Abstract
OBJECTIVES:
Limited information is available concerning the post-treatment neutrophil-lymphocyte ratio in critical limb ischemia patients who receive conservative therapy. Accordingly, this study was designed to evaluate the predictive value of the post-treatment neutrophil-lymphocyte ratio in critical limb ischemia patients without surgery.
METHOD:
From January 2009 to January 2011, critical limb ischemia patients were admitted to a vascular center. The demographic data, patient histories, comorbidities and risk factors were documented, and the differential cell count was determined at admission and seven days later after conservative therapy. The cutoff value of the post-treatment neutrophil-lymphocyte ratio was determined by an ROC curve. Patients were divided into groups A and B according to the cutoff value. Amputation-free survival was compared between groups. Univariate and multivariate analyses were used to identify independent risk factors.
RESULT:
A total of 172 patients were identified with a mean age 71.98±10.09 years; among them, 122 were male. A value of 3.8 was identified as the cutoff value of the post-treatment neutrophil-lymphocyte ratio. Groups A (post-treatment neutrophil-lymphocyte ratio ≥3.8) and B (post-treatment neutrophil-lymphocyte ratio <3.8) showed a significant difference in amputation-free survival (P<0.001). The 1-year, 2-year and 3-year amputation-free survival rates were 79.6%, 55.6% and 46.3%, respectively, in group A; however, in group B, these values were 89.7%, 79.3% and 75.9%, respectively. The post-treatment neutrophil-lymphocyte ratio was identified as an independent predictive factor for amputation in critical limb ischemia patients (P<0.001).
CONCLUSION:
The post-treatment neutrophil-lymphocyte ratio is an independent predictive factor for amputation in critical limb ischemia patients. Patients with a post-treatment neutrophil-lymphocyte ratio ≥3.8 are likely to suffer from amputation; amputation-free survival usually occurs in patients with a post-treatment neutrophil-lymphocyte ratio <3.8.
Keywords: Neutrophil-lymphocyte ratio, Critical limb ischemia, Conservative therapy, Amputation, Amputation free survival
INTRODUCTION
Critical limb ischemia (CLI) is a widespread disease in China, especially in older people. Although statistics are lacking for the Chinese population, according to epidemiological research of other countries (1), more than 5 million Chinese individuals are estimated to suffer from CLI. Due to long-term chronic ischemia, the end-point for CLI patients is usually amputation. Therefore, a method of identifying CLI patients with a high risk for amputation and performing risk stratification at an early time point is always the focus of clinical practice.
CLI is an atherosclerotic disease that is usually associated with a general inflammatory response. Based on previous studies, C-reactive protein (CRP), platelet aggregation, and the neutrophil to lymphocyte ratio (NLR) have been indicated as effective predictive markers for CLI patients (2). The NLR has universally been accepted as a predictive index for stent patency (3,4), amputation-free survival (AFS) (5), and mortality (6-8). However, these studies usually focus on preoperative or postoperative NLR in CLI patients who have undergone an operation or intervention. Little research has been performed on CLI patients who receive conservative treatment. Furthermore, the NLR has been adopted for prognosis evaluation in various medical fields including hearing loss (9), peripheral artery disease (PAD) (10,11), bladder cancer (12), prostatic hyperplasia (13), pancreatic cancer (14,15), and colorectal cancer (16,17).
However, whether the post-treatment NLR (post-NLR) can predict the prognosis in CLI patients is unclear. Therefore, the present study was designed to evaluate the predictive value of the post-NLR in CLI patients who receive conservative therapy.
METHODS
Patients and methods
The objective inclusion criteria for enrolled patients with a diagnosis of CLI consisted of more than two weeks of foot pain associated with an ankle-brachial index of 0.4 or less, an ankle systolic pressure of 50 mm Hg or less, or a toe systolic pressure of 30 mm Hg or less (Rutherford categories 4 and 5 (18). Patients with a) decisive evidence of acute limb ischemia; b) clinical symptoms or signs of sepsis, any other inflammation or a white blood cell (WBC) count >10*109/L; c) poor data integrity; d) a previous surgery history; or e) any diseases or drug that would affect the lymphocyte count, such as lymphopenia, lymphemia, acute cardinal infraction and corticosteroid use, were excluded from this study.
In this study, all of the enrolled patients who received conservative therapy were not candidates for revascularization due to poor general condition, loss of indication, severe local infection or patient unwillingness.
At admission, the demographics (age, gender), history (smoking, drug use), comorbidities and results of laboratory tests (in particular, the neutrophil, lymphocyte, and WBC counts) were recorded in a specific database by doctors and nurses. All enrolled patients received the same standardized conservative therapy plan (antiplatelet, anticoagulation and vasodilator drugs were given intravenously for approximately 7 days). The same blood tests were conducted 7 days after admission.
NLR definition
The pre-treatment WBC and differential counts were evaluated at admission before conservative therapy. The post-treatment counts evaluated taken 7 days later after conservative therapy was adopted. The NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count (19).
Follow-up after discharge
Discharged patients were followed via outpatient consultation or telephone calls. Each patient received the same unified follow-up form and was followed at the end of the 1st, 3rd, 6th, and 12th month after discharge, then annually thereafter. Telephone follow-up was performed at the same intervals. In this study, the primary end-points were amputation [above-the-knee amputation (AKA), below-the-knee amputation (BKA) or Toe] or 36 months after discharge.
Analysis method
Data analysis was performed with SPSS version 16 (SPSS Inc, Chicago, IL). Categorical data and continuous variables were compared with the chi-square test or Fisher's exact test and the independent sample t-test, respectively. The incidence of amputation was analyzed by the Kaplan-Meier method, and the difference was compared with a log-rank test. Independent risk factors were identified by multivariate Cox proportional hazards regression analysis. The calculated P-values were two-sided, and a P-value <0.05 was considered significant.
Ethics
The patients included in this study were recruited from the Vascular Department of West China Hospital, Sichuan University, between January 2009 and January 2011. The study was approved by the Ethics Committee of West China Hospital, Sichuan University.
RESULTS
Based on the exclusion criteria, 12 patients were excluded from the final analysis due to a) determined evidence of acute limb ischemia (n ϝ 1); b) clinical symptoms or signs of sepsis or any other inflammation or WBC counts > 10*109/L (n ϝ 6); c) poor data integrity (n ϝ 3); or d) a history of previous surgery (n ϝ 2). A total of 172 patients were included in the final outcome analysis.
Cutoff value of post-NLR identification
The analysis of the relationship between post-NLR and AFS was conducted using tertiles of existing data (e.g., Post-NLR <3.4, Post-NLR >6.8 and Post-NLR between 3.4 and 6.8). This grouping method showed a significant discrimination effect (P ϝ 0.006, Figure 1).
Figure 1.
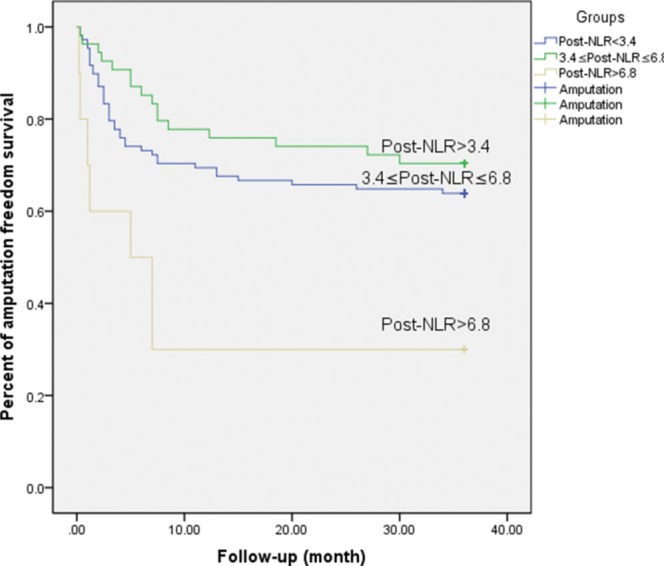
Association between post-NLR and amputation-free survival (Pϝ0.006).
The post-NLR appeared to have a significant influence on the outcome in a continuous manner. The post-NLR appeared to be negatively correlated with the outcome. An ROC curve was used to identify the cutoff value, which is shown in Figure 2. In the ROC analysis setting, amputation was set as the state variable. The C-statistic (area under the curve) was 0.740. A post-NLR of 3.8 was selected as the cutoff value because it had the maximum discriminative power (sensitivity 70.2%, specificity 71.7%). Seventy-four patients (43%) had a post-NLR ≥3.8, and 98 patients had a post-NLR <3.8.
Figure 2.
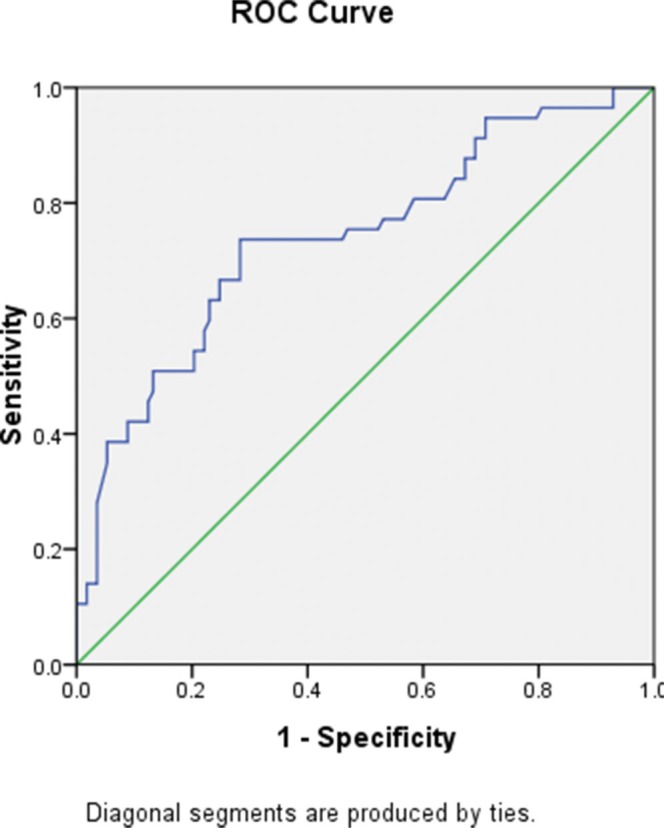
Power of diagnostic value (C-statistic is 0.740).
Baseline characteristics
A total of 172 patients with mean age of 71.98±10.09 years were included, and 70.9% (n ϝ 122) of these patients were male. The mean follow-up duration was 34.7 months (range 3.25 to 62.5 months). The 74 patients with a post-NLR ≥3.8 were collectively called group A, and the 98 patients with a post-NLR <3.8 were called group B. The mean ages in groups A and B were 71.83±11.00 and 72.00±9.52, respectively. The baseline characteristic comparison between group A and group B is shown in Table 1. The incidence of diabetes mellitus, the pre-NLR, the post-NLR, the monocyte count, and the cholesterol level were significantly different between the two groups.
Table 1.
Baseline characteristics of 172 CLI patients compared according to post-NLR.
| Factor | Group A (post-NLR ≥3.8) | Group B (post-NLR <3.8) | P-value |
|---|---|---|---|
| Gender (male/female) | 50/24 | 72/26 | NS |
| Smoking | 40 | 60 | NS |
| Hypertension | 36 | 56 | NS |
| Diabetes mellitus | 8 | 22 | 0.046 |
| Heart disease | 18 | 26 | NS |
| CAD | 10 | 14 | NS |
| Statin use | 21 | 27 | NS |
| Age | 71.83±11.00 | 72.00±9.52 | NS |
| Glucose | 7.12±3.25 | 6.52±2.45 | NS |
| Neutrophil count | 5.42±2.52 | 5.29±2.19 | NS |
| Lymphocyte count | 1.48±0.49 | 1.40±0.60 | NS |
| Pre-NLR | 6.27±5.05 | 4.37±2.49 | 0.004 |
| Post-NLR | 7.57±4.46 | 2.20±0.99 | <0.001 |
| Monocyte | 0.59±0.23 | 0.48±0.18 | 0.001 |
| WBC | 6.85±1.36 | 6.47±1.26 | NS |
| Albumin | 36.72±4.34 | 37.99±3.96 | 0.049 |
| Potassium | 3.88±0.49 | 3.90±0.49 | NS |
| Creatinine | 84.33±31.11 | 83.70±28.60 | NS |
| HDL | 1.11±0.38 | 1.17±0.39 | NS |
| LDL | 2.56±0.82 | 2.35±0.71 | NS |
| TG | 1.82±1.40 | 1.45±0.96 | NS |
| Cholesterol | 4.41±0.97 | 4.00±0.97 | 0.009 |
| Social status | NS | ||
| Bachelor or greater education | 12 | 8 | |
| Middle school education | 76 | 43 | |
| Primary school education | 18 | 15 | |
| Living condition | NS | ||
| Nursing home | 21 | 14 | |
| Private home | 85 | 52 | |
| Fontaine grade | NS | ||
| II | 2 | 4 | |
| III | 48 | 66 | |
| IV | 24 | 28 |
CAD: coronary artery disease; Pre-NLR: pre-treatment neutrophil to lymphocyte ratio; Post-NLR: post-treatment neutrophil to lymphocyte ratio; WBC: white blood cell; HDL: high density lipoprotein; LDL: low density lipoprotein; TG: triglyceride. *P<0.05 was considered a significant difference; NS indicated no significance.
Impact of the post-NLR on amputation
Overall, the AFS rates in group A (post-NLR ≥3.8) and group B (post-NLR <3.8) were 43.2% and 82.7%, respectively. Furthermore, the 1-year, 2-year and 3-year incidence rates of AFS in group A were 83.8%, 67.6% and 43.2%, respectively. By contrast, the respective 1-year, 2-year and 3-year AFS rates were 89.8%, 82.7% and 82.7% in group B. Thus, 42 patients suffered from amputation during follow-up in group A, whereas only 17 patients received amputations in group B (P<0.001). A detailed comparison of amputation instances is shown in Table 2. Moreover, the survival outcome showed a significant difference between groups A and B (P<0.001), which is shown in Figure 3.
Table 2.
Amputation comparison between Groups A and B
| Group A (post-NLR ≥3.8) | Group B (post-NLR <3.8) | P-value | |
|---|---|---|---|
| Amputation/Total | 42 | 17 | <0.001 |
| AKA | 1 | 1 | NS |
| BKA | 12 | 5 | 0.016 |
| Toe | 29 | 11 | <0.001 |
AKA: above-knee amputation; BKA: below-knee amputation.
P<0.05 was considered a significant different; NS indicated no significance.
Figure 3.
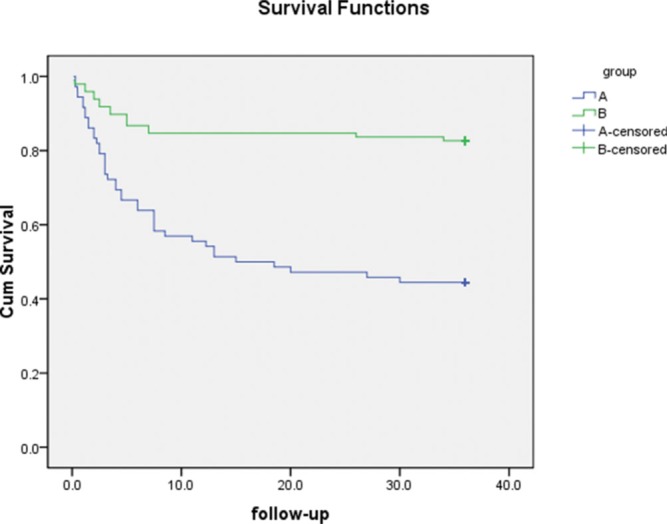
Survival outcome comparison between groups A and B (P<0.001).
Independent risk factor identification
Risk factors for CLI patients, including diabetes mellitus, pre-NLR, post-NLR, monocyte albumin and cholesterol level, were included in the multivariate analysis (Cox proportional hazards model). The analysis results showed that the post-NLR and albumin were independent risk factors of amputation (hazard ratios [95% confidence interval]: 1.140 [1.086, 1.197] and 0.914 [0.856, 0.976], respectively). These data are shown in Table 3.
Table 3.
Multivariate analysis of factors affecting overall amputation in patients with critical limb ischemia
| Factor | Hazard ratio(95% confidence interval) | P-value |
|---|---|---|
| Post-NLR | 1.140 (1.086, 1.197) | <0.001 |
| Albumin | 0.914 (0.856, 0.976) | 0.007 |
| Diabetes mellitus, pre-NLR, Monocyte and cholesterol | NS |
pre-NLR: pre-treatment neutrophil-lymphocyte ratio; post-NLR: post-treatment neutrophil-lymphocyte ratio.
P<0.05 indicated a significant difference. NS indicated no significance.
DISCUSSION
The post-NLR was identified as an independent predictive factor of amputation in CLI patients who did not undergo surgery. CLI patients usually suffer from malnutrition (20) due to sleeplessness and lack of appetite, leading to chronic ischemic pain. Moreover, variable albumin is a crucial index for evaluating a patient's nutritional condition, and hypoproteinemia is a risk factor for amputation in PAD (21,22). In this study, albumin was identified as an independent protective factor, which is in accordance with previous studies.
With respect to previous studies, major lower limb amputations (AKA or BKA) at 1 year occur in approximately 20% of untreated CLI patients (23,24). In this study, the 1-year amputation rate was 16.2% in group A (post-NLR ≥3.8) and 10.2% in group B (post-NLR <3.8). Therefore, no significant difference in the final outcome was observed for untreated CLI patients and patients who received conservative therapy. This result needs to be confirmed by a systematic review or randomized controlled trial in the future. However, this study suggests that CLI patients with a higher post-NLR have a worse prognosis.
A risk-stratification model is an important clinical tool that uses existing clinical data to predict a patient's prognosis and provides a rational discrimination between risky and non-risky patients. The NLR contains crucial information regarding the patient's inflammatory condition. Moreover, the NLR is different from other inflammation markers. It is an inexpensive and readily available marker that is directly calculated from the neutrophil and lymphocyte counts and can easily be obtained from a complete blood cell test on admission.
Furthermore, CLI is a form of chronic atherosclerosis, and fibrosis progression is associated with the general immune-inflammatory response. Repeated local inflammatory responses involving the neutrophils and lymphocytes lead to fibrosis, which aggravates ischemia. The NLR is an effective systematic inflammatory marker that represents the inflammatory condition. When severe ischemia occurs and infection and immune disease are simultaneously excluded, a systematic increase in the post-NLR could represent an irreversible ischemic condition that may lead to an endpoint event (e.g., amputation). The NLR predicts endpoint events in other medical fields, including myocardial ischemia (25) and severe sepsis (26).
In this study, all of the enrolled patients underwent conservative therapy; however, compared with all CLI patients over the same period of time, the proportion of patients receiving conservative therapy was still low. Previous studies have shown that revascularization procedures (e.g., surgery and angioplasty/stenting) can produce a better prognosis than conservative therapy (27-29). In terms of patients who are suitable candidates for revascularization, surgery, intervention or a hybrid procedure is still the optimal choice.
Although this study is prospective with respect to patient collection and discharge follow-up, selection bias cannot be denied. Nearly all of the enrolled patients used antiplatelet therapy, but only 48 subjects (27.9%) received statins. In addition, the post-NLR could not be compared with other inflammatory markers, such as C-reactive protein or the erythrocyte sedimentation rate, because they are not routine tests performed at admission. Moreover, isolated patients with anemia and a hemoglobin level of less than 70 g/L received transfusion therapy. This confounding factor was not included in the analysis in this study.
The post-NLR is an effective marker for amputation in CLI patients who do not undergo an operation. When the post-NLR ≥3.8, CLI patients are more likely to suffer from amputation compared with patients with a post-NLR <3.8. To avoid amputation and increase AFS, revascularization procedures are still the optimal choice.
No potential conflict of interest was reported.
These authors contributed equally to this manuscript.
REFERENCES
- 1.Nehler MR, Duval S, Diao L, Annex BH, Hiatt WR, Rogers K, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60((3)):686–95.e2. doi: 10.1016/j.jvs.2014.03.290. [DOI] [PubMed] [Google Scholar]
- 2.Tasoglu I, Sert D, Colak N, Uzun A, Songur M, Ecevit A. Neutrophil-Lymphocyte Ratio and the Platelet-Lymphocyte Ratio Predict the Limb Survival in Critical Limb Ischemia. Clinical and applied thrombosis/hemostasis: official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2013;20((6)):645–50. doi: 10.1177/1076029613475474. [DOI] [PubMed] [Google Scholar]
- 3.Chan C, Puckridge P, Ullah S, Delaney C, Spark JI. Neutrophil-lymphocyte ratio as a prognostic marker of outcome in infrapopliteal percutaneous interventions for critical limb ischemia. J Vasc Surg. 2014;60((3)):661–8. doi: 10.1016/j.jvs.2014.03.277. [DOI] [PubMed] [Google Scholar]
- 4.Chang SH, Tsai YJ, Chou HH, Wu TY, Hsieh CA, Cheng ST, et al. Clinical predictors of long-term outcomes in patients with critical limb ischemia who have undergone endovascular therapy. Angiology. 2014;65((4)):315–22. doi: 10.1177/0003319713515544. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Fajardo JA, Brizuela-Sanz JA, Aguirre-Gervas B, Merino-Diaz B, Del Rio-Sola L, Martin-Pedrosa M, et al. Prognostic significance of an elevated neutrophil-lymphocyte ratio in the amputation-free survival of patients with chronic critical limb ischemia. Annals of vascular surgery. 2014;28((4)):999–1004. doi: 10.1016/j.avsg.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Erturk M, Cakmak HA, Surgit O, Celik O, Aksu HU, Akgul O, et al. The predictive value of elevated neutrophil to lymphocyte ratio for long-term cardiovascular mortality in peripheral arterial occlusive disease. J Cardiol. 2014;64((5)):371–6. doi: 10.1016/j.jjcc.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Botta C, Barbieri V, Ciliberto D, Rossi A, Rocco D, Addeo R, et al. Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non-small cell lung cancer patients. Cancer Biol Ther. 2013;14((6)):469–75. doi: 10.4161/cbt.24425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinato DJ, Sharma R. An inflammation-based prognostic index predicts survival advantage after transarterial chemoembolization in hepatocellular carcinoma. Transl Res. 2012;160((2)):146–52. doi: 10.1016/j.trsl.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Ozler GS. Increased neutrophil-lymphocyte ratio in patients with idiopathic sudden sensorineural hearing loss. J Craniofac Surg. 2014;25((3)):e260–3. doi: 10.1097/SCS.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 10.Siracuse JJ, Gill HL, Jones DW, Schneider DB, Connolly PH, Parrack I, et al. Risk factors for protracted postoperative length of stay after lower extremity bypass for critical limb ischemia. Ann Vasc Surg. 2014;28((6)):1432–8. doi: 10.1016/j.avsg.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Tasoglu I, Cicek OF, Lafci G, Kadirogullari E, Sert DE, Demir A, et al. Usefulness of neutrophil/lymphocyte ratio as a predictor of amputation after embolectomy for acute limb ischemia. Ann Vasc Surg. 2014;28((3)):606–13. doi: 10.1016/j.avsg.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Mano R, Baniel J, Shoshany O, Margel D, Bar-On T, Nativ O, et al. Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non-muscle-invasive bladder cancer. Urol Oncol. 2015;33((2)):67.e1–7. doi: 10.1016/j.urolonc.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Tanik S, Albayrak S, Zengin K, Borekci H, Bakirtas H, Imamoglu MA, et al. Is the neutrophil-lymphocyte ratio an indicator of progression in patients with benign prostatic hyperplasia? Asian Pac J Cancer Prev. 2014;15((15)):6375–9. doi: 10.7314/apjcp.2014.15.15.6375. [DOI] [PubMed] [Google Scholar]
- 14.Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109((2)):416–21. doi: 10.1038/bjc.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang DS, Luo HY, Qiu MZ, Wang ZQ, Zhang DS, Wang FH, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29((5)):3092–100. doi: 10.1007/s12032-012-0226-8. [DOI] [PubMed] [Google Scholar]
- 16.Chang Z, Zheng J, Ma Y, Zhao J, Wang C, Liu Z. The neutrophil-to-lymphocyte ratio as a predictor for recurrence of colorectal liver metastases following radiofrequency ablation. Med Oncol. 2014;31((3)):855. doi: 10.1007/s12032-014-0855-1. [DOI] [PubMed] [Google Scholar]
- 17.Gohil R, Rishi M, Tan BH. Pre-operative serum albumin and neutrophil-lymphocyte ratio are associated with prolonged hospital stay following colorectal cancer surgery. Br J Med Med Res. 2014;4((1)) doi: 10.9734/BJMMR/2014/5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santilli JD, Santilli SM. Chronic critical limb ischemia: diagnosis, treatment and prognosis. Am Fam Physician. 1999;59((7)):1899–908. [PubMed] [Google Scholar]
- 19.Spark JI, Sarveswaran J, Blest N, Charalabidis P, Asthana S. An elevated neutrophil-lymphocyte ratio independently predicts mortality in chronic critical limb ischemia. J Vasc Surg. 2010;52((3)):632–636. doi: 10.1016/j.jvs.2010.03.067. [DOI] [PubMed] [Google Scholar]
- 20.Shah M, Martin A, Myers B, MacSweeney S, Richards T. Recognising anaemia and malnutrition in vascular patients with critical limb ischaemia. Ann R Coll Surg Engl. 2010;92((6)):495–8. doi: 10.1308/003588410X12664192075738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doumit JH, Nasser RN, Hanna DR. Nutritional and health status among nursing home residents in Lebanon: comparison across gender in a national cross sectional study. BMC Public Health. 2014;14:629. doi: 10.1186/1471-2458-14-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii H, Aoyama T, Takahashi H, Kamoi D, Tanaka M, Yoshikawa D, et al. Serum albumin and C-reactive protein levels predict clinical outcome in hemodialysis patients undergoing endovascular therapy for peripheral artery disease. Atherosclerosis. 2013;227((1)):130–4. doi: 10.1016/j.atherosclerosis.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 23.Chung J, Timaran DA, Modrall JG, Ahn C, Timaran CH, Kirkwood ML, et al. Optimal medical therapy predicts amputation-free survival in chronic critical limb ischemia. J Vasc Surg. 2013;58((4)):972–80. doi: 10.1016/j.jvs.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 24.Marston WA, Davies SW, Armstrong B, Farber MA, Mendes RC, Fulton JJ, et al. Natural history of limbs with arterial insufficiency and chronic ulceration treated without revascularization. J Vasc Surg. 2006;44((1)):108–14. doi: 10.1016/j.jvs.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Muhmmed Suliman MA, Bahnacy Juma AA, Ali Almadhani AA, Pathare AV, Alkindi SS, Uwe Werner F. Predictive value of neutrophil to lymphocyte ratio in outcomes of patients with acute coronary syndrome. Arch Med Res. 2010;41((8)):618–22. doi: 10.1016/j.arcmed.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Yilmaz H, Cakmak M, Inan O, Darcin T, Akcay A. 2014. Can neutrophil-lymphocyte ratio be independent risk factor for predicting acute kidney injury in patients with severe sepsis? Ren Fail; pp. 1–5. [DOI] [PubMed] [Google Scholar]
- 27.Ignatovich IN, Kondratenko GG, Sergeev GG, Kornievich SN. (3) 2012. [Revascularization surgery in the treatment of the diabetic foot syndrome]. Khirurgiia; pp. 38–42. [PubMed] [Google Scholar]
- 28.Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg. 2012;55((1)):90–7. doi: 10.1016/j.jvs.2011.06.107. [DOI] [PubMed] [Google Scholar]
- 29.Koch M, Trapp R, Hepp W. Impact of femoropopliteal bypass surgery on the survival and amputation rate of end-stage renal disease patients with critical limb ischemia. Med Klin (Munich) 2007;102((2)):107–111. doi: 10.1007/s00063-007-1021-8. [DOI] [PubMed] [Google Scholar]


