Abstract
Rationale: Former smoking history and chronic obstructive pulmonary disease (COPD) are potential risk factors for osteoporosis and fractures. Under existing guidelines for osteoporosis screening, women are included but men are not, and only current smoking is considered.
Objectives: To demonstrate the impact of COPD and smoking history on the risk of osteoporosis and vertebral fracture in men and women.
Methods: Characteristics of participants with low volumetric bone mineral density (vBMD) were identified and related to COPD and other risk factors. We tested associations of sex and COPD with both vBMD and fractures adjusting for age, race, body mass index (BMI), smoking, and glucocorticoid use.
Measurements and Main Results: vBMD by calibrated quantitative computed tomography (QCT), visually scored vertebral fractures, and severity of lung disease were determined from chest CT scans of 3,321 current and ex-smokers in the COPDGene study. Low vBMD as a surrogate for osteoporosis was calculated from young adult normal values. Male smokers had a small but significantly greater risk of low vBMD (2.5 SD below young adult mean by calibrated QCT) and more fractures than female smokers. Low vBMD was present in 58% of all subjects, was more frequent in those with worse COPD, and rose to 84% among subjects with very severe COPD. Vertebral fractures were present in 37% of all subjects and were associated with lower vBMD at each Global Initiative for Chronic Obstructive Lung Disease stage of severity. Vertebral fractures were most common in the midthoracic region. COPD and especially emphysema were associated with both low vBMD and vertebral fractures after adjustment for steroid use, age, pack-years of smoking, current smoking, and exacerbations. Airway disease was associated with higher bone density after adjustment for other variables. Calibrated QCT identified more subjects with abnormal values than the standard dual-energy X-ray absorptiometry in a subset of subjects and correlated well with prevalent fractures.
Conclusions: Male smokers, with or without COPD, have a significant risk of low vBMD and vertebral fractures. COPD was associated with low vBMD after adjusting for race, sex, BMI, smoking, steroid use, exacerbations, and age. Screening for low vBMD by using QCT in men and women who are smokers will increase opportunities to identify and treat osteoporosis in this at-risk population.
Keywords: low bone density, COPD, vertebral fractures, quantitative computed tomography, smoking
On the basis of the results of several small studies, osteoporosis and vertebral fractures appear to coexist with chronic obstructive pulmonary disease (COPD), and the relationship is often assumed to be due to glucocorticoids (1–4). Current smoking is known to negatively affect bone density, but a direct association of osteoporosis with COPD has proven elusive (5, 6). In a large study, the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE), researchers failed to demonstrate an association of reduced bone attenuation values with COPD after adjusting for smoking history and demographics. More recently, however, a subset of men from the Dutch-Belgian Randomized Lung Cancer Screening Trial (i.e., the NELSON study) was noted to have an association of bone attenuation values obtained by computed tomography (CT) as a surrogate for bone mineral density (BMD) with COPD status after adjusting for age and smoking (7). The explanations for an association of osteoporosis and COPD are not well defined, but they potentially include smoking effects on bone (5, 8), inactivity (1), nutritional deficits, glucocorticoid use (9), and chronic inflammation (10, 11).
COPD is the third leading cause of death in the United States (12), and up to 49% of the population over age 45 years is a current or ex-smoker (13). However, neither smokers nor patients with COPD are routinely screened for osteoporosis. Because of insufficient evidence, the American College of Physicians (14) does not recognize COPD as a risk factor for osteoporosis; in addition, the U.S. Preventive Services Task Force (USPSTF) recommendations regarding osteoporosis screening do not address COPD or smoking and, owing to a lack of adequate supporting data, do not include men (15). COPD is not included in the World Health Organization (WHO) FRAX fracture risk assessment tool (16), and only current smoking, and not former smoking, is considered as a risk factor. Women are typically screened, but in spite of increased recognition that men have some risk of osteoporosis and fracture (17), there are few large studies in which their risk in relation to smoking (current and former) or COPD has been assessed. Inadequate data regarding fracture risk in men may be compounded by use of dual-energy X-ray absorptiometry (DXA) as the measurement standard, given that it appears to be less effective in risk prediction for men (18, 19).
In a large cohort of smokers with or without COPD, we determined volumetric BMD (vBMD) by using calibrated quantitative CT (QCT) and scored vertebral fractures. We investigated the relationships of age, sex, race, steroid use, smoking, and COPD (airflow obstruction, CT-confirmed emphysema, and CT-confirmed airway disease) to reduced bone density and vertebral fractures. We hypothesized that COPD would be associated with both decreased bone density and an increased prevalence of vertebral fractures.
Methods
Study Design
A convenience sample of 3,321 subjects from the COPDGene study had a bone density calibrated chest CT scan, and these subjects constituted our analysis group. A comparison group of 63 never-smokers were included in this group and the analysis. COPDGene is a multicenter study that enrolled 10,300 subjects aged 45 to 80 years to identify genetic determinants of COPD (20). Subjects were non-Hispanic white (NHW) or African American (AA), current smokers or ex-smokers, had a minimum 10–pack-year smoking history, and a COPD diagnosis based on spirometry (see Table E1 in the online supplement). Subjects reported chronic inhaled and oral steroid use; a history of severe respiratory exacerbations (treated with antibiotics or steroids and hospital care) and other exacerbations treated with steroids; and a history of a physician’s diagnosis of coronary artery disease (CAD), diabetes mellitus (DM), hypertension (HTN), and gastroesophageal reflux disease (GERD). A 6-minute-walk test was administered (21). Subjects reported current medications.
CT Scans
CT scans were acquired using multidetector CT scanners and standardized protocols (22). Quantitative analysis of CT scans was performed using 3DSlicer software (http://www.slicer.org/) to assess emphysema (determined by percentage of voxels representing low attenuation area less than −950 Hounsfield units [HU] [LAA% < −950]) and thickness of segmental airway walls (assessed on the basis of the wall area percentage [WA%]) using Pulmonary Workstation Plus (VIDA Diagnostics, Coralville, IA) as previously reported (23). INTable CT scanner pads (Image Analysis Inc., Columbia, KY) containing calcium hydroxyapatite (CaHA) calibration rods were included in the field of view for all CT scans used in this study. Use of the calibration phantom included within CT scans allowed comparisons of vBMD to be made across different CT scanners.
Bone Density Analysis
BMD was measured with automated software (N-Vivo; Image Analysis Inc., Columbia, KY) that calibrated voxel density to the CaHA rods. Trabecular vBMD was quantified for each of the selected vertebrae where an automated region of interest (ROI) was positioned (Figure E1). The ROI was located within the vertebral body, positioned at least 2–3 mm from the cortical bone, thus including only trabecular bone. Obviously fractured vertebrae were excluded from measurement, and vBMD was obtained for vertebrae from T6 to L1 using a minimum of three vertebral bodies to calculate a mean vBMD in milligrams per cubic millimeter. Criteria for classifying the extent of bone loss were derived using young adult, sex-specific QCT reference data published by Budoff and colleagues measured with the same software and calibration phantom (15). Low bone density was defined as a T-scoreQCT of −2.5 or less using the conventional 2.5 standard deviations below the young adult population mean (24). Intermediate bone density was defined as a T-scoreQCT between −1 and −2.5.
Fracture Scoring
A team of six trained readers and two adjudicators reviewed CT scans to score vertebral fractures using AquariusNET software (TeraRecon, Foster City, CA). Readers identified central compression and wedge vertebral fractures on T1-L1 vertebrae using the semiquantitative method of Genant and colleagues (25). Only moderate or severe fractures as determined by the Genant method were scored. A minimum of two readers using a three-dimensional reconstruction recorded a score for each vertebra. Lack of agreement was adjudicated to a consensus score. There was complete agreement between readers for 1,887 scans, and 1,430 scans required adjudication for differences in fracture assessment at one or more levels. Scans requiring adjudication were much more likely to have fractures identified.
Comparison DXA Measurements
There were 110 subjects (54 male and 57 female) from a single clinical center who had standard DXA bone density screening of the lumbar spine done within 12 months of the COPDGene CT scan for comparison. Areal BMD (aBMD) in milligrams per square centimeter, T-scores based on population values for lumbar spine, and categorization of subjects as having osteoporosis (T-score less than −2.5), osteopenia (T-score greater than −2.5 and less than −1.0), or normal (T-score greater than −1.0) were recorded and compared with the QCT results for the thoracic spine. Fracture percentages in each of these categories were determined.
Statistical Analysis
Data were analyzed using JMP 10.0 software (SAS Institute, Cary, NC). Univariate comparisons between groups were made with Student’s t test for continuous variables and a chi-squared or Fisher’s exact test for categorical variables. Multivariable regression modeling was used to evaluate the relationship of BMD to COPD using emphysema and WA%. Logistic regression was performed to determine factors associated with low bone density as a surrogate for osteoporosis and vertebral fractures in this cohort. For regression modeling, the three categories of bone disease were collapsed into two (normal and intermediate versus low). All of the models for BMD and fracture were initially tested for associations with age, sex, race, body mass index (BMI), glucocorticoid use (both inhaled and oral), pack-years of smoking, current smoking status, severe exacerbations, and self-reports of four comorbid conditions (CAD, DM, HTN, and GERD).
Results
Study Participants
A total of 3,321 CT scans were analyzed for vBMD, and 3,317 scans had complete fracture scoring. The classification of subjects based on Global Initiative for Chronic Obstructive Lung Disease (GOLD) (26) criteria for airway obstruction in COPD is shown in Table E1. The BMD group was similar to the remaining COPDGene cohort (Table E2), with the exceptions that fewer AAs were included in the BMD group (31% AAs compared with 34% AA in the remainder group) than the remainder group and subjects with BMD had a greater mean 6-minute-walk distance.
Bone Disease Groups
The subjects were classified by bone density based on T-scoreQCT (Table 1). A total of 380 subjects(11%) were found to have normal bone density (T-scoreQCT greater than −1.0), 1,017 (31%) had intermediate bone density (T-scoreQCT greater than −2.5 and less than −1.0), and 1,924 (58%) had low bone density (T-scoreQCT less than −2.5). Mean age was inversely associated with bone density. The proportion of males was higher in the low bone density group, and the proportion of AAs was lowest (16.6%) in that group. Pack-years of smoking were greater in the low bone density group, but the proportion of current smokers was lower in the intermediate and low bone density groups. Mean vBMD was 211.6 mg/cm3 in the normal bone density group, 154.2 mg/cm3 in the intermediate bone density group, and 95.1 mg/cm3 in the low bone density group. Chronic oral steroid use was similar among groups, but the proportion of subjects using inhaled steroids and treated with steroids for exacerbations was highest in the low bone density group.
Table 1.
Classification of COPDGene subjects by bone disease
| Normal (T-scoreQCT greater than −1.0) | Intermediate Bone Density (T-scoreQCT less than −1.0 and greater than −2.5) | Low Bone Density (T-scoreQCT less than −2.5) | |
|---|---|---|---|
| Number of subjects | 380 | 1,017 | 1,924 |
| Age, yr | 52.8 (6.8) | 56.3 (7.7)* | 62.3 (8.8)*† |
| Sex, % male | 31.3% | 52.5%* | 55.7%*† |
| Race, % African American | 69.7% | 44.6%* | 16.6%*† |
| BMI | 31.6 (7.3) | 29.6 (6.4)* | 27.7 (5.5)*† |
| Current smoker | 70.8% | 60.0%* | 43.4%*† |
| Pack-yr of smoking | 36.6 (22.6) | 40.3 (21.7) | 46.9 (25.6)† |
| Chronic oral steroid use | 2.5% | 1.5% | 2.7% |
| Chronic inhaled steroid use | 16.8% | 18.6%* | 27.2%* |
| Flare-up of chest trouble | 19.0% | 21.5% | 27.6%‡ |
| Treated with steroid | 13.2% | 14.4% | 18.9%‡ |
| Severe exacerbations | 10.8% | 11.1% | 12.5% |
| QCT BMD, mg/cm3 | 211.6 (25.1) | 154.2 (14.7)* | 95.1 (24.7)*† |
| FEV1, % predicted | 82.6 (20.7) | 81.5 (22.9) | 73.8 (27.2)*† |
| FVC, % predicted | 88.1 (17.3) | 88.7 (17.3) | 86.9 (18.5)‡ |
| FEV1/FVC | 0.74 (0.11) | 0.71 (0.13) | 0.64 (0.18)*† |
| COPD, % GOLD Stages 2–4 | 20.5% | 26.4%* | 42.7%*† |
| 6-min-walk distance, ft | 1,337.7 (353.6) | 1,429.5 (380.4)* | 1,407.0 (396.5) |
| Airway wall area, % segmental | 62.5 (3.45) | 61.5 (3.3)* | 60.9 (3.2)*† |
| LAA% < −950 HU (emphysema) | 2.4 (4.2) | 4.0 (7.4) | 7.1 (10.7)*† |
| Osteoporosis diagnosis | 2.6% (n = 10) | 2.9% (n = 29) | 13% (n = 251)*† |
| Treated with bisphosphonate | 0.5% (n = 2) | 0.3% (n = 3) | 2.3% (n = 45)*† |
| Coronary artery disease | 10% | 10.8% | 13.8%‡ |
| Diabetes | 14.7% | 13.2% | 11.6% |
Definition of abbreviations: BMD = bone mineral density; BMI = body mass index; COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; HU = Hounsfield units; LAA% = low attenuation area percentage; QCT = quantitative computed tomography
Values are mean and standard deviation, except where percent is shown. Boldface type indicates P < 0.05 for comparisons with normal bone density group.
P < 0.0001 for comparisons with normal bone density group.
P < 0.0001 for comparison of low bone density group with intermediate bone density group.
P < 0.05 for comparison of low bone density group with intermediate bone density group.
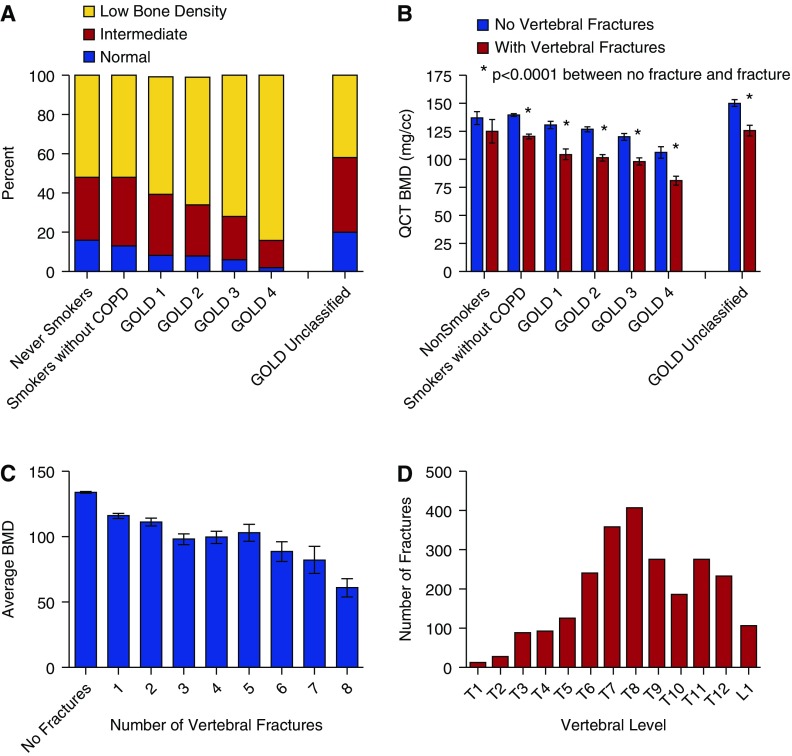
The proportion of subjects who had COPD (GOLD Stages 2–4) was higher in association with low bone density (from 20.5% in the normal bone density group to 26.4% in the intermediate and 42.7% in the low bone density groups). There were divergent associations of vBMD with subtypes of COPD. Emphysema was associated with low bone density, and airway wall thickness measured as WA% was greatest in the subjects with normal bone density in this unadjusted analysis. CAD and GERD were more frequent in the low bone density group. Six-minute-walk distance was increased in the intermediate and low bone density groups, which was likely related to a higher proportion of men in those groups. Figure 1A shows the distribution of bone disease by GOLD stage, with increased proportions of low bone density in the patients with severe COPD.
Figure 1.
Bone density and fractures in the COPDGene cohort. (A) The proportion of subjects with normal bone density, intermediate bone density (T-scoreQCT less than −1.0 and greater than −2.5), and low bone density (T-scoreQCT less than −2.5) as measured by calibrated quantitative computed tomography (QCT) is shown in relation to the severity of chronic obstructive pulmonary disease (COPD) in the cohort of smokers. Subjects with COPD are shown by Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage of severity and have higher proportions of subjects with low bone density, particularly in the more severe GOLD Stages 3 and 4 groups. GOLD unclassified subjects appeared to be somewhat resistant to the impact of smoking on bone density, but this group had a higher proportion of African American subjects. (B) At each GOLD stage, subjects with fractures had lower mean volumetric bone density than those without fractures. This was significant in all groups, including the smokers without COPD, except never-smokers, whose number was small (n = 68). (C) As the number of vertebral fractures identified increased, the mean volumetric bone mineral density (vBMD) was found to decrease across the cohort. Although fractures occur as a result of both reduced bone strength and the application of mechanical force, this graph demonstrates a relationship between decreasing BMD and number of vertebral fractures sustained by an individual. A history of a previous fracture is a strong predictor of future fractures and supports the value of screening for bone density so that treatment can be initiated and future fractures prevented. (D) Fractures were more frequent in the midthoracic to lower thoracic region and much less common in the upper thoracic region, possibly due to differences in mechanical loading on the vertebral bodies. Of the 3,317 CT scans analyzed for fractures, the figure shows the number of fractures identified at each vertebral level. There were 2,435 fractures identified in total. T8 had the greatest number of fractures (n = 407), possibly representing the increased mechanical load on the midthoracic vertebrae. Eighty-five percent of the fractures occurred in T6-T12.
Vertebral Fractures and Associations with vBMD
Of the 3,317 CT scans scored for fractures (Table 2), 37% had one or more vertebral fractures. The mean vBMD for subjects with fractures was 110.1 mg/cm3 compared with 141.4 mg/cm3 (P < 0.0001) for subjects with no fracture. Eight-five percent of the fractures occurred in the lower thoracic vertebrae (T6-T12) (Figure 1D). Those with fractures were significantly older, were disproportionately male, included more current smokers, had more pack-years of smoking, and comprised more NHW subjects (Table 2). Lower vBMD was associated with more fractures within a subject (Figure 1C). At each GOLD stage, subjects with vertebral fractures had significantly lower vBMD values than subjects with no fracture (Figure 1B).
Table 2.
Differences between subjects with no fracture and those with one or more vertebral fractures
| No Fracture | Fracture | P Value | |
|---|---|---|---|
| Number of subjects | 2,249 | 1,048 | |
| Age, yr | 58.6 (8.9) | 61.2 (9.0) | <0.0001 |
| Sex, % male | 48% | 60% | <0.0001 |
| Race, % African American | 35.6% | 21.6% | <0.0001 |
| BMI | 28.9 (6.3) | 28.6 (6.0) | 0.26 |
| Current smoker | 46.8% | 52% | 0.005 |
| Pack-yr of smoking | 42.3 (24.1) | 46.6 (24.8) | <0.0001 |
| 6-min-walk distance, ft | 1,404.3 (399) | 1,373.1 (418) | 0.04 |
| Imaging | |||
| QCT: BMD | 141.4 (47.2) | 110.1 (43.5) | <0.0001 |
| QCT: T-score | −2.4 (1.3) | −3.1 (1.3) | <0.0001 |
| Low BMD/osteoporosis based on QCT BMD and T-score | 51.3% | 72% | <0.0001 |
| Angle of kyphosis, T1-T12 | 35.9 (10.8) | 41.2 (11.9) | <0.0001 |
| LAA% < −950 HU (emphysema) | 5.0 (8.8) | 6.9 (10.3) | <0.0001 |
| Wall area % (airway disease) | 61.2 (3.3) | 61.3 (3.26) | 0.67 |
| Spirometry | |||
| FEV1, % predicted | 78.5 (25.3) | 74.0 (26.1) | <0.0001 |
| FVC, % predicted | 87.7 (18.0) | 87.2 (18.1) | 0.53 |
| FEV1/FVC | 0.68 (0.16) | 0.64 (0.17) | <0.0001 |
| Steroid use | |||
| Chronic oral steroid use | 2.2% | 2.6% | 0.5 |
| Inhaled steroid use | 21.8% | 27.4% | 0.0005 |
| Any flare-up of chest troubles | 24.3% | 25.8% | 0.36 |
| Any episode treated with steroids | 16.2% | 18.1% | 0.2 |
| Severe exacerbations | 12.0% | 11.7% | 0.8 |
| Self-reported comorbid disease | |||
| GERD | 25.6% | 27.2% | 0.33 |
| Diabetes | 12.5% | 12.8% | 0.82 |
| Coronary artery disease | 11.5% | 14.7% | 0.009 |
| Hypertension | 42% | 46.2% | 0.03 |
Definition of abbreviations: BMD = bone mineral density; BMI = body mass index; GERD = gastroesophageal reflux disease; HU = Hounsfield units; LAA% = low attenuation area percentage; QCT = quantitative computed tomography
Values are mean and standard deviation, except where percent is shown.
Race, Sex, and GOLD Stage
Sex and race differences were observed in vBMD, fractures, and categories of bone density based on vBMD. A stratified analysis (Table E3) revealed that the sex difference (more males than females had low bone density) in BMD remained present in each group, but that it was statistically significant only in NHWs. NHW men in each GOLD group had lower mean vBMD, higher rates of low BMD, and more fractures. Vertebral fractures were significantly more common in NHW men in GOLD Stage 0, GOLD unclassified, and GOLD Stage 2. AAs with COPD did not have a statistically significant sex difference in vertebral fractures.
Multivariable Models of vBMD, Low Bone Density, and Vertebral Fractures
Volumetric BMD in this cohort was strongly associated with age, race, and BMI (Table 3). After adjustment for these factors and oral glucocorticoid use (inhaled was nonsignificant) we found that the CT imaging characteristics of emphysema and airway thickening had strong but divergent associations with bone disease in this cohort. Emphysema was associated with lower vBMD, and airway wall thickening was associated with higher vBMD. Pack-years of smoking, respiratory exacerbations, and current smoking were also significantly associated with vBMD after adjusting for all other variables. Male sex remained a strong predictor of lower vBMD after adjustment for all other factors.
Table 3.
Multivariable predictors of thoracic BMD and vertebral fractures in the COPDGene cohort
| Predictors of Bone Mineral Density (QCT-BMD)* | ||||
|---|---|---|---|---|
| Term | Scaled Parametric Estimate | P Value | ||
| R2 = 0.38 for the model | |
|
||
| Age | −31.5 |
<0.0001 |
||
| Male sex | −4.6 |
<0.0001 |
||
| Non-Hispanic white | −16.3 |
<0.0001 |
||
| Pack-yr of smoking | −11.6 |
0.01 |
||
| Current smoking | −3.4 |
<0.0001 |
||
| BMI | 35.0 |
<0.0001 |
||
| History of severe exacerbation | −2.94 |
0.007 |
||
| Oral steroid use | −6.2 |
0.005 |
||
| Airway disease (wall area percent at segmental level) | 11.9 |
<0.0001 |
||
| Emphysema (LAA% < −950 HU) | −8.5 |
0.0003 |
||
|
Predictors of Vertebral
Fractures* | ||||
| Chi-Squared Test | P value | Odds† Ratio | 95% Confidence Interval | |
| Sex ratio (male:female) | 24.297 | 0.00019 | 1.48 | 1.27–1.74 |
| Race (NHW:AA) | 10.08 | 0.001 | 1.4 | 1.14–1.72 |
| Current smoking status (current:former) | 5.65 | 0.02 | 1.23 | 1.03–1.46 |
| BMI (unit odds) | 6.59 | 0.02 | 1.02 | 1.004–1.03 |
| QCT-BMD (mg/cm3) (unit odds) | 118.67 | <0.0001 | 0.988 | 0.986–0.99 |
| Emphysema (LAA% < −950 HU) (unit odds) | 6.727 | 0.01 | 1.01 | 1.003–1.02 |
Definition of abbreviations: AA = African Americans; BMD = bone mineral density; BMI = body mass index; HU = Hounsfield units; LAA% = low attenuation area percentage; NHW = non-Hispanic whites; QCT = quantitative computed tomography
Models were fit using age, sex, race, current smoking, pack-years of smoking, BMI, case–control status, oral and inhaled steroids, airway wall area percentage at the segmental level, and extent of emphysema (percentage low area attenuation at < −950 Hounsfield units). Backward stepwise regression was used to establish significant factors in the models.
Odds of fracture versus no fracture.
Modeling the relationship of low bone density as a surrogate for osteoporosis and the associated factors showed results similar to those for vBMD (Table 4). Advancing age (unit odds ratio [OR], 1.08), male sex (OR, 1.38), and NHW race (OR, 4.1) were all significant factors for low bone density. Higher BMI (OR, 0.94) was protective against low bone density, as was airway wall thickening (OR, 0.92). Current smoking (OR, 1.3), pack-years of smoking (unit OR, 1.004), oral steroid use (OR, 1.95), and positive case–control status for COPD based on spirometry (OR, 1.48) were significant factors for low bone density.
Table 4.
Factors associated with low bone density category
| Predictors of Low Bone Density
Category | ||||
|---|---|---|---|---|
| Chi-Squared Test | P Value | Odds* Ratio | 95% Confidence Interval | |
| Age, unit odds | 149.2 | <0.001 | 1.08 | 1.07–1.09 |
| Sex, male:female | 12.7 | <0.001 | 1.38 | 1.15–1.6 |
| Race, NHW:AA | 201.3 | <0.001 | 4.1 | 3.4–5.0 |
| BMI, unit odds | 69.3 | <0.001 | 0.94 | 0.92–0.95 |
| Current smoking | 5.98 | 0.015 | 1.3 | 1.05–1.6 |
| Pack-yr of smoking | 3.9 | 0.048 | 1.004 | 1.0003–1.008 |
| Chronic oral steroids | 4.3 | 0.037 | 1.95 | 1.04–3.8 |
| Airway wall thickening | 27.4 | <0.001 | 0.92 | 0.89–0.95 |
| Case–control status | 12.6 | 0.03 | 1.48 | 1.19–1.85 |
Definition of abbreviations: AA = African Americans; BMI = body mass index; NHW = non-Hispanic whites
Data tested and found to be nonsignificant in model were emphysema (percentage low area attenuation < −950 Hounsfield units) and severe exacerbations.
Odds of low bone density versus normal/intermediate bone density.
There was a strong association with vertebral fractures and vBMD in a multivariable model (Table 3). Glucocorticoid use, FEV1, and pack-years of smoking were not significant predictors of fracture risk. Emphysema was a significant factor (unit OR, 1.01, 95% confidence interval [CI], 1.003–1.02), but airway wall thickness was not. Male sex had an OR of 1.48 (95% CI, 1.27–1.7), and higher BMI was associated with increased risk of fracture (unit OR, 1.02, 95% CI, 1.002–1.03).
Comparison of QCT with DXA
In the 111 subjects who had both QCT-confirmed thoracic vBMD and DXA-confirmed lumbar aBMD measures, we compared T-scores derived from each method and the association with prevalent fractures. There was a moderate correlation between the two methods for T-scores (all subjects: R2 = 0.4, males: R2 = 0.37, females: R2 = 0.46). DXA identified fewer subjects as osteoporotic and categorized more as normal (Table 5). However, of the 52 subjects identified by DXA as being normal, QCT identified 14 as having intermediate bone density, and 6% of these had vertebral fractures, whereas 35 had low bone density, among whom 37% had vertebral fractures. In the group of 44 subjects who had DXA results classified as osteopenic (T-score in the lumbar spine of −1 to −2.5), QCT found 41 with low bone density, and 51% of that group had prevalent fractures. QCT identified more subjects with reduced BMD, and these subjects had greater numbers of fractures.
Table 5.
Lumbar DXA and thoracic QCT categories compared for association to prevalent fractures
| Thoracic QCT | |||||
|---|---|---|---|---|---|
| Lumbar DXA | Normal | Intermediate Bone Density | Low Bone Density | Totals | |
| Normal | 3 (Fx = 0%) | 14 (Fx = 6%) | 35 (Fx = 37%) | 52 | |
| Osteopenia | – | 3 (Fx = 0%) | 41 (Fx = 51%) | 44 | |
| Osteoporosis | – | – | 15 (Fx = 64%) | 15 | |
| Totals | 3 | 17 | 91 | ||
Definition of abbreviations: DXA = dual-energy X-ray absorptiometry; Fx = fractures; QCT = quantitative computed tomography
Prior Diagnosis of Osteoporosis and Use of Bisphosphonates
The number of subjects who reported having a prior physician diagnosis of osteoporosis or treatment for osteoporosis was small (Table 1). There were 10 subjects (2.6%) in the normal group who reported having a physician’s diagnosis of osteoporosis and 29 subjects (2.9%) in the intermediate group with this diagnosis. In the low bone density group, 251 subjects (13%) reported having a prior diagnosis of osteoporosis. Of these, 61 were male and 190 were female. There were 50 subjects who reported taking bisphosphonates, and the majority of these (n = 45) were in the low bone density group.
Discussion
In our large cohort of middle-aged to elderly smokers, men had lower bone density and more vertebral fractures than women. Both current smoking and pack-years of smoking were risk factors for low bone density, but COPD, by a variety of measures, was independently related to low bone density and vertebral fracture risk. Risk factors for prevalent vertebral fractures were similar to, but slightly different from, those for bone density. Measured bone density was important, but advancing age, NHW race, current smoking, and the degree of emphysema were additional factors that predicted fracture. Increased BMI was associated with higher bone density but more risk of fracture. This counterintuitive finding has been noted previously (27) and may be due to higher mechanical loads on the vertebral bodies.
Advancing age, low BMI, use of oral corticosteroids, and a history of severe respiratory exacerbations were also significant in predicting low bone density; however, the association with COPD and emphysema remained after adjusting for these factors. The prevalence of low vBMD and fractures in male smokers with versus without COPD is different from that in the general population (28), and our large sample size allowed us to confirm the results of smaller previous studies that suggested that men as well as women with COPD are at an increased risk of reduced bone mass.
Li and colleagues found that, in 179 subjects with severe COPD, 62% of the women and 70% of the men had osteoporosis based on DXA (4). Iqbal and coworkers studied 171 men with COPD with or without steroid use and reported that men with COPD had reduced spine and hip aBMD by DXA (3). Sin and colleagues studied both men and women in the National Health and Nutrition Examination Survey III and found that airflow obstruction was associated with greater osteoporosis in both sexes (worse in women), but these investigators did not stratify by smoking history (1).
In the ECLIPSE study, comprising 1,634 subjects with COPD and 259 control smokers without COPD and slightly more males than females (64% male in the COPD group and 58% male in the control group), thoracic vertebral bone attenuation was measured and compared by group. The investigators in that study found that sex was nonsignificant in predicting bone attenuation (29), and they did not find an association of reduced bone attenuation with COPD after adjusting for age, sex, and pack-years of smoking. Although women overall achieve a lower peak bone mass and lose bone faster than men, an earlier onset of smoking may reduce peak bone mass in men, and the rate of decline for both sexes may be tied to both smoking intensity and lung inflammation (30).
Male smokers also had more fractures than women smokers in our large cohort. In a previous study, McEvoy and colleagues reported that vertebral fractures were common in a group of 312 men with COPD (49% had thoracic fractures and 16.5% had lumbar fractures), but they focused on subjects with concomitant glucocorticoid use and did not include women or a control group without COPD (9). Kjensli and coworkers found similar rates of fractures in 465 men and women with COPD broken down by GOLD stage and adjusted for age, BMI, and glucocorticoid use (31). In our present study, the multivariable model identified emphysema as a specific risk factor for fractures, along with male sex and NHW race. AA smokers generally had higher vBMD and sustained fewer fractures compared with NHW smokers; however, low BMD and fractures did increase with GOLD stage, reinforcing an association between bone loss and COPD.
We selected emphysema and airway disease (measured as WA%) as measures of COPD severity because they capture different aspects of the COPD syndrome (32). Emphysema was associated with lower vBMD, and increased airway wall thickness was associated with higher vBMD. The relationship of emphysema to low BMD has been noted in two previous small studies (33, 34). However, a relationship of airway thickening to higher vBMD is novel. We postulate that airway thickening without emphysema may represent a distinct subtype of lung disease that is not associated with bone loss or that it may represent an early phase of COPD that precedes bone loss. Overall, we found that smoking exposure, severity of obstruction, emphysema, and airway disease were all related to vBMD. Mechanisms for these effects may include chronic inflammation (35), oxidative stress (36, 37), and/or the impact of smoking on vitamin D (38).
Our study has a number of strengths, including multiple centers and a large sample size, which provided statistical power to define associations between COPD and bone loss. The large number of subjects, including both men and women, allowed us to examine the impact of COPD and sex on bone density while taking into account other key factors such as steroid use and smoking. However, the study also has some limitations. COPDGene is not a population-based study and has only a small group of never-smoker controls. It is possible that we selected a group of smokers with unusual degrees of bone loss. The cohort was recruited based on smoking history but was enriched for COPD severity and AAs, giving us adequate subjects with significant smoking exposure for study of COPD in relation to bone disease. Our subset of subjects with BMD data available was not a randomly selected sample; however, this subset of subjects was not significantly different from the main cohort in terms of key subject characteristics. Use of bisphosphonates that might confound the results was surprisingly rare in our group. This and the sparse number of subjects who reported receiving a physician’s diagnosis of osteoporosis suggest that there is underdiagnosis of bone disease in association with smoking and COPD.
We used CT scans for BMD measurements and fracture assessment, and, in the majority of cohort studies in which BMD and fracture risk have been studied, DXA scans of either the lumbar spine or hip have been used to assess bone density and lateral thoracic and lumbar spine radiographs to determine fracture prevalence. CT has the advantage of measuring trabecular bone and avoids the overestimation of BMD by DXA due to degenerative spine disease and increased tissue density (39, 40). QCT may be a better predictor of fracture risk than DXA, which is more commonly used in clinical practice (41, 42). The impact of this difference may be greater in males than in females, such that our QCT method identifies more males with low bone density. As we conducted a cross-sectional study, we are not able to predict future fractures on the basis of these data. However, the existence of vertebral fractures in association with reduced BMD reinforces the utility of the QCT measurements. Fracture assessment based on a CT reconstruction may be more sensitive than DXA scout images or plain X-rays, although we used standard criteria and saw close agreement of fractures with decreased BMD. We found that, compared with DXA, QCT had stronger associations with fractures.
There is active debate regarding the use of diagnostic labels such as “osteoporosis.” We avoided using the terms osteopenia and osteoporosis because of their association with the WHO-endorsed and DXA-derived aBMD measurement. Instead, we used low bone density as an equivalent term. DXA has a lower radiation dose and may be preferable for general population screening, but for current and ex-smokers who meet the USPSTF criteria for lung cancer screening (43), CT may be a convenient way to screen for both cancer and low BMD (7).
In a large and well-characterized cohort of smokers with or without COPD, we have demonstrated that the emphysema subtype of COPD is associated with lower vBMD and increased vertebral fractures and that both male and female smokers are at risk of reduced bone density and vertebral fractures. Smoking history, as well as current smoking and COPD, should be considered in planning bone density screening strategies for these populations. Expanding screening to include men with a smoking history or COPD and starting treatment in those with bone disease may prevent fractures, improve quality of life, and reduce health care costs.
Additional material
Supplementary data supplied by authors.
Acknowledgments
Acknowledgment
The authors acknowledge Dr. Paul Docktor, Jaleh Akhaven, George Castillo, Taavish Sharma, Jacob Mann, Scott Tarrant, and Brian Hattel, as well as Image Analysis Inc. for the donation of INTable Calcium Calibration pads and analysis software.
Footnotes
Supported by Award R01 HL089897 and Award R01 HL089856 from the National Heart, Lung, and Blood Institute, National Institutes of Health. The COPDGene project is also supported by the COPD Foundation through contributions made to an industry advisory board composed of AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, Siemens, Sunovion, and GlaxoSmithKline.
Author Contributions: study design: E.A.R. and J.D.J. Interpretation of data and manuscript preparation: J.D.J., C.W., D.J.S., D.A.L., R.P.B., S.L., J.M.B., B.A., M.-L.N.M., G.R.W., E.S.W., D.L.D., M.G.F., X.S., S.E.L., N.E.L., H.K.G., E.K.S., J.E.H., B.J.M., J.D.C., and E.A.R. Data acquisition and analysis: J.D.J., S.E.L., S.L., C.W., D.J.S., and E.A.R. CT image analysis: J.D.J., S.E.L., D.A.L., E.A.R., and G.R.W.
This article has an online supplement, which is accessible from the issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Sin DD, Man JP, Man SF. The risk of osteoporosis in Caucasian men and women with obstructive airways disease. Am J Med. 2003;114:10–14. doi: 10.1016/s0002-9343(02)01297-4. [DOI] [PubMed] [Google Scholar]
- 2.Biskobing DM. COPD and osteoporosis. Chest. 2002;121:609–620. doi: 10.1378/chest.121.2.609. [DOI] [PubMed] [Google Scholar]
- 3.Iqbal F, Michaelson J, Thaler L, Rubin J, Roman J, Nanes MS. Declining bone mass in men with chronic pulmonary disease: contribution of glucocorticoid treatment, body mass index, and gonadal function. Chest. 1999;116:1616–1624. doi: 10.1378/chest.116.6.1616. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Brennan KJ, Gaughan JP, Ciccolella DE, Kuzma AM, Criner GJ. African Americans and men with severe COPD have a high prevalence of osteoporosis. COPD. 2008;5:291–297. doi: 10.1080/15412550802363329. [DOI] [PubMed] [Google Scholar]
- 5.Hollenbach KA, Barrett-Connor E, Edelstein SL, Holbrook T. Cigarette smoking and bone mineral density in older men and women. Am J Public Health. 1993;83:1265–1270. doi: 10.2105/ajph.83.9.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, Fujiwara S, Kroger H, McCloskey EV, Mellstrom D, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16:155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 7.de Jong WU, de Jong PA, Vliegenthart R, Isgum I, Lammers JW, Oudkerk M, van der Aalst C, de Koning HJ, Mohamed Hoesein FA. Association of chronic obstructive pulmonary disease and smoking status with bone density and vertebral fractures in male lung cancer screening participants. J Bone Miner Res. 2014;29:2224–2229. doi: 10.1002/jbmr.2248. [DOI] [PubMed] [Google Scholar]
- 8.Lorentzon M, Mellström D, Haug E, Ohlsson C. Smoking is associated with lower bone mineral density and reduced cortical thickness in young men. J Clin Endocrinol Metab. 2007;92:497–503. doi: 10.1210/jc.2006-1294. [DOI] [PubMed] [Google Scholar]
- 9.McEvoy CE, Ensrud KE, Bender E, Genant HK, Yu W, Griffith JM, Niewoehner DE. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:704–709. doi: 10.1164/ajrccm.157.3.9703080. [DOI] [PubMed] [Google Scholar]
- 10.de Pablo P, Cooper MS, Buckley CD. Association between bone mineral density and C-reactive protein in a large population-based sample. Arthritis Rheum. 2012;64:2624–2631. doi: 10.1002/art.34474. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson AL, Movérare-Skrtic S, Ljunggren Ö, Karlsson M, Mellström D, Ohlsson C. High-sensitivity CRP is an independent risk factor for all fractures and vertebral fractures in elderly men: the MrOS Sweden study. J Bone Miner Res. 2014;29:418–423. doi: 10.1002/jbmr.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miniño AM, Xu J, Kochanek KD. Deaths: preliminary data for 2008. Natl Vital Stat Rep. 2010;59:1–52. [PubMed] [Google Scholar]
- 13.Behavioral Risk Factor Surveillance System. http://www.cdc.gov/brfss/data_tool.shtm
- 14.Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Forciea MA, Owens DK Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:680–684. doi: 10.7326/0003-4819-148-9-200805060-00008. [DOI] [PubMed] [Google Scholar]
- 15.US Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/osteoporosis-screening
- 16.Kanis JA World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK. http://www.shef.ac.uk/FRAX/tool.aspx?country=9
- 17.Drake MT, Khosla S. Male osteoporosis. Endocrinol Metab Clin North Am. 2012;41:629–641. doi: 10.1016/j.ecl.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ettinger B, Ensrud KE, Blackwell T, Curtis JR, Lapidus JA, Orwoll ES Osteoporotic Fracture in Men (MrOS) Study Research Group. Performance of FRAX in a cohort of community-dwelling, ambulatory older men: the Osteoporotic Fractures in Men (MrOS) study. Osteoporos Int. 2013;24:1185–1193. doi: 10.1007/s00198-012-2215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orwoll ES, Oviatt SK, Mann T. The impact of osteophytic and vascular calcifications on vertebral mineral density measurements in men. J Clin Endocrinol Metab. 1990;70:1202–1207. doi: 10.1210/jcem-70-4-1202. [DOI] [PubMed] [Google Scholar]
- 20.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 22.Zach JA, Newell JD, Jr, Schroeder J, Murphy JR, Curran-Everett D, Hoffman EA, Westgate PM, Han MK, Silverman EK, Crapo JD, et al. COPDGene Investigators. Quantitative computed tomography of the lungs and airways in healthy nonsmoking adults. Invest Radiol. 2012;47:596–602. doi: 10.1097/RLI.0b013e318262292e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroeder JD, McKenzie AS, Zach JA, Wilson CG, Curran-Everett D, Stinson DS, Newell JD, Jr, Lynch DA. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol. 2013;201:W460–W470. doi: 10.2214/AJR.12.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelke K, Adams JE, Armbrecht G, Augat P, Bogado CE, Bouxsein ML, Felsenberg D, Ito M, Prevrhal S, Hans DB, et al. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11:123–162. doi: 10.1016/j.jocd.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 26.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 27.Ong T, Sahota O, Tan W, Marshall L. A United Kingdom perspective on the relationship between body mass index (BMI) and bone health: a cross sectional analysis of data from the Nottingham Fracture Liaison Service. Bone. 2014;59:207–210. doi: 10.1016/j.bone.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Looker AC. Femur neck bone mineral density and fracture risk by age, sex, and race or Hispanic origin in older US adults from NHANES III. Arch Osteoporos. 2013;8:141. doi: 10.1007/s11657-013-0141-4. [DOI] [PubMed] [Google Scholar]
- 29.Romme EA, Murchison JT, Edwards LD, van Beek E, Jr, Murchison DM, Rutten EP, Smeenk FW, Williams MC, Wouters EF, MacNee W Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Study Investigators. CT-measured bone attenuation in patients with chronic obstructive pulmonary disease: relation to clinical features and outcomes. J Bone Miner Res. 2013;28:1369–1377. doi: 10.1002/jbmr.1873. [DOI] [PubMed] [Google Scholar]
- 30.Taes Y, Lapauw B, Vanbillemont G, Bogaert V, De Bacquer D, Goemaere S, Zmierczak H, Kaufman JM. Early smoking is associated with peak bone mass and prevalent fractures in young, healthy men. J Bone Miner Res. 2010;25:379–387. doi: 10.1359/jbmr.090809. [DOI] [PubMed] [Google Scholar]
- 31.Kjensli A, Falch JA, Ryg M, Blenk T, Armbrecht G, Diep LM, Ellingsen I. High prevalence of vertebral deformities in COPD patients: relationship to disease severity. Eur Respir J. 2009;33:1018–1024. doi: 10.1183/09031936.00073908. [DOI] [PubMed] [Google Scholar]
- 32.Castaldi PJ, Dy J, Ross J, Chang Y, Washko GR, Curran-Everett D, Williams A, Lynch DA, Make BJ, Crapo JD, et al. Cluster analysis in the COPDGene study identifies subtypes of smokers with distinct patterns of airway disease and emphysema. Thorax. 2014;69:415–422. doi: 10.1136/thoraxjnl-2013-203601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohara T, Hirai T, Muro S, Haruna A, Terada K, Kinose D, Marumo S, Ogawa E, Hoshino Y, Niimi A, et al. Relationship between pulmonary emphysema and osteoporosis assessed by CT in patients with COPD. Chest. 2008;134:1244–1249. doi: 10.1378/chest.07-3054. [DOI] [PubMed] [Google Scholar]
- 34.Bon J, Fuhrman CR, Weissfeld JL, Duncan SR, Branch RA, Chang CC, Zhang Y, Leader JK, Gur D, Greenspan SL, et al. Radiographic emphysema predicts low bone mineral density in a tobacco-exposed cohort. Am J Respir Crit Care Med. 2011;183:885–890. doi: 10.1164/rccm.201004-0666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev. 2005;208:207–227. doi: 10.1111/j.0105-2896.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 36.Folchini F, Nonato NL, Feofiloff E, D’Almeida V, Nascimento O, Jardim JR. Association of oxidative stress markers and C-reactive protein with multidimensional indexes in COPD. Chron Respir Dis. 2011;8:101–108. doi: 10.1177/1479972310391284. [DOI] [PubMed] [Google Scholar]
- 37.Wilson C. Bone: oxidative stress and osteoporosis. Nat Rev Endocrinol. 2014;10:3. doi: 10.1038/nrendo.2013.225. [DOI] [PubMed] [Google Scholar]
- 38.Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr. 1999;53:920–926. doi: 10.1038/sj.ejcn.1600870. [DOI] [PubMed] [Google Scholar]
- 39.Yu EW, Bouxsein ML, Roy AE, Baldwin C, Cange A, Neer RM, Kaplan LM, Finkelstein JS. Bone loss after bariatric surgery: discordant results between DXA and QCT bone density. J Bone Miner Res. 2014;29:542–550. doi: 10.1002/jbmr.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauman WA, Schwartz E, Song IS, Kirshblum S, Cirnigliaro C, Morrison N, Spungen AM. Dual-energy X-ray absorptiometry overestimates bone mineral density of the lumbar spine in persons with spinal cord injury. Spinal Cord. 2009;47:628–633. doi: 10.1038/sc.2008.169. [DOI] [PubMed] [Google Scholar]
- 41.Rehman Q, Lang T, Modin G, Lane NE. Quantitative computed tomography of the lumbar spine, not dual x-ray absorptiometry, is an independent predictor of prevalent vertebral fractures in postmenopausal women with osteopenia receiving long-term glucocorticoid and hormone-replacement therapy. Arthritis Rheum. 2002;46:1292–1297. doi: 10.1002/art.10277. [DOI] [PubMed] [Google Scholar]
- 42.Li N, Li XM, Xu L, Sun WJ, Cheng XG, Tian W. Comparison of QCT and DXA: osteoporosis detection rates in postmenopausal women. Int J Endocrinol. 2013;2013:895474. doi: 10.1155/2013/895474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.US Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/lung-cancer-screening
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.