Abstract
Pulmonary surfactant is essential for life as it lines the alveoli to lower surface tension, thereby preventing atelectasis during breathing. Surfactant is enriched with a relatively unique phospholipid, termed dipalmitoylphosphatidylcholine, and four surfactant-associated proteins, SP-A, SP-B, SP-C, and SP-D. The hydrophobic proteins, SP-B and SP-C, together with dipalmitoylphosphatidylcholine, confer surface tension–lowering properties to the material. The more hydrophilic surfactant components, SP-A and SP-D, participate in pulmonary host defense and modify immune responses. Specifically, SP-A and SP-D bind and partake in the clearance of a variety of bacterial, fungal, and viral pathogens and can dampen antigen-induced immune function of effector cells. Emerging data also show immunosuppressive actions of some surfactant-associated lipids, such as phosphatidylglycerol. Conversely, microbial pathogens in preclinical models impair surfactant synthesis and secretion, and microbial proteinases degrade surfactant-associated proteins. Deficiencies of surfactant components are classically observed in the neonatal respiratory distress syndrome, where surfactant replacement therapies have been the mainstay of treatment. However, functional or compositional deficiencies of surfactant are also observed in a variety of acute and chronic lung disorders. Increased surfactant is seen in pulmonary alveolar proteinosis, a disorder characterized by a functional deficiency of the granulocyte-macrophage colony-stimulating factor receptor or development of granulocyte-macrophage colony-stimulating factor antibodies. Genetic polymorphisms of some surfactant proteins such as SP-C are linked to interstitial pulmonary fibrosis. Here, we briefly review the composition, antimicrobial properties, and relevance of pulmonary surfactant to lung disorders and present its therapeutic implications.
Keywords: surfactant, infection, immune responses, pulmonary host defense
It is established that pulmonary surfactant reduces surface tension at the air–water interface in the alveoli, thereby preventing collapse of these structures at end-expiration. In this manner, surfactant reduces the work associated with breathing. Although surfactant and its surface active properties were discovered relatively early in the 1920s (1), its components and mechanism of action only began to be elucidated in the 1950s by Pattle (2) and Clements (3). The breakthrough by Avery and Said helped identify a fundamental discovery linking pulmonary surfactant deficiency to infants who died of respiratory distress syndrome (RDS) (4). Indeed, these critical findings helped propel surfactant replacement therapy as an approach that has revolutionized treatment of RDS. However, during the 1990s, investigators uncovered several additional important biological properties of this surface-active material in the area of host immunity against microbial infection and immunomodulatory activity.
Surfactant Composition and Function
Composition
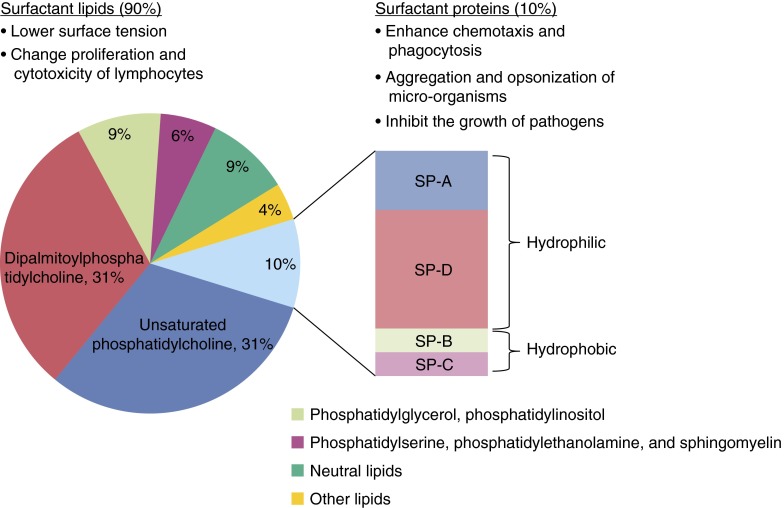
Pulmonary surfactant is composed primarily of phospholipids and key proteins (5). Lipids compose 80 to 90% of its molecular weight, of which the most abundant species are phosphatidylcholine, phosphatidylglycerol, and phosphatidylinositol (Figure 1); specifically, phosphatidylcholine constitutes approximately 70% of the lipid portion of surfactant, and it exists as a relatively unique form, known as dipalmitoylphosphatidylcholine (DPPC). Together with surfactant proteins, DPPC provides the surface activity of surfactant (6–8). The remaining types of lipid, including phosphatidylserine, phosphatidylethanolamine, and sphingomyelin, appear to be present in relatively small amounts. This lipid composition is well conserved among vertebrates (7).
Figure 1.
The composition and function of surfactant. Surfactant is composed of 90% lipid and 10% protein. The lipid content contains primarily phospholipid, specifically dipalmitoylphosphatidylcholine, which is responsible for the biophysical function of surfactant. The large hydrophilic proteins, surfactant protein (SP)-A and SP-D, play an important role in host defense and immune modulation, whereas SP-B and SP-C primarily partake in modulating biophysical properties.
Surfactant contains four associated proteins, surfactant protein (SP)-A, SP-B, SP-C, and SP-D. Two of these proteins, SP-A and SP-D, are hydrophilic, and the others are hydrophobic (9). SP-A and SP-D are members of a family of innate immune proteins, termed collectins (10, 11). These proteins have in common an NH2-terminal collagen-like region and a C-terminal lectin domain that binds carbohydrates in a calcium-dependent manner. Binding sites for these lectin domains are found on bacterial and viral surfaces (12), and this in part is responsible for the role collectins play in innate and adaptive immunity.
The hydrophobic surfactant proteins, SP-B and SP-C, are stored and secreted along with surfactant phospholipids (13, 14). SP-B is an indispensable protein that plays a role in enhancing the surface tension–reducing properties of surfactant (14) and also appears to have some antimicrobial activity (15–17). The role of SP-C, one of the most hydrophobic peptides known, is uncertain, but its high degree of conservation among species suggests an integral function (17).
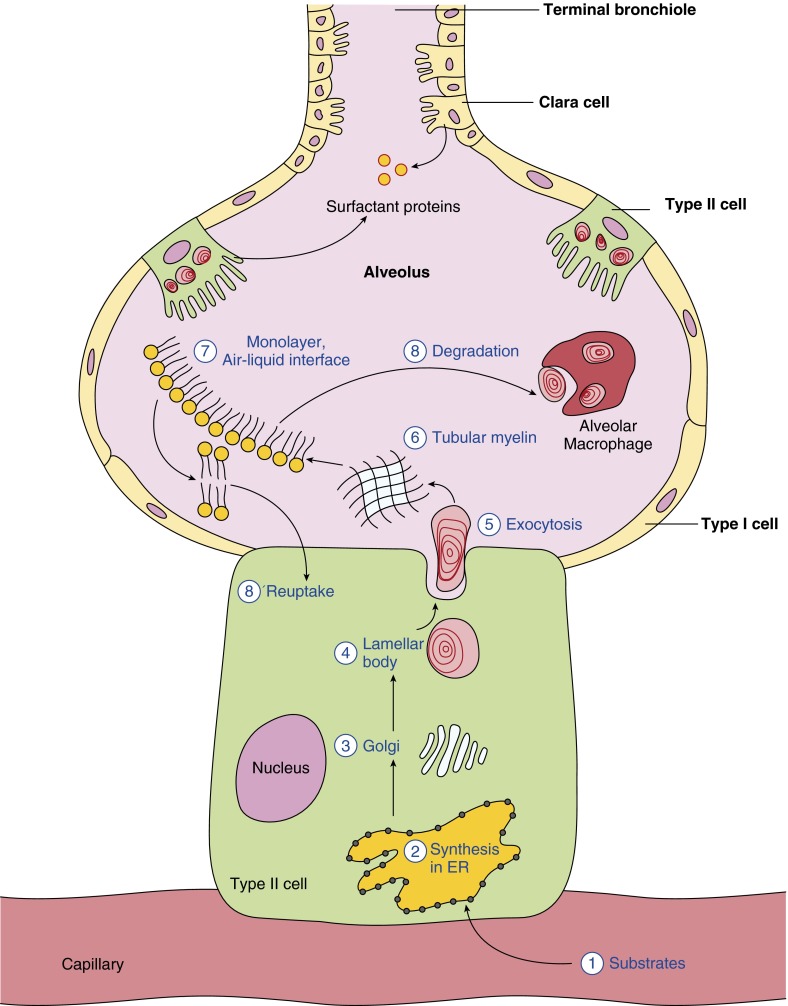
Surfactant components are synthesized primarily by the alveolar type II cell, which produces surfactant lipids and surfactant proteins (5, 18), and the airway club cell, which synthesizes surfactant proteins SP-A, SP-B, and SP-D (19–21) (Figure 2).
Figure 2.
Surfactant life cycle—synthesis, secretion, and recycling. Alveolar type II cells, which cover about 7% of alveolar epithelial surface, are mainly responsible for surfactant production using dietary substrates (1). Surfactant is synthesized in the endoplasmic reticulum (ER) (2) of alveolar type II cells, and transported to the Golgi (3) for further modification. Most of the surfactant components are stored in the lamellar bodies (4) until they are secreted into liquid hypophase on the alveoli by exocytosis (5). Surfactant forms a lattice-like structure, called tubular myelin (6), which is transported to the air–liquid interface to form a monolayer of surfactant film (7). The phospholipids are either internalized and degraded by macrophages (8) or recycled back to the type II cells for reuse (8′). Note that surfactant protein (SP)-A, SP-B, and SP-D are also synthesized in club cells in terminal bronchioles.
Function
The main functions of surfactant are as follows: (1) lowering surface tension at the air–liquid interface and thus preventing alveolar collapse at end-expiration, (2) interacting with and subsequent killing of pathogens or preventing their dissemination, and (3) modulating immune responses.
The drastic change in surface area of alveoli throughout the respiratory cycle dictates that alveolar surface tension needs to be less than 2 mN/m at end-expiration to prevent alveolar collapse (22). This critical function of surfactant is achieved through its maintenance of a film highly enriched in DPPC, which produces extremely low surface tension (<1 mN/m) on compression (17). These biophysical properties have led to modified exogenous surfactant replacement therapies that have impacted outcomes of neonatal RDS in many studies (23, 24).
Surfactant also plays a vital role in host defense against infection. The collectins SP-A and SP-D enhance bacterial and viral clearance. As previously mentioned, the C-terminal lectin domains of these proteins preferentially bind nonhost oligosaccharides found on viruses and bacteria. The most well-described function of the collectins is their ability to opsonize pathogens and facilitate their phagocytosis by cells of the innate immune system, such as macrophages and monocytes, as well as regulate the production of cell-derived mediators (11, 25). Studies have shown that mice deficient in SP-A exhibit impaired clearance against various bacterial and viral infections, including group B Streptococcus (26, 27), Pseudomonas aeruginosa (28), and respiratory syncytial virus (29). More recently, SP-A and SP-D have also been demonstrated to have direct antibacterial activity against Escherichia coli, Klebsiella pneumoniae, and Enterobacter aerogenes (30), as well as antifungal activity against Histoplasma capsulatum (31), through increasing membrane permeability of the microbes. In humans there exist two genes, SP-A1 and SP-A2, that encode for SP-A1 and SP-A2 proteins, respectively (32). This suggests a possibility that there may be human subpopulations with differential vulnerabilities to microbial infection based on these SP-A isoforms.
In addition to facilitating and activating the immune system, the lung collectins also act as immunomodulators. SP-A can inhibit dendritic cell maturation (33) and inhibit eosinophil release of IL-8 (34). Studies have shown that SP-A and SP-D inhibit allergen-induced lymphocyte proliferation via multiple mechanisms and that this effect is blunted in activated lymphocytes from children with asthma (35). SP-A and SP-D also bind directly to allergens and particles such as pollen grains (36), house dust mite allergen (37), and Aspergillus fumigatus allergen (38), inhibiting specific IgE binding to allergens and subsequently decreasing allergen-induced histamine release.
Pulmonary Disorders Related to Surfactant Dysfunction or Deficiency
Abnormalities in surfactant production or function are associated with several pulmonary diseases, and, at the same time, pulmonary infections alter surfactant metabolism. The most well-known disorder of surfactant deficiency is RDS in preterm infants. As discussed earlier, preterm neonates who are born before they produce enough surfactant develop RDS, which can be treated with exogenous surfactant. There are several genetic disorders that cause surfactant dysfunction. The mode of their inheritance is either autosomal dominant (involving the gene encoding SP-C or thyroid transcription factor 1) or autosomal recessive (involving the gene encoding SP-B or the gene encoding ATP-binding cassette protein member A3) (39). Most neonates with these genetic disorders do not survive without lung transplantation. For adults, several human observational studies show that subjects with acute respiratory distress syndrome (ARDS) have altered composition and function of surfactant (40, 41). Unfortunately, exogenous surfactant did not show a mortality benefit in randomized controlled trials (RCTs) (42).
Although the disorders mentioned above are related to inadequate or dysfunctional surfactant, an overabundance of surfactant can also cause clinical disease. Pulmonary alveolar proteinosis, a rare disease caused by gene mutations leading to dysfunction of the granulocyte-macrophage colony-stimulating factor receptor or development of granulocyte-macrophage colony-stimulating factor antibodies, results in accumulation of surfactant within the alveoli and the terminal airways and can cause impairment of gas exchange. Varying levels of SP-A and SP-D from bronchoalveolar lavage in different pulmonary disorders are summarized in Table 1. It was previously believed that surfactant components existed only in the lungs. Animal models and human observation studies have shown, however, that surfactant proteins leak into the vascular space when alveolocapillary membranes are injured (43–46). Importantly, circulating surfactant protein levels may have clinical usefulness. One study demonstrated that surfactant protein levels can be used as an indicator of lung injury and poor outcomes in H1N1 viral infections (47), and another showed that SP-A and SP-D levels are elevated in those with pulmonary fibrosis compared with healthy volunteers (48).
Table 1.
Levels of SP-A and SP-D from bronchoalveolar lavage in pulmonary disease
| SP-A Levels | SP-D Levels | Lipid Levels | References | |
|---|---|---|---|---|
| RDS in neonates | ↓ | N/A | ↓ | 140–143 |
| PAP | ↑ | ↑ | ↑ | 144–146 |
| ARDS | ↓ | N/A | ↓ | 40, 147 |
| IPF | ↓ | = | ↓ | 145, 148–150 |
| Sarcoidosis | ↑ | = | = | 145, 149, 151, 152 |
| Bacterial pneumonia | ↓ | N/A | ↓ | 153, 154 |
| Smokers | ↓ | ↓ | = | 155, 156 |
| Asthma | ↓ | N/A | = | 157 |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; IPF = idiopathic pulmonary fibrosis; N/A = not available; PAP = pulmonary alveolar proteinosis; RDS = respiratory distress syndrome.
↓ indicates decrease; ↑ indicates increase; = indicates unchanged.
Genetic polymorphisms of surfactant proteins are known to be associated with a higher prevalence of idiopathic pulmonary fibrosis (49, 50) but also a reduced prevalence of interstitial lung disease in systemic sclerosis (51). Additionally, several studies also describe the association between genetic polymorphisms for surfactant proteins and high-altitude pulmonary edema (52), ARDS (53), lung carcinoma (54), and bronchopulmonary dysplasia (55). A rare missense mutation in SFTPA2, the gene encoding SP-A2, is associated with development of familial idiopathic pulmonary fibrosis and lung cancer (56).
On the other hand, numerous respiratory infections have been shown to modify surfactant composition. For example, P. aeruginosa inhibits surfactant biosynthesis (57, 58), decreases its host defense and biophysical function (59), and secretes elastase to degrade surfactant proteins A and D (60, 61). Also, LPS, a major cell wall component of gram-negative bacteria, inhibits phospholipid synthesis and secretion (57, 58). Surfactant inhibition by bacteria seems to be associated with host cell cytokines such as tumor necrosis factor-α, which leads to degradation of surfactant biosynthetic enzymes. Human adenovirus disrupts the trafficking of surfactant phosphatidylcholine (62), whereas A. fumigatus down-regulates SP-B and SP-C protein and mRNA expression in mice (63). Respiratory syncytial virus (RSV)-infected bronchial epithelial cells have decreased SP-A protein levels through reduced mRNA translation efficiency (64).
Antimicrobial Function
Bacteria
The hydrophilic proteins SP-A and SP-D play a major role in host defense by inhibiting bacterial growth, facilitating bacterial uptake by host cells, and aggregating and opsonizing pathogens (65). These surfactant proteins can bind to both gram-negative and gram-positive bacteria. SP-A and/or SP-B interact with LPS derived from K. pneumoniae (30, 66), E. coli (30, 67), P. aeruginosa (68–70), and Legionella pneumophila (71), which consequently result in agglutination, enhancement of pathogen uptake, and growth inhibition. These surfactant proteins also bind with peptidoglycan, a cell wall component of gram-positive bacteria derived from Staphylococcus aureus (72) and Streptococcus pneumoniae (26, 27), as well as Mycobacterium avium, Mycobacterium tuberculosis, and Mycoplasma pneumoniae to enhance uptake by phagocytes and inhibit their growth (73–78).
Fungi
Both SP-A and SP-D are able to bind to a variety of fungi, mostly opportunistic pathogens, to facilitate agglutination and phagocytosis by host cells. Animal studies demonstrate that pulmonary collectins (SP-A and SP-D) increase the permeability of the cell membrane of H. capsulatum, inhibiting its growth directly (31). They also bind to A. fumigatus (79), Blastomyces dermatitidis (80), Coccidioides posadasii (81), Cryptococcus neoformans (82, 83), and Pneumocystis jiroveci (carinii) (84, 85), which results in agglutination and enhanced uptake. Interestingly, this effect appears to be microbe specific, as the binding of pulmonary collectins to Candida albicans inhibits phagocytosis by alveolar macrophages while still inhibiting the fungal growth (86, 87).
Virus
Pulmonary collectins (SP-A and SP-D) bind to viruses to facilitate pathogen removal. Viruses are unique compared with many microorganisms in that they require entrance into host cells to replicate. As SP-A and SP-D are present in the mucus layer and alveolar surface, they are well positioned to prevent infection of epithelial cells through viral neutralization, agglutination, and enhanced phagocytosis. SP-A and/or SP-D bind to hemagglutinin and neuraminidase of influenza A virus to inhibit their activity (88–90). Interestingly, the hemagglutinin of pandemic influenza viruses has a low binding activity for surfactant protein D compared with that of a seasonal influenza strain (91). Pulmonary collectins also bind to glycoproteins of viruses, including HIV (92, 93), RSV (94), and severe acute respiratory syndrome coronavirus (95). Recent studies indicate that, in addition to pulmonary collectins, the surfactant lipid components also inhibit RSV infection (96).
Therapeutic Applications and Implications
The primary indication for surfactant replacement therapy is RDS in preterm infants. Several human observational studies and RCTs demonstrate reduced mortality and morbidity, including interstitial emphysema and pneumothorax, when exogenous surfactant is administered to preterm infants born at less than 30 weeks’ gestation who are at high risk for RDS (97–99). Both synthetic and natural types of surfactant are effective, but natural preparations that retain surfactant protein B and C analogs have been shown to be superior in terms of decreasing mortality and lowering the rate of RDS complications in preterm infants (100, 101). Currently the 2014 American Academy of Pediatrics guidelines recommend initial nasal continuous positive airway pressure immediately after birth for all preterm infants and subsequent intubation with prophylactic or early surfactant administration in select patients (102). Endotracheal instillation remains a widely accepted technique of surfactant administration (103). However, this technique may be complicated by episodes of severe airway obstruction (104). Noninvasive or less-invasive techniques, including aerosolized surfactant, laryngeal mask airway-aided delivery, pharyngeal instillation, and the use of thin intratracheal catheters, are being evaluated (105–109).
For adult patients, both synthetic and natural animal surfactants have been tried for the treatment of ARDS, via either intratracheal instillation or aerosolized delivery. However, studies did not demonstrate a significant mortality benefit or a consistent improvement in oxygenation with this approach (42, 110–114). Initially it was believed that exogenous surfactant could be beneficial to patients with ARDS because they have decreased surfactant levels and persistent atelectasis contributing to gas exchange abnormalities. Patients with ARDS also have altered composition and function of surfactant, which is compounded further by mechanical ventilation (40, 41, 115). Despite the theoretical soundness of exogenous surfactant administration in patients with ARDS, this therapeutic option has limited justification at this time. Given the fact that neonates start surfactant therapy early in the course of the disease before RDS becomes severe, it may be worthwhile to consider studying an approach with early surfactant administration, but this depends on the development of effective biomarkers that can identify or predict patients with ARDS early in the course of disease. Contrary to RDS, ARDS is a heterogeneous syndrome with various degrees of inflammation and tissue remodeling depending on the individual patient, which may explain differential responses to surfactant therapy. Alternatively, the utility of novel proteolytically stable surfactant preparations as replacement therapies might be an area of future study.
Exogenous surfactant also has been examined in a variety of lung diseases such as asthma and pneumonia (116). Although a pilot study for aerosolized natural surfactant showed improved lung function during an acute asthma exacerbation (117), it did not show clinical benefit in patients with stable asthma (118). One case report demonstrated oxygenation improvement with intrabronchial instillation of surfactant in an adult patient with gram-negative lobar pneumonia (119). Other case reports demonstrate similar oxygen improvements in HIV-infected infants with P. carinii pneumonia (120, 121) or RSV pneumonia (122). One RCT of a 2-week treatment course with aerosolized synthetic surfactant showed improved pulmonary function in adult patients with stable chronic bronchitis (123). These observations need to be confirmed with larger well-controlled studies in subjects with respiratory illness.
One potential therapeutic implication of surfactant replacement therapy is immunosuppression. Animal studies and limited human data show that exogenous surfactant decreases cytokine release (124), DNA synthesis of inflammatory mediators (125, 126), lymphocyte proliferation (127), immunoglobulin production (128), and expression of adhesion molecules (129). Intratracheal administration of a surfactant–amikacin mixture to rats with Pseudomonas pneumonia showed improved antiinflammatory effects compared with amikacin alone (130). These observations suggest the possibility that surfactant may be used to modulate immune responses during inflammatory lung disease, but further studies are necessary.
Outside of exogenous surfactant therapy, there is also evidence that certain pharmacologic agents may enhance endogenous surfactant levels, although the current data are limited. Corticosteroids have been widely used in women at risk for preterm delivery, as they reduce neonatal morbidity and mortality from RDS. Antenatal steroids accelerate development of type 2 pneumocytes and thus increase the production of surfactant proteins and enzymes necessary for phospholipid synthesis. Corticosteroids also induce pulmonary β-receptors, which play a role in surfactant release and alveolar fluid absorption when stimulated (131). Thyroid hormone also has a synergistic effect on phospholipid synthesis with corticosteroids in animal models (132, 133). Ambroxol may also act to increase surfactant release and is under investigation for use in RDS (134). Hydroxychloroquine has been anecdotally reported to successfully treat children with SP-C deficiency with or without corticosteroid use (135–137). The mechanism of action is unclear, but it may be related to hydroxychloroquine’s inhibition of the intracellular processing of SP-C precursors leading to late accumulation of SP-C (138). Other agents such as keratinocyte growth factor have been shown to increase surfactant secretion or its synthesis (139).
Conclusions
In summary, pulmonary surfactant has important functions beyond reducing surface tension and altering mechanical properties that lead to decreased work of breathing. As the lung epithelium is in constant exposure to the environment, surfactant provides a crucial first line of defense against infection by enhancing the removal of pathogens, modulating the response of inflammatory cells, and optimizing lung biophysical activity. Hydrophilic proteins, which constitute a small portion of surfactant, play a major role in antimicrobial activity. Although surfactant is an established treatment for RDS in preterm infants, there has been no compelling clinical benefit for use of exogenous surfactant in adult patients with ARDS thus far. Further studies need to be performed to explore the possibility of surfactants as an immune modulating therapy or designing small molecules that modulate availability of surfactant components in respiratory illness.
Summary
-
•
Surfactant has many biological functions, including its tension-reducing property at the air–water interface, antimicrobial activity, and immunomodulation.
-
•
Although surfactant is an established treatment for RDS in preterm infants, no clinical benefit has been shown in adult patients with ARDS.
-
•
Animal studies and limited anecdotal reports suggest surfactant could be used to treat infectious and inflammatory lung disease; however, further preclinical and clinical studies are necessary.
Footnotes
Supported by a Merit Review Award from the U.S. Department of Veterans Affairs, the Flight Attendants Medical Research Institute, and from the National Institutes of Health R01 grants HL096376, HL097376, HL098174, HL081784, 1UH2HL123502, and P01 HL114453 (R.K.M.).
Author Contributions: S.H. drafted the manuscript; R.K.M. made editorial revisions.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Neergaard K. Neue Auffassungen über einen Grundbegriff der Atemmechanik. Z Ges Exp Med. 1929;66:373–394. [Google Scholar]
- 2.Pattle RE. Properties, function and origin of the alveolar lining layer. Nature. 1955;175:1125–1126. doi: 10.1038/1751125b0. [DOI] [PubMed] [Google Scholar]
- 3.Clements JA. Surface tension of lung extracts. Proc Soc Exp Biol Med. 1957;95:170–172. doi: 10.3181/00379727-95-23156. [DOI] [PubMed] [Google Scholar]
- 4.Avery ME, Said S. Surface phenomena in lungs in health and disease. Medicine (Baltimore) 1965;44:503–526. doi: 10.1097/00005792-196511000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Wright JR, Clements JA. Metabolism and turnover of lung surfactant. Am Rev Respir Dis. 1987;136:426–444. doi: 10.1164/ajrccm/136.2.426. [DOI] [PubMed] [Google Scholar]
- 6.King RJ, Clements JA. Surface active materials from dog lung. I. Method of isolation. Am J Physiol. 1972;223:707–714. doi: 10.1152/ajplegacy.1972.223.3.707. [DOI] [PubMed] [Google Scholar]
- 7.Veldhuizen R, Nag K, Orgeig S, Possmayer F. The role of lipids in pulmonary surfactant. Biochim Biophys Acta. 1998;1408:90–108. doi: 10.1016/s0925-4439(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 8.Clements J, Tierney D.Alveolar instability associated with altered surface tension Fenn W, Rahn H.Handbook of physiology Section 3: respiration. Vol IIWashington, D.C.American Physiological Society; 19651565–1583. [Google Scholar]
- 9.Weaver TE, Whitsett JA. Function and regulation of expression of pulmonary surfactant-associated proteins. Biochem J. 1991;273:249–264. doi: 10.1042/bj2730249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crouch E, Wright JR. Surfactant proteins a and d and pulmonary host defense. Annu Rev Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 11.Crouch E, Hartshorn K, Ofek I. Collectins and pulmonary innate immunity. Immunol Rev. 2000;173:52–65. doi: 10.1034/j.1600-065x.2000.917311.x. [DOI] [PubMed] [Google Scholar]
- 12.Crouch EC. Collectins and pulmonary host defense. Am J Respir Cell Mol Biol. 1998;19:177–201. doi: 10.1165/ajrcmb.19.2.140. [DOI] [PubMed] [Google Scholar]
- 13.Mingarro I, Lukovic D, Vilar M, Pérez-Gil J. Synthetic pulmonary surfactant preparations: new developments and future trends. Curr Med Chem. 2008;15:393–403. doi: 10.2174/092986708783497364. [DOI] [PubMed] [Google Scholar]
- 14.Schürch D, Ospina OL, Cruz A, Pérez-Gil J. Combined and independent action of proteins SP-B and SP-C in the surface behavior and mechanical stability of pulmonary surfactant films. Biophys J. 2010;99:3290–3299. doi: 10.1016/j.bpj.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulugeta S, Beers MF. Surfactant protein C: its unique properties and emerging immunomodulatory role in the lung. Microbes Infect. 2006;8:2317–2323. doi: 10.1016/j.micinf.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Blanco O, Pérez-Gil J. Biochemical and pharmacological differences between preparations of exogenous natural surfactant used to treat Respiratory Distress Syndrome: role of the different components in an efficient pulmonary surfactant. Eur J Pharmacol. 2007;568:1–15. doi: 10.1016/j.ejphar.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Gil J, Weaver TE. Pulmonary surfactant pathophysiology: current models and open questions. Physiology (Bethesda) 2010;25:132–141. doi: 10.1152/physiol.00006.2010. [DOI] [PubMed] [Google Scholar]
- 18.Weaver TE. Pulmonary surfactant-associated proteins. Gen Pharmacol. 1988;19:361–368. doi: 10.1016/0306-3623(88)90029-8. [DOI] [PubMed] [Google Scholar]
- 19.Voorhout WF, Veenendaal T, Kuroki Y, Ogasawara Y, van Golde LM, Geuze HJ. Immunocytochemical localization of surfactant protein D (SP-D) in type II cells, Clara cells, and alveolar macrophages of rat lung. J Histochem Cytochem. 1992;40:1589–1597. doi: 10.1177/40.10.1527377. [DOI] [PubMed] [Google Scholar]
- 20.Kalina M, Mason RJ, Shannon JM. Surfactant protein C is expressed in alveolar type II cells but not in Clara cells of rat lung. Am J Respir Cell Mol Biol. 1992;6:594–600. doi: 10.1165/ajrcmb/6.6.594. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Souza P, Kuliszewski M, Tanswell AK, Post M. Expression of surfactant proteins in embryonic rat lung. Am J Respir Cell Mol Biol. 1994;10:222–229. doi: 10.1165/ajrcmb.10.2.7509164. [DOI] [PubMed] [Google Scholar]
- 22.Clements JA. Lung surfactant: a personal perspective. Annu Rev Physiol. 1997;59:1–21. doi: 10.1146/annurev.physiol.59.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Soll R, Ozek E. Prophylactic protein free synthetic surfactant for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2010;1:CD001079. doi: 10.1002/14651858.CD001079.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soll RF. Prophylactic synthetic surfactant for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2000;(2):CD001079. doi: 10.1002/14651858.CD001079. [DOI] [PubMed] [Google Scholar]
- 25.Wright JR. Immunomodulatory functions of surfactant. Physiol Rev. 1997;77:931–962. doi: 10.1152/physrev.1997.77.4.931. [DOI] [PubMed] [Google Scholar]
- 26.LeVine AM, Bruno MD, Huelsman KM, Ross GF, Whitsett JA, Korfhagen TR. Surfactant protein A-deficient mice are susceptible to group B streptococcal infection. J Immunol. 1997;158:4336–4340. [PubMed] [Google Scholar]
- 27.LeVine AM, Kurak KE, Wright JR, Watford WT, Bruno MD, Ross GF, Whitsett JA, Korfhagen TR. Surfactant protein-A binds group B Streptococcus enhancing phagocytosis and clearance from lungs of surfactant protein-A-deficient mice. Am J Respir Cell Mol Biol. 1999;20:279–286. doi: 10.1165/ajrcmb.20.2.3303. [DOI] [PubMed] [Google Scholar]
- 28.LeVine AM, Kurak KE, Bruno MD, Stark JM, Whitsett JA, Korfhagen TR. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 1998;19:700–708. doi: 10.1165/ajrcmb.19.4.3254. [DOI] [PubMed] [Google Scholar]
- 29.LeVine AM, Gwozdz J, Stark J, Bruno M, Whitsett J, Korfhagen T. Surfactant protein-A enhances respiratory syncytial virus clearance in vivo. J Clin Invest. 1999;103:1015–1021. doi: 10.1172/JCI5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H, Kuzmenko A, Wan S, Schaffer L, Weiss A, Fisher JH, Kim KS, McCormack FX. Surfactant proteins A and D inhibit the growth of gram-negative bacteria by increasing membrane permeability. J Clin Invest. 2003;111:1589–1602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCormack FX, Gibbons R, Ward SR, Kuzmenko A, Wu H, Deepe GS., Jr Macrophage-independent fungicidal action of the pulmonary collectins. J Biol Chem. 2003;278:36250–36256. doi: 10.1074/jbc.M303086200. [DOI] [PubMed] [Google Scholar]
- 32.Floros J, Wang G, Mikerov AN. Genetic complexity of the human innate host defense molecules, surfactant protein A1 (SP-A1) and SP-A2—impact on function. Crit Rev Eukaryot Gene Expr. 2009;19:125–137. doi: 10.1615/critreveukargeneexpr.v19.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinker KG, Garner H, Wright JR. Surfactant protein A modulates the differentiation of murine bone marrow-derived dendritic cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L232–L241. doi: 10.1152/ajplung.00187.2002. [DOI] [PubMed] [Google Scholar]
- 34.Cheng G, Ueda T, Nakajima H, Nakajima A, Kinjyo S, Motojima S, Fukuda T. Suppressive effects of SP-A on ionomycin-induced IL-8 production and release by eosinophils. Int Arch Allergy Immunol. 1998;117:59–62. doi: 10.1159/000053574. [DOI] [PubMed] [Google Scholar]
- 35.Wang JY, Shieh CC, You PF, Lei HY, Reid KB. Inhibitory effect of pulmonary surfactant proteins A and D on allergen-induced lymphocyte proliferation and histamine release in children with asthma. Am J Respir Crit Care Med. 1998;158:510–518. doi: 10.1164/ajrccm.158.2.9709111. [DOI] [PubMed] [Google Scholar]
- 36.Malhotra R, Haurum J, Thiel S, Jensenius JC, Sim RB. Pollen grains bind to lung alveolar type II cells (A549) via lung surfactant protein A (SP-A) Biosci Rep. 1993;13:79–90. doi: 10.1007/BF01145960. [DOI] [PubMed] [Google Scholar]
- 37.Wang JY, Kishore U, Lim BL, Strong P, Reid KB. Interaction of human lung surfactant proteins A and D with mite (Dermatophagoides pteronyssinus) allergens. Clin Exp Immunol. 1996;106:367–373. doi: 10.1046/j.1365-2249.1996.d01-838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madan T, Kishore U, Shah A, Eggleton P, Strong P, Wang JY, Aggrawal SS, Sarma PU, Reid KB. Lung surfactant proteins A and D can inhibit specific IgE binding to the allergens of Aspergillus fumigatus and block allergen-induced histamine release from human basophils. Clin Exp Immunol. 1997;110:241–249. doi: 10.1111/j.1365-2249.1997.tb08323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faro A, Hamvas A. Lung transplantation for inherited disorders of surfactant metabolism. NeoReviews. 2008;9:e468–e476. [Google Scholar]
- 40.Gregory TJ, Longmore WJ, Moxley MA, Whitsett JA, Reed CR, Fowler AA, III, Hudson LD, Maunder RJ, Crim C, Hyers TM. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Invest. 1991;88:1976–1981. doi: 10.1172/JCI115523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hallman M, Spragg R, Harrell JH, Moser KM, Gluck L. Evidence of lung surfactant abnormality in respiratory failure: study of bronchoalveolar lavage phospholipids, surface activity, phospholipase activity, and plasma myoinositol. J Clin Invest. 1982;70:673–683. doi: 10.1172/JCI110662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidson WJ, Dorscheid D, Spragg R, Schulzer M, Mak E, Ayas NT. Exogenous pulmonary surfactant for the treatment of adult patients with acute respiratory distress syndrome: results of a meta-analysis. Crit Care. 2006;10:R41. doi: 10.1186/cc4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chida S, Phelps DS, Soll RF, Taeusch HW. Surfactant proteins and anti-surfactant antibodies in sera from infants with respiratory distress syndrome with and without surfactant treatment. Pediatrics. 1991;88:84–89. [PubMed] [Google Scholar]
- 44.Doyle IR, Nicholas TE, Bersten AD. Serum surfactant protein-A levels in patients with acute cardiogenic pulmonary edema and adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995;152:307–317. doi: 10.1164/ajrccm.152.1.7599839. [DOI] [PubMed] [Google Scholar]
- 45.Jobe A, Ikegami M, Jacobs H, Jones S, Conaway D. Permeability of premature lamb lungs to protein and the effect of surfactant on that permeability. J Appl Physiol. 1983;55:169–176. doi: 10.1152/jappl.1983.55.1.169. [DOI] [PubMed] [Google Scholar]
- 46.Robertson B, Curstedt T, Herting E, Sun B, Akino T, Schäfer KP. Alveolar-to-vascular leakage of surfactant protein A in ventilated immature newborn rabbits. Biol Neonate. 1995;68:185–190. doi: 10.1159/000244236. [DOI] [PubMed] [Google Scholar]
- 47.Delgado C, Krötzsch E, Jiménez-Alvarez LA, Ramírez-Martínez G, Márquez-García JE, Cruz-Lagunas A, Morán J, Hernández C, Sierra-Vargas P, Avila-Moreno F, et al. Serum Surfactant Protein D (SP-D) is a Prognostic Marker of Poor Outcome in Patients with A/H1N1 Virus Infection. Lung. 2015;193:25–30. doi: 10.1007/s00408-014-9669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuroki Y, Takahashi H, Chiba H, Akino T. Surfactant proteins A and D: disease markers. Biochim Biophys Acta. 1998;1408:334–345. doi: 10.1016/s0925-4439(98)00079-9. [DOI] [PubMed] [Google Scholar]
- 49.Selman M, Lin HM, Montaño M, Jenkins AL, Estrada A, Lin Z, Wang G, DiAngelo SL, Guo X, Umstead TM, et al. Surfactant protein A and B genetic variants predispose to idiopathic pulmonary fibrosis. Hum Genet. 2003;113:542–550. doi: 10.1007/s00439-003-1015-4. [DOI] [PubMed] [Google Scholar]
- 50.Lawson WE, Grant SW, Ambrosini V, Womble KE, Dawson EP, Lane KB, Markin C, Renzoni E, Lympany P, Thomas AQ, et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax. 2004;59:977–980. doi: 10.1136/thx.2004.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sumita Y, Sugiura T, Kawaguchi Y, Baba S, Soejima M, Murakawa Y, Hara M, Kamatani N. Genetic polymorphisms in the surfactant proteins in systemic sclerosis in Japanese: T/T genotype at 1580 C/T (Thr131Ile) in the SP-B gene reduces the risk of interstitial lung disease. Rheumatology (Oxford) 2008;47:289–291. doi: 10.1093/rheumatology/kem355. [DOI] [PubMed] [Google Scholar]
- 52.Saxena S, Kumar R, Madan T, Gupta V, Muralidhar K, Sarma PU. Association of polymorphisms in pulmonary surfactant protein A1 and A2 genes with high-altitude pulmonary edema. Chest. 2005;128:1611–1619. doi: 10.1378/chest.128.3.1611. [DOI] [PubMed] [Google Scholar]
- 53.Lin Z, Pearson C, Chinchilli V, Pietschmann SM, Luo J, Pison U, Floros J. Polymorphisms of human SP-A, SP-B, and SP-D genes: association of SP-B Thr131Ile with ARDS. Clin Genet. 2000;58:181–191. doi: 10.1034/j.1399-0004.2000.580305.x. [DOI] [PubMed] [Google Scholar]
- 54.Seifart C, Lin HM, Seifart U, Plagens A, DiAngelo S, von Wichert P, Floros J. Rare SP-A alleles and the SP-A1-6A(4) allele associate with risk for lung carcinoma. Clin Genet. 2005;68:128–136. doi: 10.1111/j.1399-0004.2005.00470.x. [DOI] [PubMed] [Google Scholar]
- 55.Pavlovic J, Papagaroufalis C, Xanthou M, Liu W, Fan R, Thomas NJ, Apostolidou I, Papathoma E, Megaloyianni E, DiAngelo S, et al. Genetic variants of surfactant proteins A, B, C, and D in bronchopulmonary dysplasia. Dis Markers. 2006;22:277–291. doi: 10.1155/2006/817805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, DiMaio JM, Kinch LN, Grishin NV, Garcia CK. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84:52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henderson FC, Miakotina OL, Mallampalli RK. Proapoptotic effects of P. aeruginosa involve inhibition of surfactant phosphatidylcholine synthesis. J Lipid Res. 2006;47:2314–2324. doi: 10.1194/jlr.M600284-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Wu Y, Xu Z, Henderson FC, Ryan AJ, Yahr TL, Mallampalli RK. Chronic Pseudomonas aeruginosa infection reduces surfactant levels by inhibiting its biosynthesis. Cell Microbiol. 2007;9:1062–1072. doi: 10.1111/j.1462-5822.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- 59.Malloy JL, Veldhuizen RA, Thibodeaux BA, O’Callaghan RJ, Wright JR. Pseudomonas aeruginosa protease IV degrades surfactant proteins and inhibits surfactant host defense and biophysical functions. Am J Physiol Lung Cell Mol Physiol. 2005;288:L409–L418. doi: 10.1152/ajplung.00322.2004. [DOI] [PubMed] [Google Scholar]
- 60.Mariencheck WI, Alcorn JF, Palmer SM, Wright JR. Pseudomonas aeruginosa elastase degrades surfactant proteins A and D. Am J Respir Cell Mol Biol. 2003;28:528–537. doi: 10.1165/rcmb.2002-0141OC. [DOI] [PubMed] [Google Scholar]
- 61.Kuang Z, Hao Y, Walling BE, Jeffries JL, Ohman DE, Lau GW. Pseudomonas aeruginosa elastase provides an escape from phagocytosis by degrading the pulmonary surfactant protein-A. PLoS One. 2011;6:e27091. doi: 10.1371/journal.pone.0027091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miakotina OL, McCoy DM, Shi L, Look DC, Mallampalli RK. Human adenovirus modulates surfactant phospholipid trafficking. Traffic. 2007;8:1765–1777. doi: 10.1111/j.1600-0854.2007.00641.x. [DOI] [PubMed] [Google Scholar]
- 63.Haczku A, Atochina EN, Tomer Y, Chen H, Scanlon ST, Russo S, Xu J, Panettieri RA, Jr, Beers MF. Aspergillus fumigatus-induced allergic airway inflammation alters surfactant homeostasis and lung function in BALB/c mice. Am J Respir Cell Mol Biol. 2001;25:45–50. doi: 10.1165/ajrcmb.25.1.4391. [DOI] [PubMed] [Google Scholar]
- 64.Bruce SR, Atkins CL, Colasurdo GN, Alcorn JL. Respiratory syncytial virus infection alters surfactant protein A expression in human pulmonary epithelial cells by reducing translation efficiency. Am J Physiol Lung Cell Mol Physiol. 2009;297:L559–L567. doi: 10.1152/ajplung.90507.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nayak A, Dodagatta-Marri E, Tsolaki AG, Kishore U. An insight into the diverse roles of surfactant proteins, SP-A and SP-D in innate and adaptive immunity. Front Immunol. 2012;3:131. doi: 10.3389/fimmu.2012.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kabha K, Schmegner J, Keisari Y, Parolis H, Schlepper-Schaeffer J, Ofek I. SP-A enhances phagocytosis of Klebsiella by interaction with capsular polysaccharides and alveolar macrophages. Am J Physiol. 1997;272:L344–L352. doi: 10.1152/ajplung.1997.272.2.L344. [DOI] [PubMed] [Google Scholar]
- 67.Kuan SF, Rust K, Crouch E. Interactions of surfactant protein D with bacterial lipopolysaccharides. Surfactant protein D is an Escherichia coli-binding protein in bronchoalveolar lavage. J Clin Invest. 1992;90:97–106. doi: 10.1172/JCI115861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuang Z, Hao Y, Hwang S, Zhang S, Kim E, Akinbi HT, Schurr MJ, Irvin RT, Hassett DJ, Lau GW. The Pseudomonas aeruginosa flagellum confers resistance to pulmonary surfactant protein-A by impacting the production of exoproteases through quorum-sensing. Mol Microbiol. 2011;79:1220–1235. doi: 10.1111/j.1365-2958.2010.07516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim BL, Wang JY, Holmskov U, Hoppe HJ, Reid KB. Expression of the carbohydrate recognition domain of lung surfactant protein D and demonstration of its binding to lipopolysaccharides of gram-negative bacteria. Biochem Biophys Res Commun. 1994;202:1674–1680. doi: 10.1006/bbrc.1994.2127. [DOI] [PubMed] [Google Scholar]
- 70.Restrepo CI, Dong Q, Savov J, Mariencheck WI, Wright JR. Surfactant protein D stimulates phagocytosis of Pseudomonas aeruginosa by alveolar macrophages. Am J Respir Cell Mol Biol. 1999;21:576–585. doi: 10.1165/ajrcmb.21.5.3334. [DOI] [PubMed] [Google Scholar]
- 71.Sawada K, Ariki S, Kojima T, Saito A, Yamazoe M, Nishitani C, Shimizu T, Takahashi M, Mitsuzawa H, Yokota S, et al. Pulmonary collectins protect macrophages against pore-forming activity of Legionella pneumophila and suppress its intracellular growth. J Biol Chem. 2010;285:8434–8443. doi: 10.1074/jbc.M109.074765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McNeely TB, Coonrod JD. Comparison of the opsonic activity of human surfactant protein A for Staphylococcus aureus and Streptococcus pneumoniae with rabbit and human macrophages. J Infect Dis. 1993;167:91–97. doi: 10.1093/infdis/167.1.91. [DOI] [PubMed] [Google Scholar]
- 73.Hartshorn KL, Crouch E, White MR, Colamussi ML, Kakkanatt A, Tauber B, Shepherd V, Sastry KN. Pulmonary surfactant proteins A and D enhance neutrophil uptake of bacteria. Am J Physiol. 1998;274:L958–L969. doi: 10.1152/ajplung.1998.274.6.L958. [DOI] [PubMed] [Google Scholar]
- 74.Piboonpocanun S, Chiba H, Mitsuzawa H, Martin W, Murphy RC, Harbeck RJ, Voelker DR. Surfactant protein A binds Mycoplasma pneumoniae with high affinity and attenuates its growth by recognition of disaturated phosphatidylglycerols. J Biol Chem. 2005;280:9–17. doi: 10.1074/jbc.M411570200. [DOI] [PubMed] [Google Scholar]
- 75.Ferguson JS, Voelker DR, McCormack FX, Schlesinger LS. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J Immunol. 1999;163:312–321. [PubMed] [Google Scholar]
- 76.Kudo K, Sano H, Takahashi H, Kuronuma K, Yokota S, Fujii N, Shimada K, Yano I, Kumazawa Y, Voelker DR, et al. Pulmonary collectins enhance phagocytosis of Mycobacterium avium through increased activity of mannose receptor. J Immunol. 2004;172:7592–7602. doi: 10.4049/jimmunol.172.12.7592. [DOI] [PubMed] [Google Scholar]
- 77.Shepherd VL. Pulmonary surfactant protein D: a novel link between innate and adaptive immunity. Am J Physiol Lung Cell Mol Physiol. 2002;282:L516–L517. doi: 10.1152/ajplung.00442.2001. [DOI] [PubMed] [Google Scholar]
- 78.Ragas A, Roussel L, Puzo G, Rivière M. The Mycobacterium tuberculosis cell-surface glycoprotein apa as a potential adhesin to colonize target cells via the innate immune system pulmonary C-type lectin surfactant protein A. J Biol Chem. 2007;282:5133–5142. doi: 10.1074/jbc.M610183200. [DOI] [PubMed] [Google Scholar]
- 79.Madan T, Eggleton P, Kishore U, Strong P, Aggrawal SS, Sarma PU, Reid KB. Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect Immun. 1997;65:3171–3179. doi: 10.1128/iai.65.8.3171-3179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lekkala M, LeVine AM, Linke MJ, Crouch EC, Linders B, Brummer E, Stevens DA. Effect of lung surfactant collectins on bronchoalveolar macrophage interaction with Blastomyces dermatitidis: inhibition of tumor necrosis factor alpha production by surfactant protein D. Infect Immun. 2006;74:4549–4556. doi: 10.1128/IAI.00243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Awasthi S, Magee DM, Coalson JJ. Coccidioides posadasii infection alters the expression of pulmonary surfactant proteins (SP)-A and SP-D. Respir Res. 2004;5:28. doi: 10.1186/1465-9921-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schelenz S, Malhotra R, Sim RB, Holmskov U, Bancroft GJ. Binding of host collectins to the pathogenic yeast Cryptococcus neoformans: human surfactant protein D acts as an agglutinin for acapsular yeast cells. Infect Immun. 1995;63:3360–3366. doi: 10.1128/iai.63.9.3360-3366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walenkamp AM, Verheul AF, Scharringa J, Hoepelman IM. Pulmonary surfactant protein A binds to Cryptococcus neoformans without promoting phagocytosis. Eur J Clin Invest. 1999;29:83–92. doi: 10.1046/j.1365-2362.1999.00429.x. [DOI] [PubMed] [Google Scholar]
- 84.Yong SJ, Vuk-Pavlovic Z, Standing JE, Crouch EC, Limper AH. Surfactant protein D-mediated aggregation of Pneumocystis carinii impairs phagocytosis by alveolar macrophages. Infect Immun. 2003;71:1662–1671. doi: 10.1128/IAI.71.4.1662-1671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zimmerman PE, Voelker DR, McCormack FX, Paulsrud JR, Martin WJ., II 120-kD surface glycoprotein of Pneumocystis carinii is a ligand for surfactant protein A. J Clin Invest. 1992;89:143–149. doi: 10.1172/JCI115554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Rozendaal BA, van Spriel AB, van De Winkel JG, Haagsman HP. Role of pulmonary surfactant protein D in innate defense against Candida albicans. J Infect Dis. 2000;182:917–922. doi: 10.1086/315799. [DOI] [PubMed] [Google Scholar]
- 87.Rosseau S, Guenther A, Seeger W, Lohmeyer J. Phagocytosis of viable Candida albicans by alveolar macrophages: lack of opsonin function of surfactant protein A. J Infect Dis. 1997;175:421–428. doi: 10.1093/infdis/175.2.421. [DOI] [PubMed] [Google Scholar]
- 88.Hartshorn KL, White MR, Voelker DR, Coburn J, Zaner K, Crouch EC. Mechanism of binding of surfactant protein D to influenza A viruses: importance of binding to haemagglutinin to antiviral activity. Biochem J. 2000;351:449–458. [PMC free article] [PubMed] [Google Scholar]
- 89.Hillaire ML, Haagsman HP, Osterhaus AD, Rimmelzwaan GF, van Eijk M. Pulmonary surfactant protein D in first-line innate defence against influenza A virus infections. J Innate Immun. 2013;5:197–208. doi: 10.1159/000346374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hartshorn KL, Sastry KN, Chang D, White MR, Crouch EC. Enhanced anti-influenza activity of a surfactant protein D and serum conglutinin fusion protein. Am J Physiol Lung Cell Mol Physiol. 2000;278:L90–L98. doi: 10.1152/ajplung.2000.278.1.L90. [DOI] [PubMed] [Google Scholar]
- 91.Qi L, Kash JC, Dugan VG, Jagger BW, Lau YF, Sheng ZM, Crouch EC, Hartshorn KL, Taubenberger JK. The ability of pandemic influenza virus hemagglutinins to induce lower respiratory pathology is associated with decreased surfactant protein D binding. Virology. 2011;412:426–434. doi: 10.1016/j.virol.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meschi J, Crouch EC, Skolnik P, Yahya K, Holmskov U, Leth-Larsen R, Tornoe I, Tecle T, White MR, Hartshorn KL. Surfactant protein D binds to human immunodeficiency virus (HIV) envelope protein gp120 and inhibits HIV replication. J Gen Virol. 2005;86:3097–3107. doi: 10.1099/vir.0.80764-0. [DOI] [PubMed] [Google Scholar]
- 93.Pandit H, Gopal S, Sonawani A, Yadav AK, Qaseem AS, Warke H, Patil A, Gajbhiye R, Kulkarni V, Al-Mozaini MA, et al. Surfactant protein D inhibits HIV-1 infection of target cells via interference with gp120-CD4 interaction and modulates pro-inflammatory cytokine production. PLoS One. 2014;9:e102395. doi: 10.1371/journal.pone.0102395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hickling TP, Bright H, Wing K, Gower D, Martin SL, Sim RB, Malhotra R. A recombinant trimeric surfactant protein D carbohydrate recognition domain inhibits respiratory syncytial virus infection in vitro and in vivo. Eur J Immunol. 1999;29:3478–3484. doi: 10.1002/(SICI)1521-4141(199911)29:11<3478::AID-IMMU3478>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 95.Leth-Larsen R, Zhong F, Chow VT, Holmskov U, Lu J. The SARS coronavirus spike glycoprotein is selectively recognized by lung surfactant protein D and activates macrophages. Immunobiology. 2007;212:201–211. doi: 10.1016/j.imbio.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Numata M, Kandasamy P, Nagashima Y, Fickes R, Murphy RC, Voelker DR. Phosphatidylinositol inhibits respiratory syncytial virus infection. J Lipid Res. 2015;56:578–587. doi: 10.1194/jlr.M055723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hintz SR, Poole WK, Wright LL, Fanaroff AA, Kendrick DE, Laptook AR, Goldberg R, Duara S, Stoll BJ, Oh W NICHD Neonatal Research Network. Changes in mortality and morbidities among infants born at less than 25 weeks during the post-surfactant era. Arch Dis Child Fetal Neonatal Ed. 2005;90:F128–F133. doi: 10.1136/adc.2003.046268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoekstra RE, Jackson JC, Myers TF, Frantz ID, III, Stern ME, Powers WF, Maurer M, Raye JR, Carrier ST, Gunkel JH, et al. Improved neonatal survival following multiple doses of bovine surfactant in very premature neonates at risk for respiratory distress syndrome. Pediatrics. 1991;88:10–18. [PubMed] [Google Scholar]
- 99.Liechty EA, Donovan E, Purohit D, Gilhooly J, Feldman B, Noguchi A, Denson SE, Sehgal SS, Gross I, Stevens D, et al. Reduction of neonatal mortality after multiple doses of bovine surfactant in low birth weight neonates with respiratory distress syndrome. Pediatrics. 1991;88:19–28. [PubMed] [Google Scholar]
- 100.Seger N, Soll R. Animal derived surfactant extract for treatment of respiratory distress syndrome. Cochrane Database Syst Rev. 2009;(2):CD007836. doi: 10.1002/14651858.CD007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, Saugstad OD, Simeoni U, Speer CP, Halliday HL European Association of Perinatal Medicine. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants - 2010 update. Neonatology. 2010;97:402–417. doi: 10.1159/000297773. [DOI] [PubMed] [Google Scholar]
- 102.Committee on Fetus and Newborn; American Academy of Pediatrics. Respiratory support in preterm infants at birth. Pediatrics. 2014;133:171–174. doi: 10.1542/peds.2013-3442. [DOI] [PubMed] [Google Scholar]
- 103.Polin RA, Carlo WA Committee on Fetus and Newborn; American Academy of Pediatrics. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133:156–163. doi: 10.1542/peds.2013-3443. [DOI] [PubMed] [Google Scholar]
- 104.Tarawneh A, Kaczmarek J, Bottino MN, Sant'anna GM. Severe airway obstruction during surfactant administration using a standardized protocol: a prospective, observational study. J Perinatol. 2012;32:270–275. doi: 10.1038/jp.2011.89. [DOI] [PubMed] [Google Scholar]
- 105.Al Ethawi Y. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. J Clin Neonatol. 2012;1:66–68. doi: 10.4103/2249-4847.96744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dargaville PA, Aiyappan A, Cornelius A, Williams C, De Paoli AG. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch Dis Child Fetal Neonatal Ed. 2011;96:F243–F248. doi: 10.1136/adc.2010.192518. [DOI] [PubMed] [Google Scholar]
- 107.Abdel-Latif ME, Osborn DA. Laryngeal mask airway surfactant administration for prevention of morbidity and mortality in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst Rev. 2011;7:CD008309. doi: 10.1002/14651858.CD008309.pub2. [DOI] [PubMed] [Google Scholar]
- 108.Göpel W, Kribs A, Ziegler A, Laux R, Hoehn T, Wieg C, Siegel J, Avenarius S, von der Wense A, Vochem M, et al. German Neonatal Network. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet. 2011;378:1627–1634. doi: 10.1016/S0140-6736(11)60986-0. [DOI] [PubMed] [Google Scholar]
- 109.Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics. 2013;131:e502–e509. doi: 10.1542/peds.2012-0603. [DOI] [PubMed] [Google Scholar]
- 110.Spragg RG, Lewis JF, Wurst W, Häfner D, Baughman RP, Wewers MD, Marsh JJ. Treatment of acute respiratory distress syndrome with recombinant surfactant protein C surfactant. Am J Respir Crit Care Med. 2003;167:1562–1566. doi: 10.1164/rccm.200207-782OC. [DOI] [PubMed] [Google Scholar]
- 111.Weg JG, Balk RA, Tharratt RS, Jenkinson SG, Shah JB, Zaccardelli D, Horton J, Pattishall EN. Safety and potential efficacy of an aerosolized surfactant in human sepsis-induced adult respiratory distress syndrome. JAMA. 1994;272:1433–1438. [PubMed] [Google Scholar]
- 112.Anzueto A, Baughman RP, Guntupalli KK, Weg JG, Wiedemann HP, Raventós AA, Lemaire F, Long W, Zaccardelli DS, Pattishall EN Exosurf Acute Respiratory Distress Syndrome Sepsis Study Group. Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome. N Engl J Med. 1996;334:1417–1421. doi: 10.1056/NEJM199605303342201. [DOI] [PubMed] [Google Scholar]
- 113.Spragg RG, Lewis JF, Walmrath HD, Johannigman J, Bellingan G, Laterre PF, Witte MC, Richards GA, Rippin G, Rathgeb F, et al. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med. 2004;351:884–892. doi: 10.1056/NEJMoa033181. [DOI] [PubMed] [Google Scholar]
- 114.Gregory TJ, Steinberg KP, Spragg R, Gadek JE, Hyers TM, Longmore WJ, Moxley MA, Cai GZ, Hite RD, Smith RM, et al. Bovine surfactant therapy for patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;155:1309–1315. doi: 10.1164/ajrccm.155.4.9105072. [DOI] [PubMed] [Google Scholar]
- 115.Gunther A, Siebert C, Schmidt R, Ziegler S, Grimminger F, Yabut M, Temmesfeld B, Walmrath D, Morr H, Seeger W. Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med. 1996;153:176–184. doi: 10.1164/ajrccm.153.1.8542113. [DOI] [PubMed] [Google Scholar]
- 116.Griese M. Pulmonary surfactant in health and human lung diseases: state of the art. Eur Respir J. 1999;13:1455–1476. doi: 10.1183/09031936.99.13614779. [DOI] [PubMed] [Google Scholar]
- 117.Kurashima K, Ogawa H, Ohka T, Fujimura M, Matsuda T, Kobayashi T. A pilot study of surfactant inhalation in the treatment of asthmatic attack. Arerugi. 1991;40:160–163. [PubMed] [Google Scholar]
- 118.Oetomo SB, Dorrepaal C, Bos H, Gerritsen J, van der Mark TW, Koëter GH, van Aalderen WM. Surfactant nebulization does not alter airflow obstruction and bronchial responsiveness to histamine in asthmatic children. Am J Respir Crit Care Med. 1996;153:1148–1152. doi: 10.1164/ajrccm.153.3.8630559. [DOI] [PubMed] [Google Scholar]
- 119.Mikawa K, Maekawa N, Nishina K, Takao Y, Yaku H, Obara H. Selective intrabronchial instillation of surfactant in a patient with pneumonia: a preliminary report. Eur Respir J. 1993;6:1563–1566. [PubMed] [Google Scholar]
- 120.Marriage SC, Underhill H, Nadel S. Use of natural surfactant in an HIV-infected infant with Pneumocystis carinii pneumonia. Intensive Care Med. 1996;22:611–612. doi: 10.1007/BF01708109. [DOI] [PubMed] [Google Scholar]
- 121.Creery WD, Hashmi A, Hutchison JS, Singh RN. Surfactant therapy improves pulmonary function in infants with Pneumocystis carinii pneumonia and acquired immunodeficiency syndrome. Pediatr Pulmonol. 1997;24:370–373. doi: 10.1002/(sici)1099-0496(199711)24:5<370::aid-ppul10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 122.Vos GD, Rijtema MN, Blanco CE. Treatment of respiratory failure due to respiratory syncytial virus pneumonia with natural surfactant. Pediatr Pulmonol. 1996;22:412–415. doi: 10.1002/(SICI)1099-0496(199612)22:6<412::AID-PPUL11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 123.Anzueto A, Jubran A, Ohar JA, Piquette CA, Rennard SI, Colice G, Pattishall EN, Barrett J, Engle M, Perret KA, et al. Effects of aerosolized surfactant in patients with stable chronic bronchitis: a prospective randomized controlled trial. JAMA. 1997;278:1426–1431. [PubMed] [Google Scholar]
- 124.Allen JN, Moore SA, Pope-Harman AL, Marsh CB, Wewers MD. Immunosuppressive properties of surfactant and plasma on alveolar macrophages. J Lab Clin Med. 1995;125:356–369. [PubMed] [Google Scholar]
- 125.Antal JM, Divis LT, Erzurum SC, Wiedemann HP, Thomassen MJ. Surfactant suppresses NF-kappa B activation in human monocytic cells. Am J Respir Cell Mol Biol. 1996;14:374–379. doi: 10.1165/ajrcmb.14.4.8600942. [DOI] [PubMed] [Google Scholar]
- 126.Thomassen MJ, Antal JM, Barna BP, Divis LT, Meeker DP, Wiedemann HP. Surfactant downregulates synthesis of DNA and inflammatory mediators in normal human lung fibroblasts. Am J Physiol. 1996;270:L159–L163. doi: 10.1152/ajplung.1996.270.1.L159. [DOI] [PubMed] [Google Scholar]
- 127.Bartmann P, Bamberger U, Pohlandt F, Gortner L. Immunogenicity and immunomodulatory activity of bovine surfactant (SF-RI 1) Acta Paediatr. 1992;81:383–388. doi: 10.1111/j.1651-2227.1992.tb12254.x. [DOI] [PubMed] [Google Scholar]
- 128.Bartmann P, Gortner L, Pohlandt F, Jaeger H. In vitro lymphocyte functions in the presence of bovine surfactant and its phospholipid fractions. J Perinat Med. 1992;20:189–196. doi: 10.1515/jpme.1992.20.3.189. [DOI] [PubMed] [Google Scholar]
- 129.Roth MD, Pinto M, Golub SH, Shau H. Pulmonary surfactant inhibits interleukin-2-induced proliferation and the generation of lymphokine-activated killer cells. Am J Respir Cell Mol Biol. 1993;9:652–658. doi: 10.1165/ajrcmb/9.6.652. [DOI] [PubMed] [Google Scholar]
- 130.Birkun AA, Kubyshkin AV, Novikov NY, Krivorutchenko YL, Fedosov MI, Postnikova ON, Snitser AA. Joint intratracheal surfactant-antibacterial therapy in experimental Pseudomonas-induced pneumonia. J Aerosol Med Pulm Drug Deliv. doi: 10.1089/jamp.2014.1161. [online ahead of print] 17 Dec 2014; DOI:10.1089/jamp.2014.1161. [DOI] [PubMed] [Google Scholar]
- 131.Ballard PL, Ballard RA. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol. 1995;173:254–262. doi: 10.1016/0002-9378(95)90210-4. [DOI] [PubMed] [Google Scholar]
- 132.Ballard PL, Hovey ML, Gonzales LK. Thyroid hormone stimulation of phosphatidylcholine synthesis in cultured fetal rabbit lung. J Clin Invest. 1984;74:898–905. doi: 10.1172/JCI111507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ikegami M, Polk D, Tabor B, Lewis J, Yamada T, Jobe A. Corticosteroid and thyrotropin-releasing hormone effects on preterm sheep lung function. J Appl Physiol (1985) 1991;70:2268–2278. doi: 10.1152/jappl.1991.70.5.2268. [DOI] [PubMed] [Google Scholar]
- 134.Gonzalez Garay AG, Reveiz L, Velasco Hidalgo L, Solis Galicia C. Ambroxol for women at risk of preterm birth for preventing neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2014;10:CD009708. doi: 10.1002/14651858.CD009708.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rosen DM, Waltz DA. Hydroxychloroquine and surfactant protein C deficiency. N Engl J Med. 2005;352:207–208. doi: 10.1056/NEJM200501133520223. [DOI] [PubMed] [Google Scholar]
- 136.Hepping N, Griese M, Lohse P, Garbe W, Lange L. Successful treatment of neonatal respiratory failure caused by a novel surfactant protein C p.Cys121Gly mutation with hydroxychloroquine. J Perinatol. 2013;33:492–494. doi: 10.1038/jp.2012.131. [DOI] [PubMed] [Google Scholar]
- 137.Rabach I, Poli F, Zennaro F, Germani C, Ventura A, Barbi E. Is treatment with hydroxychloroquine effective in surfactant protein C deficiency? Arch Bronconeumol. 2013;49:213–215. doi: 10.1016/j.arbres.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 138.Beers MF. Inhibition of cellular processing of surfactant protein C by drugs affecting intracellular pH gradients. J Biol Chem. 1996;271:14361–14370. doi: 10.1074/jbc.271.24.14361. [DOI] [PubMed] [Google Scholar]
- 139.Xu X, McCormick-Shannon K, Voelker DR, Mason RJ. KGF increases SP-A and SP-D mRNA levels and secretion in cultured rat alveolar type II cells. Am J Respir Cell Mol Biol. 1998;18:168–178. doi: 10.1165/ajrcmb.18.2.2824. [DOI] [PubMed] [Google Scholar]
- 140.Hallman M, Merritt TA, Pohjavuori M, Gluck L. Effect of surfactant substitution on lung effluent phospholipids in respiratory distress syndrome: evaluation of surfactant phospholipid turnover, pool size, and the relationship to severity of respiratory failure. Pediatr Res. 1986;20:1228–1235. doi: 10.1203/00006450-198612000-00008. [DOI] [PubMed] [Google Scholar]
- 141.Stevens PA, Schadow B, Bartholain S, Segerer H, Obladen M. Surfactant protein A in the course of respiratory distress syndrome. Eur J Pediatr. 1992;151:596–600. doi: 10.1007/BF01957730. [DOI] [PubMed] [Google Scholar]
- 142.Moya FR, Montes HF, Thomas VL, Mouzinho AM, Smith JF, Rosenfeld CR. Surfactant protein A and saturated phosphatidylcholine in respiratory distress syndrome. Am J Respir Crit Care Med. 1994;150:1672–1677. doi: 10.1164/ajrccm.150.6.7952631. [DOI] [PubMed] [Google Scholar]
- 143.Hallman M, Merritt TA, Akino T, Bry K. Surfactant protein A, phosphatidylcholine, and surfactant inhibitors in epithelial lining fluid. Correlation with surface activity, severity of respiratory distress syndrome, and outcome in small premature infants. Am Rev Respir Dis. 1991;144:1376–1384. doi: 10.1164/ajrccm/144.6.1376. [DOI] [PubMed] [Google Scholar]
- 144.Singh G, Katyal SL, Bedrossian CW, Rogers RM. Pulmonary alveolar proteinosis. Staining for surfactant apoprotein in alveolar proteinosis and in conditions simulating it. Chest. 1983;83:82–86. doi: 10.1378/chest.83.1.82. [DOI] [PubMed] [Google Scholar]
- 145.Honda Y, Kuroki Y, Matsuura E, Nagae H, Takahashi H, Akino T, Abe S. Pulmonary surfactant protein D in sera and bronchoalveolar lavage fluids. Am J Respir Crit Care Med. 1995;152:1860–1866. doi: 10.1164/ajrccm.152.6.8520747. [DOI] [PubMed] [Google Scholar]
- 146.Akino T, Okano G, Ohno K. Alveolar phospholipids in pulmonary alveolar proteinosis. Tohoku J Exp Med. 1978;126:51–62. doi: 10.1620/tjem.126.51. [DOI] [PubMed] [Google Scholar]
- 147.Pison U, Obertacke U, Seeger W, Hawgood S. Surfactant protein A (SP-A) is decreased in acute parenchymal lung injury associated with polytrauma. Eur J Clin Invest. 1992;22:712–718. doi: 10.1111/j.1365-2362.1992.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 148.McCormack FX, King TE, Jr, Voelker DR, Robinson PC, Mason RJ. Idiopathic pulmonary fibrosis. Abnormalities in the bronchoalveolar lavage content of surfactant protein A. Am Rev Respir Dis. 1991;144:160–166. doi: 10.1164/ajrccm/144.1.160. [DOI] [PubMed] [Google Scholar]
- 149.Honda Y, Tsunematsu K, Suzuki A, Akino T. Changes in phospholipids in bronchoalveolar lavage fluid of patients with interstitial lung diseases. Lung. 1988;166:293–301. doi: 10.1007/BF02714060. [DOI] [PubMed] [Google Scholar]
- 150.Robinson PC, Watters LC, King TE, Mason RJ. Idiopathic pulmonary fibrosis. Abnormalities in bronchoalveolar lavage fluid phospholipids. Am Rev Respir Dis. 1988;137:585–591. doi: 10.1164/ajrccm/137.3.585. [DOI] [PubMed] [Google Scholar]
- 151.Hamm H, Lührs J, Guzman y Rotaeche J, Costabel U, Fabel H, Bartsch W. Elevated surfactant protein A in bronchoalveolar lavage fluids from sarcoidosis and hypersensitivity pneumonitis patients. Chest. 1994;106:1766–1770. doi: 10.1378/chest.106.6.1766. [DOI] [PubMed] [Google Scholar]
- 152.Low RB, Davis GS, Bell DY, Giancola MS, Vacek PM. Lipids in bronchoalveolar lavage fluid from patients with sarcoidosis. Thorax. 1987;42:926–932. doi: 10.1136/thx.42.12.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Baughman RP, Sternberg RI, Hull W, Buchsbaum JA, Whitsett J. Decreased surfactant protein A in patients with bacterial pneumonia. Am Rev Respir Dis. 1993;147:653–657. doi: 10.1164/ajrccm/147.3.653. [DOI] [PubMed] [Google Scholar]
- 154.Baughman RP, Stein E, MacGee J, Rashkin M, Sahebjami H. Changes in fatty acids in phospholipids of the bronchoalveolar fluid in bacterial pneumonia and in adult respiratory distress syndrome. Clin Chem. 1984;30:521–523. [PubMed] [Google Scholar]
- 155.Honda Y, Takahashi H, Kuroki Y, Akino T, Abe S. Decreased contents of surfactant proteins A and D in BAL fluids of healthy smokers. Chest. 1996;109:1006–1009. doi: 10.1378/chest.109.4.1006. [DOI] [PubMed] [Google Scholar]
- 156.Low RB, Davis GS, Giancola MS. Biochemical analyses of bronchoalveolar lavage fluids of healthy human volunteer smokers and nonsmokers. Am Rev Respir Dis. 1978;118:863–875. doi: 10.1164/arrd.1978.118.5.863. [DOI] [PubMed] [Google Scholar]
- 157.van de Graaf EA, Jansen HM, Lutter R, Alberts C, Kobesen J, de Vries IJ, Out TA. Surfactant protein A in bronchoalveolar lavage fluid. J Lab Clin Med. 1992;120:252–263. [PubMed] [Google Scholar]