Abstract
Elevated serum levels of cardiac troponin and C-reactive protein are associated with all-cause and cardiovascular mortality in patients with end-stage renal disease. However, the relationship between these two biomarker levels and mortality in patients with chronic kidney disease remains unclear. We conducted a meta-analysis to quantify the association of cardiac troponin and C-reactive protein levels with all-cause and cardiovascular mortality in patients with chronic kidney disease. Relevant studies were identified by searching the MEDLINE database through November 2013. Studies were included in the meta-analysis if they reported the long-term all-cause or cardiovascular mortality of chronic kidney disease patients with abnormally elevated serum levels of cardiac troponin or C-reactive protein. Summary estimates of association were obtained using a random-effects model. Thirty-two studies met our inclusion criteria. From the pooled analysis, cardiac troponin and C-reactive protein were significantly associated with all-cause (HR 2.93, 95% CI 1.97-4.33 and HR 1.21, 95% CI 1.14-1.29, respectively) and cardiovascular (HR 3.27, 95% CI 1.67-6.41 and HR 1.19, 95% CI 1.10-1.28, respectively) mortality. In the subgroup analysis of cardiac troponin and C-reactive protein, significant heterogeneities were found among the subgroups of population for renal replacement therapy and for the proportion of smokers and the C-reactive protein analysis method. Elevated serum levels of cardiac troponin and C-reactive protein are significant associated with higher risks of all-cause and cardiovascular mortality in patients with chronic kidney disease. Further studies are warranted to explore the risk stratification in chronic kidney disease patients.
Keywords: Renal insufficiency, Mortality, Troponin, C-reactive protein, Meta-analysis
INTRODUCTION
The number of patients with chronic kidney disease (CKD), including end-stage renal disease (ESRD), has increased in recent years (1), and the mortality of these patients is much higher than that of the normal population. CKD is associated with an increased risk for cardiovascular mortality (2), and cardiovascular causes account for almost half of all deaths in patients with ESRD (3). Cardiovascular risk-factor control is urged for addressing the heavy burden of cardiovascular diseases and adverse events in patients with CKD (1). Studies have shown that diabetes, hypertension, smoking, age, and serum albumin, calcium, phosphorus, and parathyroid hormone levels are associated with all-cause and cardiovascular mortality in patients with CKD (4-10). Novel cardiovascular risk factors, including elevated serum C-reactive protein (CRP) and cardiac troponin (cTn) levels, are prevalent in these CKD patients (4). As a marker of inflammation that is critical to the pathogenesis of atherosclerosis (11), the serum level of CRP is elevated in more than 70% of patients with ESRD (12), and the serum level of cTn, a marker of myocardial infarction (13), may predict death in these patients (14).
Some studies have shown that elevated serum levels of CTn and CRP are associated with all-cause and cardiovascular mortality in patients with ESRD (14,15). However, the relationship between the serum levels of these two biomarkers and mortality in patients with CKD remains unclear. Some studies have shown that the serum levels of these two biomarkers were significantly associated with mortality in CKD patients (16-19), whereas different results were found in other studies (4,20,21). We conducted an up-to-date meta-analysis of published studies to quantify the association of these two biomarker levels with long-term mortality among CKD patients.
METHODS
The meta-analysis was conducted according to the checklist of the Meta-Analysis of Observational Studies in Epidemiology group (22). We performed a systematic search of relevant studies published through November 2013 in the MEDLINE database.
Search strategy
Accessing the MEDLINE database, we performed a literature search for studies published until November 4, 2013 using the following search terms and key words: “C-reactive protein”, “CRP”, “troponin”, “mortality” and “chronic kidney disease”. We manually checked the reference list of retrieved articles to identify any studies that were not identified from the preliminary literature searches.
Inclusion and exclusion criteria
Studies were included in the meta-analysis if they met the following criteria: 1) published in the English language; 2) had a randomized controlled trial or a prospective observational study design; 3) enrolled patients with CKD, which was defined as an estimated glomerular filtration rate of less than 60 mL/min per 1.73 m2 (23); 4) reported the long-term all-cause mortality or cardiovascular mortality of CKD patients who had abnormal elevated serum levels of CRP or cTn; and 5) presented estimates of risk ratios (RRs) or hazard ratios (HRs) with 95% confidence intervals (CIs) or reported the data necessary to calculate these values. Animal, autopsy, and phantom studies were excluded. If the studies excluded patients with a history of myocardial infarction, they were also excluded.
Data extraction
Two authors independently extracted the following data from each retrieved article: study characteristics (design, population, duration of follow-up, CRP analyzed as a continuous variable or elevated variable, cardiac troponin assays, and adjustment), patient characteristics (mean age, sex, proportion of cardiovascular death, renal replacement therapy, history of diabetes mellitus, history of cardiovascular disease, history of hypertension, history of tobacco use), and outcomes (all-cause and cardiovascular mortality).
Statistical analysis
We directly extracted or indirectly estimated hazard ratios (HRs) from each study and pooled HRs using a random-effects meta-analysis method. Subgroup analyses for all-cause and cardiovascular mortalities were conducted according to the following factors: the baseline population (≥500 vs. <500); proportion of male patients (≥50% vs. <50%); mean or average age (≥60 years vs. <60 years); duration of follow-up (≥2 years vs. <2 years); study design (prospective observational studies vs. randomized controlled trials); renal replacement therapy (hemodialysis vs. peritoneal dialysis vs. hemodialysis or peritoneal dialysis vs. receiving or not receiving renal replacement therapy vs. not receiving renal replacement therapy, or ESRD vs. not receiving renal replacement therapy); adjustment (no adjustment vs. appropriate adjustment vs. inappropriate adjustment; appropriate adjustment was defined as adjusting for age, history of diabetes mellitus, and history of cardiovascular diseases at the same time, whereas inappropriate adjustment was defined as not adjusting for age, history of diabetes mellitus, and history of cardiovascular diseases at the same time); CRP analysis method (elevated variable vs. continuous variable); troponin assays (cardiac troponin T vs. cardiac troponin I); prevalence of diabetes mellitus (≥30% vs. <30%); prevalence of cardiovascular diseases (≥30% vs. <30%); prevalence of hypertension (≥50% vs. <50% or ≥80% vs. <80%); proportion of smokers (≥30% vs. <30%); and proportion of cardiovascular deaths (≥50% vs. <50%).
To perform the quality assessment, two authors independently assessed the study quality using the Downs-Black criteria (24). The Downs-Black criteria is an instrument comprising 27 questions that evaluate reporting, external validity, internal validity (bias and confounding), and statistical power. All questions received a score of 0 or 1, with the exception of question 5, which received a score ranging from 0 to 2, depending on whether the statistical power of the survey was explicitly stated in the article as being at least 80%. Thus, the maximum score achievable by an article was 27 points. Disagreements were resolved by consensus.
Statistical heterogeneity between studies was evaluated with the I2 statistic (25). Publication bias was assessed using the Egger asymmetry test (p<0.05 indicating statistical significance) (26). P-values that were less than 0.05 were considered statistically significant. All statistical analyses were carried out with STATA, version 12.0 (Stata Corp, College Station, Texas).
RESULTS
Literature search
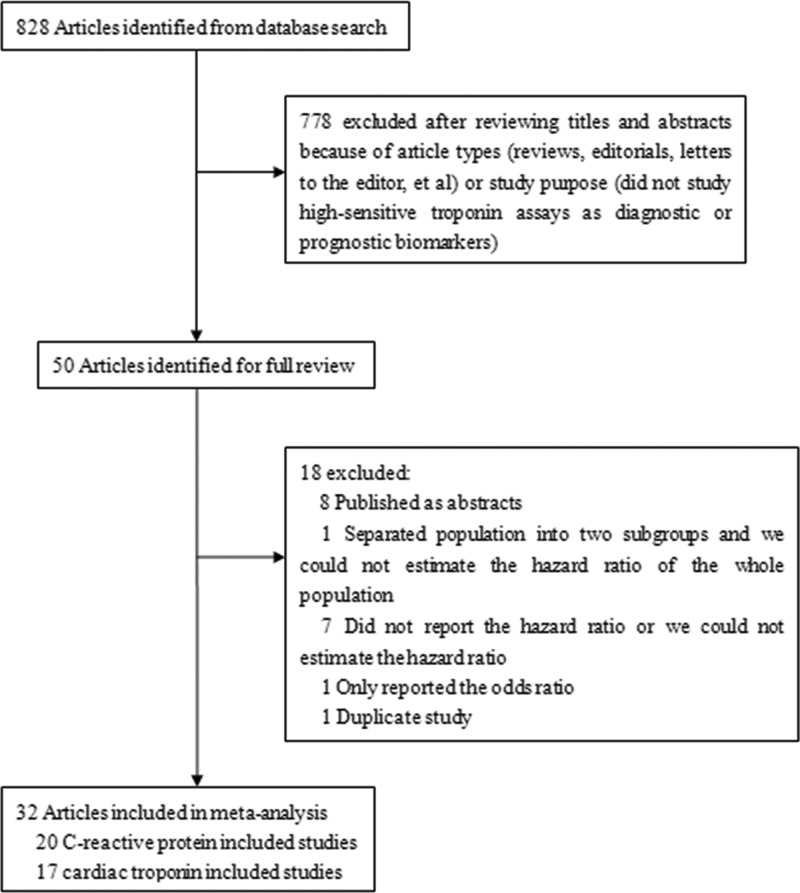
The details of the search steps are shown in Figure 1. We identified and screened 828 articles from our preliminary search, and 50 clinical trials were identified for title and abstract analysis. Eight articles were published as abstracts only and were excluded. Seven studies were excluded because they did not report the hazard ratio or we could not estimate the hazard ratio due to incomplete statistical data. One duplicate study and one study that only reported the odds ratio were also excluded. Among the included studies, 20 studies were included into the CRP analysis (4,16,17,27–43), and 17 studies were included into the cTn analysis (18–20,29,30,32,34,37,44–52).
Figure 1.
Selection of included studies.
Study characteristics
The characteristics of the included studies are shown in Table 1, and the patient characteristics are shown in Table 2. The total population of the included studies was 21,047, and most of the patients were male (53.93%). Most studies were prospective observational studies, and there were 3 randomized controlled studies, which studied CRP (16,17,38). Aside from the unavailable data, 2,321 cardiac deaths and 2,633 all-cause deaths were reported.
Table 1.
Characteristics of the included articles
| Studies | Study design | Total patients | Duration of follow-up (months) | Analyzed CRP as continuous variable/elevated variable | Cardiac troponin assays (T or I) | Adjustment | Downs-Black criteria scores |
|---|---|---|---|---|---|---|---|
| Böger 2005, Germany (27) | Prospective observational study | 445 | 46.8 | Elevated variable | N/A | Age, hemodialysis duration, gender, smoking history, body mass index, medication, history of diabetes mellitus, history of coronary artery disease | 19 |
| Menon 2005, USA (16) | Randomized controlled study | 697 | 125 | Elevated variable | N/A | Age, gender, race, randomization to different protein diets and blood pressure strata, diabetes, current smoking, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, body mass index, systolic blood pressure, etiology of kidney disease | 16 |
| Tripepi 2005, Italy (28) | Randomized controlled study | 217 | 41 | Elevated variable | N/A | Cardiovascular death, namely age, gender, smoking, diabetes, systolic BP, previous cardiovascular events, asymmetric dimethylarginine | 17 |
| Hocher 2008, Germany (29) | Randomized controlled study | 230 | 52 | Elevated variable | T | No adjustment | 15 |
| Hocher 2003, Germany (30) | Randomized controlled study | 245 | 25 | Elevated variable | T | No adjustment | 16 |
| Panichi 2008, Italy (31) | Randomized controlled study | 757 | 30 | Elevated variable | N/A | No adjustment | 14 |
| Boulier 2004, France (32) | Randomized controlled study | 191 | 13 | Elevated variable | I | Age, sex, duration of dialysis, diabetes, smoking, hypertension, dialysis center, HD modality, total cholesterol, triglycerides | 16 |
| Shlipak 2005, USA (4) | Randomized controlled study | 1249 | 103.2 | Continuous variable | N/A | Age, sex, race, education, prevalent cardiovascular disease, elevated systolic blood pressure, diabetes, current smoking, HDL of 40 mg/dL or less, LDL of 130 mg/dL or more, triglycerides, alcohol use, obesity, physical activity, left ventricular hypertrophy, and diuretic use | 18 |
| Zoccali 2011, Italy (33) | Randomized controlled study | 537 | 29 | Elevated variable | N/A | Age, gender, cardiovascular comorbidities, Kt/V and dialysis duration | 18 |
| Paniagua 2010, Mexico (34) | Randomized controlled study | 753 | 16 | Continuous variable | T | No adjustment | 15 |
| Rao 2008, USA (35) | Randomized controlled study | 182 | 78 | Continuous variable | N/A | No adjustment | 14 |
| Kawaguchi 2011, International (36) | Randomized controlled study | 2181 | 24 | Continuous variable | N/A | Age, sex, vintage, BMI, spKt/V, hemoglobin, albumin, calcium, phosphorus, nPCR, BUN, creatinine, white blood cell count, total cholesterol, uric acid, ferritin, dialysis time, coronary artery disease, diabetes mellitus and other comorbidities | 18 |
| deFilippi 2003, USA (37) | Randomized controlled study | 224 | 27 | Elevated variable | T | Age, white race, sex, length of time receiving dialysis, history of smoking, coronary disease, or diabetes, levels of albumin, dialysis dose (Kt/V), BSA | 20 |
| McMurray 2011, International (17) | Randomized controlled study | 4038 | 42.5 | Elevated variable | N/A | Age, serum albumin, coronary heart disease, diabetes complications, arrhythmia, glycated hemoglobin, reticulocytes, BUN, cerebrovascular disease, gender, race, hemoglobin level, treatment, smoking status, heart rate, BMI, and other confounding factors | 20 |
| Schneider 2013, International (38) | Randomized controlled study | 2776 | 38.4 | Continuous variable | N/A | No adjustment | 18 |
| McMahon 2012, Australia (39) | Randomized controlled study | 302 | 24 | Continuous variable | N/A | Age, duration of dialysis, peripheral vascular disease, ESA dose, hemoglobin | 17 |
| Stenvinkel 2002, International (40) | Randomized controlled study | 663 | 33±1 | Elevated variable | N/A | Age, body mass index, renal center, gender | 15 |
| Kalantar-Zadeh 2004, USA (41) | Randomized controlled study | 378 | 12 | Elevated variable | N/A | Age, gender, race, ethnicity, insurance status, diabetes mellitus, Charlson co-morbidity score, dialysis vintage, dialysis dose, BMI history of cardiovascular disease | 18 |
| Nascimento 2004, International (42) | Randomized controlled study | 180 | 21 | Continuous variable | N/A | Event-free times for age, SGA, CRP, S-Alb, fibrinogen, gender | 17 |
| Grootendorst 2007, International (43) | Randomized controlled study | 840 | 36 | Elevated variable | N/A | Age, gender, PKD, Khan comorbidity score, BMI, smoking habit, GFR | 16 |
| Acharji 2012, USA (18) | Randomized controlled study | 2179 | 12 | N/A | T and I | Differences in baseline clinical, ECG, laboratory findings | 20 |
| Dierkes 2000, Germany (44) | Randomized controlled study | 102 | 24 | N/A | T | Age, time on dialysis, baseline diabetes, cerebrovascular disease | 18 |
| Apple 2002, USA (45) | Randomized controlled study | 733 | 36 | N/A | T and I | Age, pre-draw history of coronary artery disease, time since initial hemodialysis | 17 |
| Hocher 2004, Germany (46) | Randomized controlled study | 245 | 38 | N/A | T | No adjustment | 15 |
| Ooi 1999, Canada (20) | Randomized controlled study | 172 | 12 | N/A | T | No adjustment | 12 |
| Ishii 2001, Japan (47) | Randomized controlled study | 100 | 24 | N/A | T | No adjustment | 12 |
| Deegan 2001, UK (48) | Randomized controlled study | 73 | 15 | N/A | T | No adjustment | 12 |
| Peetz 2003, Germany (49) | Randomized controlled study | 104 | 6 | N/A | T and I | No adjustment | 12 |
| Yakupoglu 2002, Turkey (50) | Randomized controlled study | 38 | 48 | N/A | I | No adjustment | 13 |
| Stolear 1999, Belgium (51) | Randomized controlled study | 94 | 12 | N/A | T | No adjustment | 14 |
| Löwbeer 2002, Sweden (52) | Randomized controlled study | 26 | 48 | N/A | T | No adjustment | 13 |
| Wood 2003, UK (19) | Randomized controlled study | 96 | 24 | N/A | T | Age, sex, diabetes mellitus, history of cardiovascular disease, serum creatinine | 17 |
Table 2.
Patient characteristics in included articles.
| Studies | Average or median age (years old) | Male (%) | Cardiovascular death (%) | Renal replacement therapy | History of diabetes mellitus (%) | History of cardiovascular diseases (%) | History of hypertension (%) | History of tobacco use (%) |
| Böger 2005, Germany (27) | 67.5±8.2 | 54.83 | 38.69 | Hemodialysis | 100 | 57 | N/A | 44 |
| Menon 2005, USA (16) | 51±12 | 58.11 | 51.45 | Receiving or not receiving renal replacement therapy | 4 | N/A | N/A | 10 |
| Tripepi 2005, Italy (28) | 60.7±15.1 | 55 | 58.04 | Hemodialysis or peritoneal dialysis | 15 | 49 | 45 | 40 |
| Hocher 2008, Germany (29) | 65.6±13.7 | 48.7 | 47.5 | Hemodialysis | 33 | 27 | 91.74 | 41 |
| Hocher 2003, Germany (30) | 65.6±7.2 | 50.20 | 56.16 | Hemodialysis | 34 | 64 | 89.8 | 41 |
| Panichi 2008, Italy (31) | 66±14 | 60.5 | 45.49 | Hemodialysis | 19 | 26 | 35 | N/A |
| Boulier 2004, France (32) | 66.7 | 50.8 | 44.83 | Hemodialysis | 20 | 33 | 22.5 | 27 |
| Shlipak 2005, USA (4) | 75±6 | 47 | N/A | Receiving or not receiving renal replacement therapy | 17 | N/A | N/A | 10 |
| Zoccali 2011, Italy (33) | 63±15 | 57.73 | 63.19 | Hemodialysis | 16 | 27 | 49 | 11 |
| Paniagua 2010, Mexico (34) | 48.64±17.55 | 55.11 | 46.7 | Hemodialysis or peritoneal dialysis | 44 | 15.9 | N/A | N/A |
| Rao 2008, USA (35) | 62.2±12.3 | 47 | 35.51 | Hemodialysis | 41 | 66 | N/A | 54 |
| Kawaguchi 2011, International (36) | 62.4±12.6 | 58.6 | N/A | Hemodialysis | 33 | 29 | 72.08 | 18 |
| deFilippi 2003, USA (37) | 62 | 54 | 53 | Hemodialysis | 48 | 36 | N/A | 21 |
| McMurray 2011, International (17) | 67.5±10.76 | 42.74 | N/A | Not receiving renal replacement therapy | 65.82 | 36.5 | N/A | 43.86 |
| Schneider 2013, International (38) | 64.2±8.7 | 62.18 | N/A | Hemodialysis | 26.4 | 51.4 | N/A | 15.5 |
| McMahon 2012, Australia (39) | 60±14 | 63.9 | 88.4 | Hemodialysis or peritoneal dialysis | 18.5 | 88.4 | 88.4 | N/A |
| Stenvinkel 2002, International (40) | 59.4±1 | 56.41 | N/A | Hemodialysis or peritoneal dialysis | 0.243 | N/A | N/A | N/A |
| Kalantar-Zadeh 2004, USA (41) | 54.5±14.7 | 53.2 | 51.28 | Hemodialysis | 55.1 | 50.8 | N/A | N/A |
| Nascimento 2004, International (42) | 49±25 | 55 | 52 | Hemodialysis | 9 | N/A | 26 | N/A |
| Grootendorst 2007, International (43) | 59.1±14.9 | 60.1 | 46.9 | Hemodialysis or peritoneal dialysis | 20.7 | 34.9 | N/A | N/A |
| Acharji 2012, USA (18) | 76 (troponin positive) 75 (troponin negative) | 53.46 | 57.3 | Not receiving renal replacement therapy | 28.92 | 33.25 | 76.95 | 14.84 |
| Dierkes 2000, Germany (44) | 64±13 | 49.02 | 39.29 | Hemodialysis | 40.2 | 21 | N/A | 44 |
| Apple 2002, USA (45) | 62 | 56 | N/A | Hemodialysis | 46 | 29 | N/A | N/A |
| Hocher 2004, Germany (46) | 63.5±5.8 | 50.2 | 65.42 | Hemodialysis | 34.29 | 64.08 | 89.8 | 41.22 |
| Ooi 1999, Canada (20) | 61 (cTnT<0.1 μg/L) 64.5 (0.1 μg/L≤cTnT≤0.2 μg/L)62.8 (cTnT>0.2 μg/L) | 64.47 | 38.71 | Hemodialysis | 29.07 | 31.4 | 43.6 | N/A |
| Ishii 2001, Japan (47) | 58 | 61 | 52.63 | Hemodialysis or peritoneal dialysis | 41 | 22 | 71 | N/A |
| Deegan 2001, UK (48) | 64±18 | 57.53 | 57.14 | Hemodialysis | 16.44 | 24.66 | N/A | N/A |
| Peetz 2003, Germany (49) | 63 (male)65 (female) | 60.58 | 85 | Hemodialysis | 26.92 | 37.5 | 70.19 | N/A |
| Yakupoglu 2002, Turkey (50) | 55.9±12.9 | 42.11 | 100 | Hemodialysis | N/A | N/A | N/A | N/A |
| Stolear 1999, Belgium (51) | 62.9±1.4 | 58.51 | 58.33 | Hemodialysis | 18.09 | 30.85 | N/A | N/A |
| Löwbeer 2002, Sweden (52) | 58 | 50 | 73.33 | Peritoneal dialysis | 19 | 19 | 62 | N/A |
| Wood 2003, UK (19) | 52.4 | 66.67 | 90.48 | Not receiving renal replacement therapy | 14.6 | 24 | N/A | N/A |
CRP predicting all-cause and cardiovascular mortality
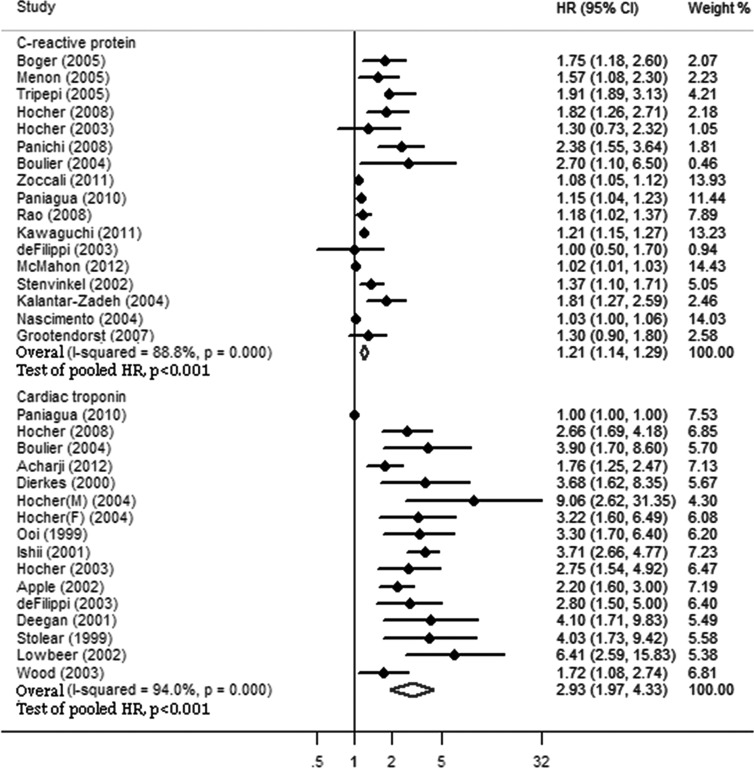
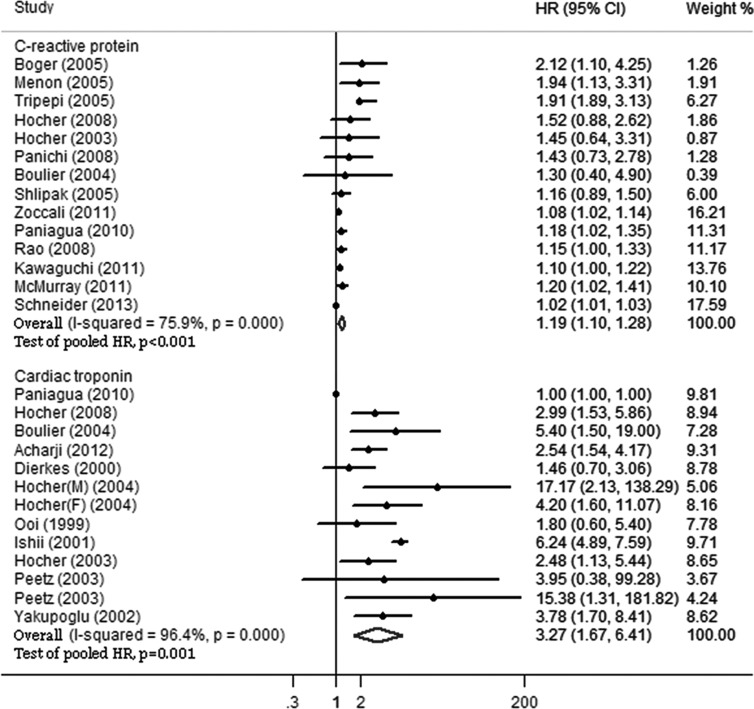
The pooled HRs of all-cause and cardiovascular mortality in CKD patients are shown in Figures 2 and 3. Elevated serum levels of CRP were significantly associated with higher all-cause (HR 1.21, 95% CI 1.14-1.29) and cardiovascular (HR 1.19, 95% CI 1.10-1.28) mortality in CKD patients. There were heterogeneities among studies for both all-cause (I2 ϝ 88.8%, p<0.001) and cardiovascular (I2 ϝ 75.9%, p<0.001) mortality.
Figure 2.
C-reactive protein and cardiac troponin for predicting all-cause mortality. Elevated serum levels of C-reactive protein and cardiac troponin were significantly associated with all-cause mortality in patients with chronic kidney disease. However, the pooled HR of cardiac troponin was higher than that of C-reactive protein. HR, hazard ratio; CI, confidence interval.
Figure 3.
C-reactive protein and cardiac troponin for predicting cardiovascular mortality. Elevated serum levels of C-reactive protein and cardiac troponin were significantly associated with cardiovascular mortality in patients with chronic kidney disease. However, the pooled HR of cardiac troponin was higher than that of C-reactive protein. HR, hazard ratio; CI confidence interval.
For all-cause mortality, significant heterogeneities were found among the subgroups of adjustment (p ϝ 0.003) and CRP analysis method (p<0.001). Studies with appropriate adjustment showed higher all-cause mortality (HR 1.52, 95% CI 1.16-1.99) compared with those with inappropriate adjustment (HR 1.07, 95% CI 1.02-1.11) and no adjustment (HR 1.39, 95% CI 1.13-1.71). When CRP was analyzed as an elevated variable, all-cause mortality was higher (HR 1.56, 95% CI 1.28-1.90) than when analyzed as a continuous variable (HR 1.10, 95% CI 1.03-1.17).
For cardiovascular mortality, significant heterogeneities were found among the population (p ϝ 0.033), CRP analysis method (p ϝ 0.012), and proportion of smokers (p ϝ 0.011) subgroups. A larger population was linked to lower cardiovascular mortality (HR 1.10, 95% CI 1.04-1.17) compared with a smaller population (HR 1.52, 95% CI 1.14-2.04). Additionally, when CRP was analyzed as an elevated variable, cardiovascular mortality was higher (HR 1.44, 95% CI 1.17-1.77) than when analyzed as a continuous variable (HR 1.09, 95% CI 1.01-1.17). Compared with studies with a smaller proportion of smokers (HR 1.07, 95% CI 1.01-1.14), those with a larger proportion of smokers (HR 1.42, 95% CI 1.15-1.75) showed higher cardiovascular mortality.
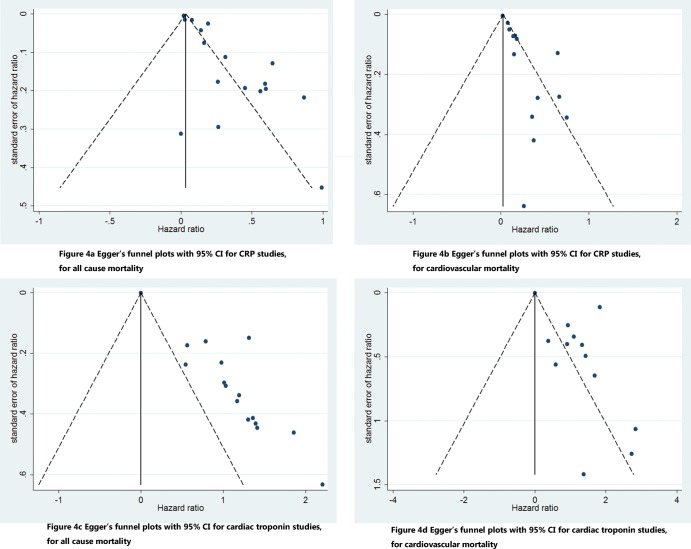
Publication bias was found in the analysis of all-cause mortality (p<0.001) and cardiovascular mortality (p<0.001), indicating the presence of publication bias or other sources of bias. The funnel plots are shown in Figures 4A and 4B.
Figure 4.
Egger's funnel plots for C-reactive protein and cardiac troponin studies. Publication bias was found in this meta-analysis. CRP, C-reactive protein; CI confidence interval.
Sensitivity analysis
During the sensitivity analysis, excluding three studies (33,39,42) did not have a significant effect on the relationship between CRP serum levels and all-cause mortality. Excluding one study that enrolled patients without ESRD (16) also did not have a significant effect on the relationship between CRP serum levels and all-cause mortality. For cardiovascular mortality, one study was excluded (33), but this exclusion did not influence the statistical significance of the results. Excluding three studies that enrolled patients without ESRD (4,16,17) did not have a significant effect on the relationship between the serum levels of CRP and cardiovascular mortality.
Cardiac troponin for predicting all-cause and cardiovascular mortality
The pooled HRs of all-cause and cardiovascular mortality in CKD patients are shown in Figures 2 and 3. Elevated serum levels of cTn were significantly associated with higher all-cause (HR 2.93, 95% CI 1.97-4.33) and cardiovascular (HR 3.27, 95% CI 1.67-6.41) mortality in CKD patients. Additionally, there were heterogeneities among studies for both all-cause (I2 ϝ 94.0%, p<0.001) and cardiovascular (I2 ϝ 96.4%, p<0.001) mortality.
For all-cause mortality, significant heterogeneities were found among the population (p ϝ 0.015) and renal replacement therapy (p ϝ 027) subgroups. Studies with a smaller population showed higher all-cause mortality (HR 3.20, 95% CI 2.65-3.87) compared with a larger population (HR 1.54, 95% CI 0.88-2.70). Additionally, studies with a larger population did not show a significant association of the serum levels of cTn with increased all-cause mortality. Studies with different renal replacement therapies showed different associations of cTn with all-cause mortality. Studies with an ESRD population had higher all-cause mortality (HR 3.21, 95% CI 2.02-5.09) compared with those with a population that did not require renal replacement therapy (HR 1.75, 95% CI 1.33-2.30).
For cardiovascular mortality, significant heterogeneities were found in the subgroup of the proportion of cardiovascular deaths (p ϝ 0.043). Studies with a higher proportion of cardiovascular deaths had higher cardiovascular mortality (HR 4.18, 95% CI 2.68-6.54) compared with those with a lower proportion of cardiovascular deaths (HR 1.88, 95% CI 1.00-3.53).
Publication bias was found in the analysis of all-cause mortality (p<0.001) and cardiovascular mortality (p ϝ 0.014), indicating the presence of publication bias or other sources of bias. The funnel plots are shown in Figures 4C and 4D.
Sensitivity analysis
Excluding two studies that enrolled patients without ESRD (18,19) did not have a significant effect on the relationship between cTn serum levels and all-cause mortality. For cardiovascular mortality, one study was excluded (41), but this exclusion did not influence the statistical significance of the results. Excluding one study that enrolled patients without ESRD (18) did not have a significant effect on the relationship between the serum levels of cTn and cardiovascular mortality.
Quality assessment
The quality assessment results for the included studies are shown in Table 1. Studies could reach a maximum of 27 points, divided into 5 different aspects – reporting, external validity, bias, confounding and power. The scores varied from 12 to 20, and the average score was 16. Because most of the included studies were not randomized controlled trials, the questions about blinding and randomizing were scored 0 in most of the studies.
DISCUSSION
Elevated serum levels of cTn and CRP were found to be significantly associated with higher risks of all-cause and cardiovascular mortality in patients with CKD, according to our meta-analysis. Compared with CRP, cTn was more strongly associated with all-cause (HR 2.93 vs. 1.21) and cardiovascular (HR 3.27 vs. 1.19) mortality.
In our meta-analysis, CRP was significantly associated with increased all-cause and cardiovascular mortality, although the association was not as strong as that for cTn. In the subgroup analyses, significant heterogeneities were found in the subgroups of adjustment, CRP analysis method and population. These results indicated that a larger population might lead to lower cardiovascular mortality and that analyzing a large population as a continuous variable would lower the all-cause and cardiovascular mortality. More appropriate adjustments would lead to higher all-cause mortality. However, notably, there were heterogeneities in the subgroups of study design, mean or average age, renal replacement therapy, and the prevalence of hypertension for cardiovascular mortality, although the p-values were >0.05. Randomized controlled trials, studies with younger patients and studies with fewer hypertension patients showed no significant results. In the subgroup of renal replacement therapy, the situation was similar. CRP is a biomarker of inflammation. CKD patients are subject to multiple nonvascular inflammatory stimuli, including chronic infection (53). Therefore, not surprisingly, elevated serum levels of CRP were found to be associated with higher all-cause and cardiovascular mortality due to inflammation. However, in the population with CKD, CRP elevation may also result from decreased renal clearance or degradation rather than increased production. This might explain the decreased statistical association of CRP with mortality (4). Thus, study characteristics, such as age, population, or renal replacement therapy, might affect the findings of the relationship between CRP and mortality, as we found in the subgroup analysis. This result suggests that screening CKD patients with elevated CRP for infectious or non-infectious inflammation and treating with proper interventions may improve patient outcomes.
We found that cTn was strongly associated with increased all-cause and cardiovascular mortality. However, patients who were not receiving renal replacement therapy showed significantly lower all-cause mortality compared with ESRD patients. Not surprisingly, ESRD patients were subject to infection, acute myocardial infarction, and death due to renal failure. Notably, in the subgroup analysis, the studies with a larger population showed no significant association of cTn with all-cause and cardiovascular mortality. Additionally, studies with younger patients, appropriate adjustment, a smaller prevalence of cardiovascular diseases, and a smaller proportion of cardiovascular deaths showed no significant association of cTn with cardiovascular mortality. cTn is specific for identifying myocardial cell injury. The elevation of cTn in CKD patients may result from microembolization (54), non-ischemic cardiomyopathy or left ventricular hypertrophy (55-57). Therefore, cTn elevation was found to be strongly associated with increased cardiovascular mortality in CKD patients. The association of cTn elevation with increased all-cause mortality might be the result of a large proportion of cardiovascular deaths in CKD patients. (3) In the population with fewer cardiovascular risk factors, as we found in the subgroup analysis, cTn elevation might not be a predictor. One possible explanation is the decreased renal clearance or degradation of cTn in CKD patients, but more mechanisms should be explored. According to the present results, screening for cardiovascular diseases and performing more active treatment may improve patient outcomes is suggested when serum levels of cTn are elevated in patients with CKD.
The strengths of this meta-analysis are the large number of patients analyzed and the robustness of the findings by sensitivity analyses. However, there were some limitations to this study. First, although we conducted an unrestricted literature search, few studies were randomized controlled trials, few studies enrolled patients who did not need to receive renal replacement therapy, and few studies included a large population. Along with causing publication bias, these limitations would tend to overinflate the pooled estimate of HR. However, we did find a significant association of cTn and CRP with mortality in the sensitive analyses. Second, the studies included in our analysis used different cut-off points to define an elevation in the serum levels of cTn and CRP. This variation in cut-off point is at least in part related to the significant heterogeneity. Third, the inclusion criteria of included studies were not well balanced. Population differences might therefore influence our results.
In conclusion, the present meta-analysis shows that elevated serum levels of cTn and CRP are significantly associated with higher risks of all-cause and cardiovascular mortality in patients with CKD. Further studies with strict standardization, long-term follow up, rigorous design, and large and representative populations are warranted to explore the risk stratification in CKD patients.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81370285), the Guangdong Province Natural Science Foundation (No.06021338), the Guangdong Province Science and Technology Program (No. 2012B031800091, 2007B031508003) and the National Ministry of Education Scholarly Exchanges Foundation (No. 200724) to Dr. Wu.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59((1 Suppl 1)):A7, e1–420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS. Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108((17)):2154–69. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 3.Renal Data System USRDS 1997 Annual Data Report. Bethesda, Md: National Institute of Diabetes and Digestive and Kidney Diseases. 1997;D1–D51 (NIH publication No. 97-3176) [Google Scholar]
- 4.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293((14)):1737–45. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 5.Mahmoodi BK, Matsushita K, Woodward M, Blankestijn PJ, Cirillo M, Ohkubo T, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet. 2012;380((9854)):1649–61. doi: 10.1016/S0140-6736(12)61272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380((9854)):1662–73. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17((7)):2034–47. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 8.Herselman M, Esau N, Kruger JM, Labadarios D, Moosa MR. Relationship between serum protein and mortality in adults on long-term hemodialysis: exhaustive review and meta-analysis. Nutrition. 2010;26((1)):10–32. doi: 10.1016/j.nut.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305((11)):1119–27. doi: 10.1001/jama.2011.308. [DOI] [PubMed] [Google Scholar]
- 10.Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308((22)):2349–60. doi: 10.1001/jama.2012.16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340((2)):115–26. doi: 10.1016/j.urolonc.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 12.de Filippi C, Wasserman S, Rosanio S, Tiblier E, Sperger H, Tocchi M, et al. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA. 2003;290((3)):353–9. doi: 10.1001/jama.290.3.353. [DOI] [PubMed] [Google Scholar]
- 13.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36((3)):959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 14.Khan NA, Hemmelgarn BR, Tonelli M, Thompson CR, Levin A. Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: a meta-analysis. Circulation. 2005;112((20)):3088–96. doi: 10.1161/CIRCULATIONAHA.105.560128. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, He J, Zhang F, Huang C, Wu Y, Han Y, et al. Prognostic role of C-reactive protein and interleukin-6 in dialysis patients: a systematic review and meta-analysis. J Nephrol. 2013;26((2)):243–53. doi: 10.5301/jn.5000169. [DOI] [PubMed] [Google Scholar]
- 16.Menon V, Greene T, Wang X, Pereira AA, Marcovina SM, Beck GJ, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68((2)):766–772. doi: 10.1111/j.1523-1755.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 17.McMurray JJ, Uno H, Jarolim P, Desai AS, de Zeeuw D, Eckardt KU, et al. Predictors of fatal and nonfatal cardiovascular events in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia: an analysis of the Trial to Reduce cardiovascular Events with Aranesp (darbepoetin-alfa) Therapy (TREAT) Am Heart J. 2011;162((4)):748–755.e3. doi: 10.1016/j.ahj.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Acharji S, Baber U, Mehran R, Fahy M, Kirtane AJ, Lansky AJ, et al. Prognostic significance of elevated baseline troponin in patients with acute coronary syndromes and chronic kidney disease treated with different antithrombotic regimens: a substudy from the ACUITY trial. Circ Cardiovasc Interv. 2012;5((2)):157–65. doi: 10.1161/CIRCINTERVENTIONS.111.963876. [DOI] [PubMed] [Google Scholar]
- 19.Wood GN, Keevil B, Gupta J, Foley R, Bubtana A, McDowell G, et al. Serum troponin T measurement in patients with chronic renal impairment predicts survival and vascular disease: a 2 year prospective study. Nephrol Dial Transplant. 2003;18((8)):1610–5. doi: 10.1038/nrendo.2010.227. [DOI] [PubMed] [Google Scholar]
- 20.Ooi DS, Veinot JP, Wells GA, House AA. Increased mortality in hemodialyzed patients with elevated serum troponin T: a one-year outcome study. Clin Biochem. 1999;32((8)):647–52. doi: 10.1016/s0009-9120(99)00064-8. [DOI] [PubMed] [Google Scholar]
- 21.Möckel M, Schindler R, Knorr L, Müller C, Heller G, Jr, Störk TV, et al. Prognostic value of cardiac troponin T and I elevations in renal disease patients without acute coronary syndromes: a 9-month outcome analysis. Nephrol Dial Transplant. 1999;14((6)):1489–95. doi: 10.1093/ndt/14.6.1489. [DOI] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283((15)):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39((2 Suppl 1)):S1–266. [PubMed] [Google Scholar]
- 24.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52((6)):377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21((11)):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315((7109)):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Böger CA, Götz A, Stubanus M, Banas B, Deinzer M, Krüger B, et al. C-reactive protein as predictor of death in end-stage diabetic nephropathy: role of peripheral arterial disease. Kidney Int. 2005;68((1)):217–27. doi: 10.1111/j.1523-1755.2005.00396.x. [DOI] [PubMed] [Google Scholar]
- 28.Tripepi G, Mallamaci F, Zoccali C. Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: searching for the best risk marker by multivariate modeling. J Am Soc Nephrol. 2005;16((Suppl 1)):S83–8. doi: 10.1681/asn.2004110972. [DOI] [PubMed] [Google Scholar]
- 29.Hocher B, Kalk P, Godes M, Liefeldt L, Ziebig R, Stasch JP, et al. Gender-dependent impact of risk factors for cardiovascular and non-cardiovascular mortality in end-stage renal disease patients on haemodialysis. Kidney Blood Press Res. 2008;31((5)):360–6. doi: 10.1159/000173718. [DOI] [PubMed] [Google Scholar]
- 30.Hocher B, Ziebig R, Altermann C, Krause R, Asmus G, Richter CM, et al. Different impact of biomarkers as mortality predictors among diabetic and nondiabetic patients undergoing hemodialysis. J Am Soc Nephrol. 2003;14((9)):2329–37. doi: 10.1097/01.asn.0000081662.64171.9b. [DOI] [PubMed] [Google Scholar]
- 31.Panichi V, Rizza GM, Paoletti S, Bigazzi R, Aloisi M, Barsotti G, et al. Chronic inflammation and mortality in haemodialysis: effect of different renal replacement therapies. Results from the RISCAVID study. Nephrol Dial Transplant. 2008;23((7)):2337–43. doi: 10.1093/ndt/gfm951. [DOI] [PubMed] [Google Scholar]
- 32.Boulier A, Jaussent I, Terrier N, Maurice F, Rivory JP, Chalabi L, et al. Measurement of circulating troponin Ic enhances the prognostic value of C-reactive protein in haemodialysis patients. Nephrol Dial Transplant. 2004;19((9)):2313–8. doi: 10.1093/ndt/gfh365. [DOI] [PubMed] [Google Scholar]
- 33.Zoccali C, Postorino M, Marino C, Pizzini P, Cutrupi S, Tripepi G, et al. Waist circumference modifies the relationship between the adipose tissue cytokines leptin and adiponectin and all-cause and cardiovascular mortality in haemodialysis patients. J Intern Med. 2011;269((2)):172–81. doi: 10.1111/j.1365-2796.2010.02288.x. [DOI] [PubMed] [Google Scholar]
- 34.Paniagua R, Ventura MD, Avila-Díaz M, Hinojosa-Heredia H, Méndez-Durán A, Cueto-Manzano A, et al. NT-proBNP, fluid volume overload and dialysis modality are independent predictors of mortality in ESRD patients. Nephrol Dial Transplant. 2010;25((2)):551–7. doi: 10.1093/ndt/gfp395. [DOI] [PubMed] [Google Scholar]
- 35.Rao M, Li L, Tighiouart H, Jaber BL, Pereira BJ, Balakrishnan VS, et al. Plasma adiponectin levels and clinical outcomes among haemodialysis patients. Nephrol Dial Transplant. 2008;23((8)):2619–28. doi: 10.1093/ndt/gfn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawaguchi T, Tong L, Robinson BM, Sen A, Fukuhara S, Kurokawa K, et al. C-reactive protein and mortality in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephron Clin Pract. 2011;117((2)):c167–78. doi: 10.1159/000320116. [DOI] [PubMed] [Google Scholar]
- 37.deFilippi C, Wasserman S, Rosanio S, Tiblier E, Sperger H, Tocchi M, et al. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA. 2003;290((3)):353–9. doi: 10.1001/jama.290.3.353. [DOI] [PubMed] [Google Scholar]
- 38.Schneider A, Jardine AG, Schneider MP, Holdaas H, Holme I, Fellstroem BC, et al. Determinants of cardiovascular risk in haemodialysis patients: post hoc analyses of the AURORA study. Am J Nephrol. 2013;37((2)):144–51. doi: 10.1159/000346710. [DOI] [PubMed] [Google Scholar]
- 39.McMahon LP, Cai MX, Baweja S, Holt SG, Kent AB, Perkovic V, et al. Mortality in dialysis patients may not be associated with ESA dose: a 2-year prospective observational study. BMC Nephrol. 2012;13:40. doi: 10.1186/1471-2369-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stenvinkel P, Wanner C, Metzger T, Heimbürger O, Mallamaci F, Tripepi G, et al. Inflammation and outcome in end-stage renal failure: does female gender constitute a survival advantage. Kidney Int. 2002;62((5)):1791–8. doi: 10.1046/j.1523-1755.2002.00637.x. [DOI] [PubMed] [Google Scholar]
- 41.Kalantar-Zadeh K, Kopple JD, Humphreys MH, Block G. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol Dial Transplant. 2004;19((6)):1507–19. doi: 10.1093/ndt/gfh143. [DOI] [PubMed] [Google Scholar]
- 42.Nascimento MM, Pecoits-Filho R, Qureshi AR, Hayashi SY, Manfro RC, Pachaly MA, et al. The prognostic impact of fluctuating levels of C-reactive protein in Brazilian haemodialysis patients: a prospective study. Nephrol Dial Transplant. 2004;19((11)):2803–9. doi: 10.1093/ndt/gfh493. [DOI] [PubMed] [Google Scholar]
- 43.Grootendorst DC, de Jager DJ, Brandenburg VM, Boeschoten EW, Krediet RT, Dekker FW, et al. Excellent agreement between C-reactive protein measurement methods in end-stage renal disease patients--no additional power for mortality prediction with high-sensitivity CRP. Nephrol Dial Transplant. 2007;22((11)):3277–84. doi: 10.1093/ndt/gfm381. [DOI] [PubMed] [Google Scholar]
- 44.Dierkes J, Domröse U, Westphal S, Ambrosch A, Bosselmann HP, Neumann KH, et al. Cardiac troponin T predicts mortality in patients with end-stage renal disease. Circulation. 2000;102((16)):1964–9. doi: 10.1161/01.cir.102.16.1964. [DOI] [PubMed] [Google Scholar]
- 45.Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation. 2002;106((23)):2941–5. doi: 10.1161/01.cir.0000041254.30637.34. [DOI] [PubMed] [Google Scholar]
- 46.Hocher B, Ziebig R, Krause R, Asmus G, Neumayer HH, Liefeldt L, et al. Relaxin is an independent risk factor predicting death in male patients with end-stage kidney disease. Circulation. 2004;109((19)):2266–8. doi: 10.1161/01.CIR.0000128598.72920.B5. [DOI] [PubMed] [Google Scholar]
- 47.Ishii J, Nomura M, Okuma T, Minagawa T, Naruse H, Mori Y, et al. Risk stratification using serum concentrations of cardiac troponin T in patients with end-stage renal disease on chronic maintenance dialysis. Clin Chim Acta. 2001;312((1-2)):69–79. doi: 10.1016/s0009-8981(01)00592-7. [DOI] [PubMed] [Google Scholar]
- 48.Deegan PB, Lafferty ME, Blumsohn A, Henderson IS, McGregor E. Prognostic value of troponin T in hemodialysis patients is independent of comorbidity. Kidney Int. 2001;60((6)):2399–405. doi: 10.1046/j.1523-1755.2001.00076.x. [DOI] [PubMed] [Google Scholar]
- 49.Peetz D, Schütt S, Sucké B, Faldum A, Wandel E, Hafner G, et al. Prognostic value of troponin T, troponin I, and CK-MBmass in patients with chronic renal failure. Med Klin (Munich) 2003;98((4)):188–92. doi: 10.1007/s00063-003-1243-3. [DOI] [PubMed] [Google Scholar]
- 50.Yakupoglu U, Ozdemir FN, Arat Z, Haberal A, Agca E, Bilgin N. Can troponin-I predict cardiovascular mortality due to myocardial injury in hemodialysis patients. Transplant Proc. 2002;34((6)):2033–4. doi: 10.1016/s0041-1345(02)02841-5. [DOI] [PubMed] [Google Scholar]
- 51.Stolear JC, Georges B, Shita A, Verbeelen D. The predictive value of cardiac troponin T measurements in subjects on regular haemodialysis. Nephrol Dial Transplant. 1999;14((8)):1961–7. doi: 10.1093/ndt/14.8.1961. [DOI] [PubMed] [Google Scholar]
- 52.Löwbeer C, Gutierrez A, Gustafsson SA, Norrman R, Hulting J, Seeberger A. Elevated cardiac troponin T in peritoneal dialysis patients is associated with CRP and predicts all-cause mortality and cardiac death. Nephrol Dial Transplant. 2002;17((12)):2178–83. doi: 10.1093/ndt/17.12.2178. [DOI] [PubMed] [Google Scholar]
- 53.Arici M, Walls J. End-stage renal disease, atherosclerosis, and cardiovascular mortality: is C-reactive protein the missing link. Kidney Int. 2001;59((2)):407–14. doi: 10.1046/j.1523-1755.2001.059002407.x. [DOI] [PubMed] [Google Scholar]
- 54.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92((3)):657–71. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 55.Mallamaci F, Zoccali C, Parlongo S, Tripepi G, Benedetto FA, Cutrupi S, et al. Diagnostic value of troponin T for alterations in left ventricular mass and function in dialysis patients. Kidney Int. 2002;62((5)):1884–90. doi: 10.1046/j.1523-1755.2002.00641.x. [DOI] [PubMed] [Google Scholar]
- 56.Iliou MC, Fumeron C, Benoit MO, Tuppin P, Courvoisier CL, Calonge VM, et al. Factors associated with increased serum levels of cardiac troponins T and I in chronic haemodialysis patients: Chronic Haemodialysis And New Cardiac Markers Evaluation (CHANCE) study. Nephrol Dial Transplant. 2001;16((7)):1452–8. doi: 10.1093/ndt/16.7.1452. [DOI] [PubMed] [Google Scholar]
- 57.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Circulation. 2012;126((16)):2020–35. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]