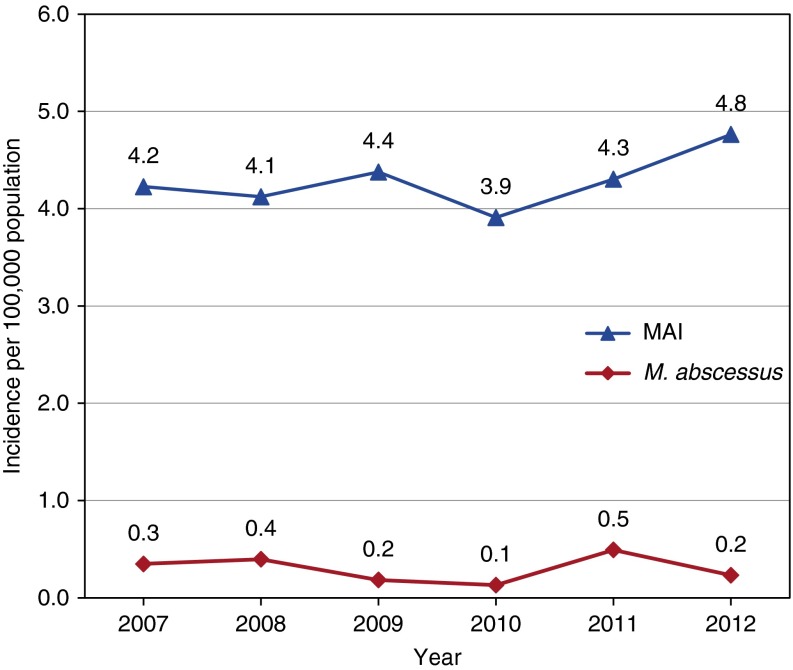
The annual incidence of pulmonary NTM disease increased from 4.8/100,000 in 2007 to 5.6/100,000 in 2012, although this increase was not statistically significant (increase of 2.2% per year, P for trend = 0.21) (Figure 1). The trend was similar for MAI incidence, but M. abscessus/chelonae complex incidence was variable (range 0.1/100,000 in 2010 to 0.5/100,000 in 2011) (Figure 2). The overall average annual incidence of pulmonary NTM disease during 2007 to 2012 was 5.0/100,000 (95% CI, 4.6–5.3), and the age-adjusted average annual incidence was 3.8/100,000 (95% CI, 3.2–4.4). By region, average disease incidence from 2007 to 2012 was 5.1/100,000 (95% CI, 4.4–5.8) in Western Oregon and 4.2/100,000 (95% CI, 3.6–4.8) in Eastern Oregon (P = 0.2 for comparison). Using data from 2011 to 2012 only, we calculated the annualized prevalence to be 5.9/100,000 (95% CI, 5.4–6.5) for pulmonary NTM disease, compared with 5.6/100,000 (95% CI, 5.0–6.1) in 2005 to 2006 as we previously reported (P = 0.6) (1).
Figure 1.
Observed incidence rate of pulmonary nontuberculous Mycobacterium disease per 100,000 population, Oregon 2007 to 2012.
Figure 2.
Observed incidence rates of Mycobacterium avium/intracellulare complex (MAI) and Mycobacterium abscessus/chelonae complex pulmonary nontuberculous Mycobacterium disease per 100,000 population, Oregon 2007 to 2012.
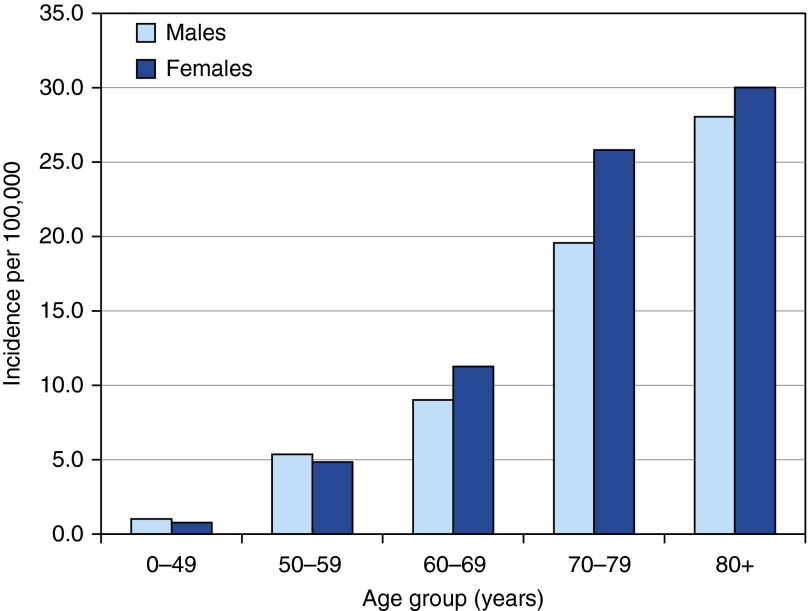
The proportion of cases that were female increased with age from 42% in patients less than 50 years old to 64% in patients 80 years or older (Table 2). Age- and sex-specific incidence rates during 2007 to 2012 are presented in Table 2 and Figure 3. Male individuals less than 60 years of age had a higher incidence rate of pulmonary NTM, although rates remained low, whereas women aged at least 60 years of age had higher incidence rates. The highest rates in both men and women occurred in those aged 80 years old or greater (30/100,000 in female cases and 28/100,000 in male cases).
Table 2.
Age- and sex-stratified cases and incidence per 100,000 population of pulmonary nontuberculous Mycobacterium disease, Oregon 2007 to 2012
| Age Group (yr) | Male Cases | Female Cases | % Female | Male Incidence | Female Incidence |
|---|---|---|---|---|---|
| 0–49 | 52 | 38 | 42.2 | 1.0 | 0.8 |
| 50–59 | 57 | 54 | 48.6 | 5.4 | 4.8 |
| 60–69 | 73 | 97 | 57.1 | 9.0 | 11.2 |
| 70–79 | 81 | 123 | 60.3 | 19.6 | 25.8 |
| 80+ | 65 | 113 | 63.5 | 28.0 | 30.0 |
Cases meeting 2007 American Thoracic Society/Infectious Disease Society of America laboratory case definition: nontuberculous mycobacteria detected in two sputum (same species) or one of any other lung or pleural fluid.
Figure 3.
Average annual age- and sex-specific incidence of pulmonary nontuberculous Mycobacterium disease in Oregon, 2007 to 2012.
Discussion
We report here the first population-based estimate of pulmonary NTM disease incidence from a region within the United States. In Oregon, we observed a slight increase in the incidence of pulmonary disease during 2007 to 2012, with the vast majority of disease due to MAI. Although prior studies and our current study have documented a female predominance of disease, we have found that incident disease in patients less than age 60 years is actually slightly more common among male subjects. Among both male and female subjects, we observed marked increased in disease incidence with older age.
The incidence of pulmonary NTM in Oregon increased gradually between 2007 and 2012. Two recent large epidemiologic studies of pulmonary NTM in North America have evaluated disease prevalence over time, with comparable results. In Ontario, Canada, Marras and colleagues identified an increasing prevalence of respiratory NTM isolates as well as pulmonary NTM disease in the last 10 to 15 years, despite a possible leveling off of disease prevalence at the end of the study in 2010 (2). The annual prevalence of NTM pulmonary disease increased from 4.9/100,000 in 1998 to a peak of 10.7 in 2009, which is slightly higher than our current prevalence estimate (2). Prevots and colleagues calculated change in NTM disease prevalence within two large Health Maintenance Organization populations in California and Washington and observed 2.6 and 2.9% increases in NTM prevalence in women and men, respectively, between 1994 and 2006 (3). This increase in prevalence was similar to the 2.2% annual increase in incidence observed within our study. The age-adjusted annual prevalence was 5.5/100,000 at all four sites included in the Prevots and colleagues study, with rates varying from 1.4/100,000 to 6.7/100,000 by site (3). Our average age-adjusted incidence falls in this range.
To better compare with our calculated annual prevalence in 2005 to 2006 within our prior study in Oregon, we calculated an annualized prevalence measure for 2011 to 2012. This estimate was 5% higher for pulmonary disease in 2011 to 2012 compared with our prior study (1). Considering that patients with NTM may not be repeatedly cultured, or convert to culture negative with treatment, the increase in prevalence is notable, although it did not reach statistical significance (4). As with other chronic diseases, increasing prevalence over time suggests that disease incidence is rising and/or individuals are living longer with their disease.
Several reasons have been proposed to explain the increasing incidence and prevalence of NTM disease, including a true increase in disease, an increase in awareness of NTM, increases in underlying risk factors, and improved laboratory and imaging diagnostics (10–12). However, in Ontario the increasing prevalence between 2003 and 2008 is not explained by aging or an increase in chronic obstructive pulmonary disease or other risk factors (11). In our study, we were able to explore whether increasing incidence was explained by surveillance bias, either due to increased numbers of patients being cultured for NTM or additional follow up of those with a single positive sputum culture. Although the total number of NTM-positive pulmonary cultures per year increased 4% per year (P for trend < 0.001, data not shown), the number of patients with isolates was stable (increasing 1.2% per yr, P for trend = 0.29), suggesting the increase in positive isolates represents closer monitoring of prevalent cases. In addition, there was no corresponding decrease in the number of patients with a single NTM sputum culture, at least after 2007 (isolation rate, 4.3/100,000 population), with stable isolation rates varying between 3.2 and 3.5/100,000 population.
In the last 40 years, the epidemiology of pulmonary NTM disease in the United States has changed. The disease was first described in men and for years believed to be more common in this group, likely due to its close association with chronic obstructive pulmonary disease and smoking (13–16). Our study in 2005 to 2006 identified a higher disease prevalence in women (1, 9) and provided evidence for the anecdotal reports from various treatment centers suggesting disease was in fact more prevalent in women, particularly in association with bronchiectasis (17, 18). However, in our present study evaluating annual disease incidence, we observed disease to be more common in male cases among individuals less than age 60 years. After this age cut-off, disease rates increased rapidly for both sexes, becoming more common in women, with the most pronounced difference between the sexes during their 70s. Incidence rates reached 30/100,000 in women and 28/100,000 in men over age 80 years.
Prevots and colleagues reported similar patterns, with annual disease prevalence rates reaching 60/100,000 in women over age 80 years (3). In Ontario in 2008, the prevalence of MAI was similarly higher for male individuals less than 50 years old (11). The reasons for a higher risk for older women are not well understood but may be related to different rates of underlying age-related disease that are associated with NTM disease, including bronchiectasis and rheumatoid arthritis (5, 10, 12, 17, 19). Differential survival between the two sexes may play a role, as men with NTM are more likely to have chronic obstructive pulmonary disease–related disease with cavitation and diminished survival relative to women, who typically have noncavitary disease (5, 10). This could potentially explain why disease is less common among men in the older age groups. Differences in care-seeking behavior between men and women, as well as diagnostic bias, might also contribute to our observations and deserve further study.
We observed 20 different species associated with cases over the 6-year period in Oregon, compared with 14 in 2005 to 2006 (1). Treatment is species specific, so is it important to pursue additional testing to ensure isolates are fully identified by the laboratory. The percentage of MAI cases (86 vs. 84% previously) was similar to the previous study, as was the percentage represented by more rare species. Two percent of patients met the case criteria for two NTM species, and more than 90% of these included MAI. The overall percentage of cases associated with M. abscessus/chelonae complex was higher in the current study (7 vs. 3% in 2005–2006). Although rare, we observed up to 19 M. abscessus/chelonae cases in a single year in the current study (incidence rate, 0.5/100,000), which is more than twice as many cases as identified in 2005 to 2006 combined. The reasons for this increase are unknown, but M. abscessus/chelonae cases represent a group of patients with disease that is often difficult to manage (4). M. lentiflavum (11 cases, 1%) was a notable species that was not present in 2005/2006. Reasons for M. lentiflavum’s arrival are unclear but could represent differences in laboratory techniques regarding specifies recovery or identification or could represent changes within the environment. Oregon also continues to see relatively few Mycobacterium kansasii and M. xenopi cases, which are associated with disease in other regions of the United States (4).
The strengths of our study are the use of a validated mechanism to identify cases of NTM and 8 years of population-based laboratory surveillance data. We confirmed using our data that very few prevalent cases were likely counted as incident, given that fewer than 1.5% of cases identified in 2005 to 2006 would have met the case definition subsequently. In addition, we believe we have likely underestimated the true disease incidence. Although a small percentage of cases identified in our study could be misclassified due to our use of the microbiologic criteria alone (we were unable to review clinical records to verify patients met the full ATS/IDSA disease criteria consisting of symptoms, radiographic changes, and the microbiologic criteria) (5), this was likely balanced or outweighed by a lack of sensitivity in our case finding. We suspect many patients with NTM disease are undiagnosed, as some patients do not fit the either of the typical profiles (middle-aged male smoker with chronic obstructive pulmonary disease or older, thin woman, “Lady Windermere”) or they have minimal symptoms and do not seek care (4, 20, 21). In addition, the pulmonary case definition requires two sputum samples, but some patients with a positive culture might not be recultured and given the opportunity to meet disease criteria. It is likely that at least some of the 806 (about 134 per yr) single positive sputum results are undiagnosed cases. These patients were slightly more likely to have M. fortuitum complex or rare or unspeciated results, but a similar large majority (82%) isolated MAI or M. abscessus/chelonae (4%) that are likely clinically significant. Our prior work suggests that just over 50% of respiratory cultures with acid-fast bacillus (other than M. gordonae) represent true NTM disease (5). Furthermore, we were unable to account for residents who might have moved out of state or died after giving their first positive sputum culture result. Last, two laboratories were missing data for the earliest years within our project, making it likely that not all cases were reported.
In summary, we estimated the population-based incidence of pulmonary NTM disease in Oregon and showed that it was relatively stable but increased slightly during a 6-year period from 2007 to 2012. Further work is needed to better understand the natural history of pulmonary NTM disease, underlying risk factors, and clinical relevance of the microbiological definition, particularly for rare species other than MAI and M. abscessus/chelonae complex.
Acknowledgments
Acknowledgment
The authors thank the laboratory staff who extracted the data necessary for this study and Sarah A. R. Siegel and Jennifer L. Ku for project support.
Footnotes
Supported by National Institutes of Health/National Institute of Allergy and Infectious Disease grant NIH/NIAID HHSN272201200005C (E.H., K.L.W.).
Author Contributions: E.H.: acquisition, analysis, and interpretation of data, developed manuscript. S.S., K.H., and K.L.W.: concept and study design, interpretation of data, reviewing manuscript critically for intellectual content. S.N.: interpretation of data, reviewing manuscript critically for intellectual content.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis. 2009;49:e124–e129. doi: 10.1086/648443. [DOI] [PubMed] [Google Scholar]
- 2.Marras TK, Mendelson D, Marchand-Austin A, May K, Jamieson FB. Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998-2010. Emerg Infect Dis. 2013;19:1889–1891. doi: 10.3201/eid1911.130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182:970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. ATS Mycobacterial Diseases Subcommittee American Thoracic Society Infectious Disease Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases Am J Respir Crit Care Med 2007175367–416. [Published erratum appears in Am J Respir Crit Care Med 175:744–745.] [DOI] [PubMed] [Google Scholar]
- 5.Winthrop KL, McNelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, Saulson A, Hedberg K. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182:977–982. doi: 10.1164/rccm.201003-0503OC. [DOI] [PubMed] [Google Scholar]
- 6.Henkle E, Schafer S, Hedberg K, Novosad S, Siegel S, Ku J, Winthrop KL. Incidence of nontuberculous mycobacteria infection In Oregon, 2007–2012 [abstract] Am J Respir Crit Care Med. 2014;189:A6528. [Google Scholar]
- 7.Portland State University, College of Urban and Public Affairs Population Research CenterPopulation estimates [updated 2012 Dec 15; accessed 2013 Sept 5]. Available from: http://www.pdx.edu/prc/population-estimates-0
- 8.National Cancer Institute Surveillance Epidemiology and End Results SystemStandard populations (millions) for age-adjustment. [accessed 2014 Nov 17]. Available from: http://seer.cancer.gov/stdpopulations/ Last Accessed 11/17/2014
- 9.Winthrop KL, Varley CD, Ory J, Cassidy PM, Hedberg K. Pulmonary disease associated with nontuberculous mycobacteria, Oregon, USA. Emerg Infect Dis. 2011;17:1760–1761. doi: 10.3201/eid1709.101929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185:881–886. doi: 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Houqani M, Jamieson F, Mehta M, Chedore P, May K, Marras TK. Aging, COPD, and other risk factors do not explain the increased prevalence of pulmonary Mycobacterium avium complex in Ontario. Chest. 2012;141:190–197. doi: 10.1378/chest.11-0089. [DOI] [PubMed] [Google Scholar]
- 12.Seitz AE, Olivier KN, Adjemian J, Holland SM, Prevots R. Trends in bronchiectasis among medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142:432–439. doi: 10.1378/chest.11-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenzweig DY. Pulmonary mycobacterial infections due to Mycobacterium intracellulare-avium complex. Clinical features and course in 100 consecutive cases. Chest. 1979;75:115–119. doi: 10.1378/chest.75.2.115. [DOI] [PubMed] [Google Scholar]
- 14.Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979;119:107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- 15.Andréjak C, Nielsen R, Thomsen VO, Duhaut P, Sørensen HT, Thomsen RW. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013;68:256–262. doi: 10.1136/thoraxjnl-2012-201772. [DOI] [PubMed] [Google Scholar]
- 16.Bates JH. A study of pulmonary disease associated with mycobacteria other than Mycobacterium tuberculosis: clinical characteristics. XX. A report of the Veterans Administration-armed forces cooperative study on the chemotherapy of tuberculosis. Am Rev Respir Dis. 1967;96:1151–1157. doi: 10.1164/arrd.1967.96.6.1151. [DOI] [PubMed] [Google Scholar]
- 17.Griffith DE, Girard WM, Wallace RJ., Jr Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993;147:1271–1278. doi: 10.1164/ajrccm/147.5.1271. [DOI] [PubMed] [Google Scholar]
- 18.Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1991;144:914–916. doi: 10.1164/ajrccm/144.4.914. [DOI] [PubMed] [Google Scholar]
- 19.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD, Merkel PA, et al. National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 20.Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest. 1992;101:1605–1609. doi: 10.1378/chest.101.6.1605. [DOI] [PubMed] [Google Scholar]
- 21.Chan ED, Iseman MD. Slender, older women appear to be more susceptible to nontuberculous mycobacterial lung disease. Gend Med. 2010;7:5–18. doi: 10.1016/j.genm.2010.01.005. [DOI] [PubMed] [Google Scholar]