Abstract
Background
Symptoms of irritable bowel syndrome (IBS) are frequently reported to be exacerbated by stress. Animal studies suggest that corticotrophin releasing hormone (CRH) mediates the effect of stress on the bowel. We have shown that stressed IBS patients with diarrhea have constricted small bowels. We hypothesized that we could mimic this effect by applying experimental stress in the form of either hand immersion in ice water or CRH injection in healthy volunteers (HV).
Methods
The postprandial effect of the cold pressor test (repeated hand immersion in ice cold water) and injection of CRH, were assessed vs control in two groups of 18 HVs.
Key Results
CRH produced a significant rise from baseline salivary cortisol levels (p = 0.004) not seen with the cold pressor test. Small bowel water content (SBWC) fell postprandially on all four treatments. SBWC was significantly reduced by both stressors but CRH caused a greater effect (anova, p < 0.003 vs p = 0.02). Ascending colon (AC) volume was greater after CRH injection compared with saline (p = 0.002) but no differences were seen with the cold pressor test vs warm water. Postprandial increase in colon volume was also reduced by CRH which also increased the sensations of distension and bloating.
Conclusions & Inferences
Two experimental stressors were shown to constrict the small bowel, mimicking the effect previously seen in IBS-D patients. CRH increased the volume of the AC. We speculate that stress accelerates transfer of water from the small bowel to the AC.
Keywords: colon, MRI, small bowel water, stress, volume
Key Messages
Previous studies have shown IBS patients with diarrhea have constricted small bowels.
The current study shows that CRH injections and cold water hand immersion stress constricts the intestine and that CRH increases the volume of the ascending colon while reducing the normal postprandial increase in ascending colon volumes.
This was associated with the sensation of distension and bloating in healthy volunteers and similar mechanisms could account for such symptoms in IBS patients.
Introduction
Irritable bowel syndrome (IBS) is a widespread functional disorder of the lower gastrointestinal tract characterized by abdominal pain, bloating, and abnormal bowel habit (both constipation and diarrhea).1 Symptoms are frequently reported to be exacerbated by stress and both psychological and pharmacological treatments of underlying stress have been reported to be helpful.2–4 Animal studies indicate that corticotrophin releasing hormone (CRH) plays an important role in mediating the effects of stress on small intestinal and colonic motility, leading to delayed gastric emptying but accelerated small and large intestinal transit.5 We have previously studied anxious IBS patients with diarrhea (IBS-D) and found, using Magnetic Resonance Imaging (MRI), that they have constricted small bowels with an acceleration of orocecal transit which correlated with their anxiety levels.6 We hypothesized that stress might cause these abnormalities; however, studying the underlying mechanisms is difficult since ethical requirements for informed consent and the freedom to withdraw from studies makes inducing real life stress in healthy volunteers (HV) difficult. Numerous experimental models which mimic some aspects of real life stress but are considered ethical have been devised for studying the effect of stress on the gut in humans including dichotomous listening tasks,7 presentations to audiences8 (Trier Social Stress Test) and pain induced by immersion of the hand into ice cold water.7,9 Previous studies suggest immersion in ice water stimulates the sympathetic nervous system and activates colonic motility thus simulating at least some of the normal response to stress. Corticotrophin releasing hormone intravenous injections in healthy humans stimulate small bowel and colonic contractions, an effect which appears exaggerated in patients with the IBS.10 We chose these two convenient models to study the effect of experimental stress on intestinal function in HV using MRI. These results have been reported in part in abstract form.11
Methods
Participants
This study involved 36 healthy, non-smoker volunteers. It was carried out according to Good Clinical Practice principles and the Declaration of Helsinki with LREC Ethics approval. All subjects gave written informed consent. There were no adverse events during the studies.
Procedure
All subjects were asked to fast from 2000 h the previous evening, with no caffeine or strenuous exercise, and to avoid alcohol for 24 h prior to the study day. They were allowed a small glass of water on waking on the day of the experiment. On arrival at the MRI unit they completed a questionnaire to investigate adherence to the study restrictions. They then provided a baseline saliva sample (for cortisol measurement) and had pulse and blood pressure readings taken (t = −10 min). Each group of 18 subjects were then exposed to their experimental stressor (defined as t = −5 min time point) with the order randomized using a Latin square design. Group 1 had non-dominant hand immersion in ice or warm water which was maintained for 20 s or until unbearable (which ever was the sooner) and immediately repeated twice more. This procedure was repeated every 45 min and followed by the hand discomfort questionnaire. Group 2 were cannulated and had a bolus injection of CRH (100 μg Corticorelin trifluoroacetate; Ferring, Saint-Praix, Switzerland) or saline. Both the subjects and investigators were blinded to this treatment which was administered by a clinician not involved in the data processing. Immediately after the injection subjects underwent a fasted MRI scan (defined as t = 0 time point). After exiting the scanner, they completed a bowel symptom questionnaire and then consumed their test meal within 20 min. At t = 45 min they had a further hand immersion (in ice/warm protocol only), a saliva sample, pulse and blood pressure readings, MRI scan and symptom questionnaires This procedure was repeated every 45 min for 270 min with a total of seven scans/subject.
Test meal
The test meal was a standard rice pudding meal as used previously12 consisting of 220 g creamed rice pudding (J. Sainsbury Plc, London, UK), 34 g seedless raspberry jam (Robertson's, Addlestone, Surrey, UK) with 15 g coarse wheat bran (Holland and Barrett Health Foods, Hinckley, Leicestershire, UK) mixed together uniformly, and a glass of 100 mL pure smooth orange juice from concentrate providing 2.9 g fructose, 3.1 g sucrose and 2.8 g glucose (J. Sainsbury Plc). The test meal had 362 kcal energy, 10% from fat, 81% from carbohydrate and 9% from protein.
Magnetic resonance imaging
All MRI scans were carried out using a 1.5 T Philips Achieva MRI scanner (Philips, Best, The Netherlands). The subjects were positioned supine with a 4-element parallel imaging body coil wrapped around the abdomen. Colonic volume was imaged as described previously13 using a coronal dual-echo, fast field echo sequence (24 contiguous slices with TE = 2.3/4.6 ms, TR = 158 ms, in-plane resolution 1.76 mm × 1.76 mm, slice thickness of 7 mm, acceleration factor = 2) during an expiration breath hold of 13 s and a transverse dual-echo fast field echo sequence (45 contiguous slices with TE = 2.3/4.6 ms, TR = 296 ms with in-plane resolution 1.76 mm × 1.76 mm, slice thickness 7 mm, acceleration factor = 2) under a 20 s expiration breath hold. Small bowel water content (SBWC) was imaged as described previously14 using a single shot fast spin echo sequence (24 contiguous slices with acquired voxel size 1.56 mm × 2.83 mm × 7 mm interpolated to 0.78 mm × 0.78 mm × 7 mm) during an expiration breath hold of 24 s.
The subjects spent only 10 min per time point supine inside the scanner and were asked to spend the rest of the time sitting upright in an adjacent room.
Data analysis and statistics
Small bowel water content was quantified using the method developed and previously validated.14 All symptom scores were assessed using a standard visual analogue score (VAS) with 0 = not at all and 100 representing the symptom maximum. Individual regional colon volumes (ascending colon [AC], transverse colon and descending colon) were measured by manual segmentation of the coronal dual echo images.13
The data are expressed as mean ± SD. Statistical analysis was carried out using Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). Normality of the data was checked using D'Agostino Pearson's normality test. Two-way anova15 was used when the data were normally distributed. When the anova was significant, post hoc test assessments of the individual time points were then performed using the Bonferroni correction to account for multiple comparisons. In those cases where normality of the data could not be assumed (nor for log-transformed data) differences were assessed using paired comparison tests, t-test or Wilcoxon-signed rank test where appropriate. The area under the curve (AUC) was calculated using the trapezoidal method as overall integration over the study period.
One subject failed to attend their second visit for the ice/warm study for personal reasons. Their first visit MRI and cortisol data were not included in the following per protocol analysis. Cortisol levels were also not available for two visits in the CRH/saline study which has therefore n = 16 in the cortisol data.
Our primary endpoint was the mean fasting SBWC for which from previous studies we found mean values of 81 mL for HV and 42 mL for IBS patients with a pooled SD of 37 mL. We calculated that using n = 18 subjects with α = 0.05 and a power of 90% we could detect a reduction of 30 mL, i.e., a 37% reduction in normal values, which we felt gave us adequate sensitivity since we anticipated a change nearer to 100% if the stress were to mimic the mental state of IBS patients.
Results
Stress response
There was no significant difference in the baseline saliva cortisol levels for the CRH or saline study days, being 0.37 ± 0.20 μg/mL and 0.40 ± 0.34 μg/mL (n = 16), respectively, nor for the warm or ice water hand immersion study days, 0.55 ± 0.31 μg/mL and 0.45 ± 0.28 μg/mL (n = 17), respectively. Salivary cortisol data are shown in Fig.1. anova analysis of the post intervention cortisol levels showed a significant increase in the cortisol levels after CRH injection lasting approximately 2 h (CRH vs saline p = 0.004). Ice water hand immersion was, however, not effective in raising salivary cortisol levels. Immersion in ice water did, however, cause a highly significant increase in perceived hand discomfort measured at all time points (ice vs warm mean AUC(SD) 11 635(3720) vs 73(128) VAS min; anova p < 0.0001).
Figure 1.
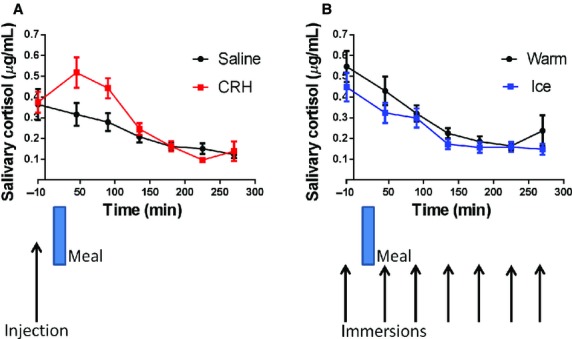
Salivary cortisol levels (mean ± SEM) vs time. (A) Pre- and post injection (saline and CRH) n = 16 (two cortisol values unavailable) showing a significant increase lasting around 2 h following CRF injection, anova p = 0.004. (B) Pre- and post immersion (warm and ice) n = 17 (one subject failed to attend for second visit) showing no effect of cold water compared to warm water and immersion anova p = 0.017.
There were no significant differences in the pre-intervention pulse and blood pressures measured. The pulse rate of the volunteers decreased slightly throughout the day with no significant difference between interventions. The systolic and diastolic blood pressures measured throughout the day also showed no significant differences.
Small bowel water content
The effect of the two interventions on SBWC is shown in Fig.2. There was a reduction in mean SBWC after CRH injection compared to saline of 40 mL and analysis of the two time series showed a significant difference (anova p = 0.003). This difference persisted for the entire 270 min of the study. There was also a reduction in SBWC after ice cold compared to warm water immersion. This reduction was smaller though still significant (anova p = 0.020). The CRH vs saline mean AUC(SD) was 17 204(7650) vs 24 136(15 106) mL/min, and the ice vs warm AUC(SD) was 21 442(14 355) vs 24 555(13 278) mL/min.
Figure 2.
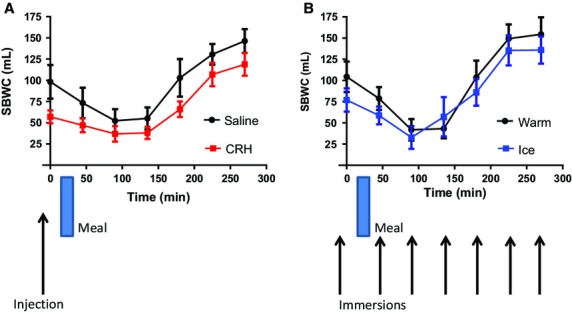
Small bowel water content (mean ± SEM) vs time. (A) Showing significant decrease after CRH but not saline injection. n = 18 anova p = 0.003. (B) Showing a smaller but still significant decrease post immersion in ice water compared to warm water. n = 17 anova p = 0.020.
Small bowel water content volumes dropped after feeding in all arms of the study up to t = 90 min, after which the volumes progressively increased above baseline values. The postprandial delta SBWC volume decrease between t = 0 and 90 was significant in each arm of the study. It was smaller for CRH (20 mL, p = 0.0446) than for saline (46 mL, p = 0.0191), ice immersion (44 mL, p = 0.0051) or warm immersion (63 mL, p = 0.0011) but the difference between arms was not significant (CRH vs saline p = 0.2227 and ice vs warm immersion p = 0.3459).
Regional colon volumes
Corticotrophin releasing hormone increased the AC volumes (Fig.3) compared to saline injection. The mean AUC(SD) was 49 817(10 770) for CRH vs 46 227(10 927) for saline, anova p = 0.002. However, the ice water immersion had no effect compared to warm water immersion, the mean AUC being 48 991(17 501) vs 48 964(16 950) respectively, p = 0.730.
Figure 3.
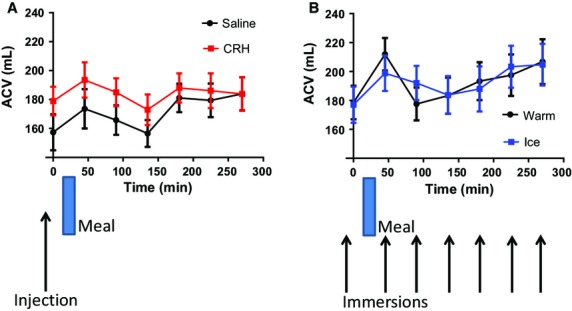
Ascending colon volumes (mean ± SEM) vs time post injection (saline and CRH) n = 18, anova p = 0.0021. (B) Post immersion (warm and ice) n = 17. anova p = 0.73. The ascending colon volume rose significantly between t = 0 and 45 min after the saline injection (p = 0.040), after the warm immersion (p = 0.020), and after ice immersion (p = 0.005). There was also a postprandial rise in volume after CRH injection but this was not statistically significant (p = 0.3).
The AC volume showed a significant postprandial rise (between t = 0 and 45 min) after the saline injection of 16(32) mL, paired t-test p = 0.040, after the warm immersion of 33(51) mL, p = 0.020, and after ice immersion of 22(27) mL, p = 0.005. There was also a postprandial rise in volume of 15(32) mL after CRH injection which was, however, not statistically significant (p = 0.3). The transverse colon volumes (TCV) are shown in Fig.4. Here, the difference between CRH and saline is not significant (p = 0.0801) whereas there is a significant drop in transverse volume ice vs warm (p = 0.0107). There were no differences seen in descending colon volumes.
Figure 4.
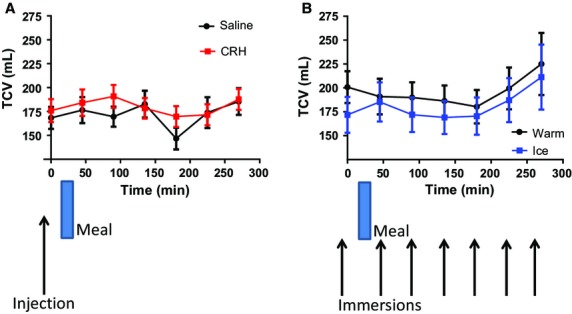
Transverse colon volumes (mean ± SEM) vs time. (A) Post injection (saline and CRH) n = 18, anova p = 0.080. (B) Post immersion (warm and ice) n = 17. anova p = 0.011.
Symptom scores
The average VAS scores for all subjects, representative of symptom severity, are summarized in Fig.5. In this group of HV the symptom scores were very low (and often zero) preventing the use of anova. We therefore used a matched pairs test to evaluate the observed VAS scores in those cases where the VAS scores were measureable but non-normally distributed. Corticotrophin releasing hormone injection increased the sensation of distension which was larger immediately post–injection 4.5 (1.75–28.5) with compared with saline 0.5 (0–6) (median values [IQR]) p = 0.043 (Wilcoxon-matched pairs signed rank test). Corticotrophin releasing hormone appeared to blunt the normal postprandial increase in distension seen after the saline injection and ice/warm immersions. This postprandial increase was significant both in the ice (from 1 [0–3.5] to 21 [4–64], median values [IQR], p < 0.0001) and warm cohort (from 1 [0–3] to 8 [2–40.5], median values [IQR], p = 0.001) (Wilcoxon-matched pairs signed rank test) but not in the saline control. The sensation of bloating was also larger immediately post injection with CRH compared with saline but this did not achieve significance and there was no difference between the warm and ice treatments. There was a significant increase in fullness post CRH injection p = 0.042 (Wilcoxon-matched pairs signed rank test), but this increase was small compared to the change caused by consumption of the test meal.
Figure 5.
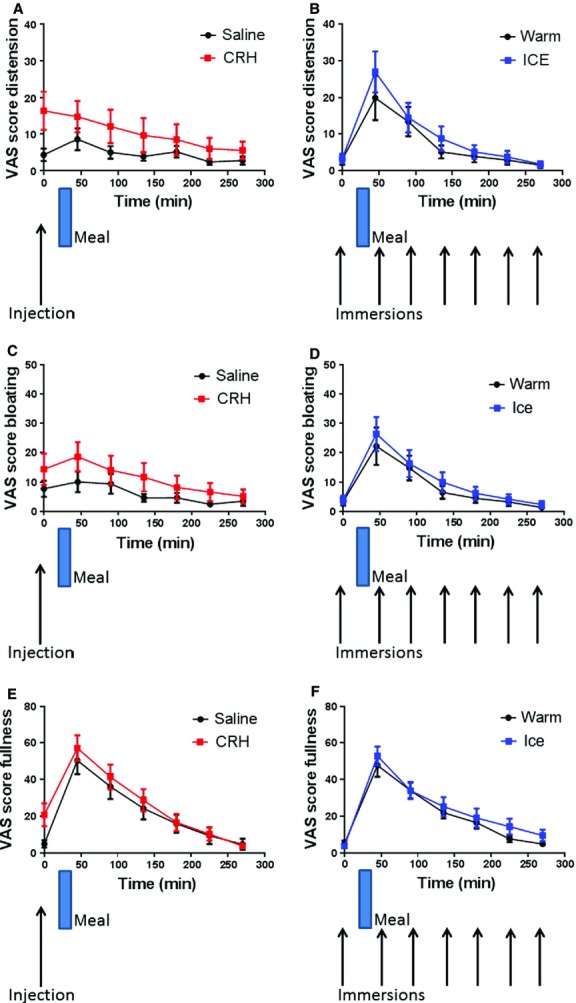
Visual analogue score (VAS) symptom scores (mean ± SEM) vs time. (A) Distension post injection (saline and CRH) n = 18. (B) Distension post immersion (warm and ice) n = 17. (C) Bloating post injection (saline and CRH) n = 18. (D) Bloating post immersion (warm and ice). (E) Fullness post injection (saline and CRH) n = 18. (F) Fullness post immersion (warm and ice).
There were no differences seen in the minimal abdominal discomfort or nausea scores for either treatment.
Discussion
We have shown for the first time that CRH injection constricts the small intestine in HV and that this is associated with an increase in both the sense of distension and the ascending and TCV. Animal studies have consistently shown CRH delays gastric emptying and accelerates colonic transit.5,16 Human studies of stress are limited but have shown that psychological stress delays gastric emptying but accelerates small bowel transit.17,18 Our findings are compatible with this and suggest that the reduction in SBWC after CRH injection is due to increased motility. Interestingly the effect on the small bowel lasted much longer than the effect on the adrenal suggesting CRH may be acting by exciting enteric nerves or perhaps mucosal mast cells19 to produce long-lasting changes in small bowel function. The increase in colonic volumes we observed may reflect the distal propulsion of chyme. Eating a meal typically stimulates ileal motility,20 causing ileal emptying and as we have previously shown a transient increase in ACV.13 This is likely to reflect a receptive relaxation, something which appears lacking in IBS-D patients.13 Interestingly, we found this receptive relaxation was much reduced by CRH and in the 60 min after CRH injection subjects experienced an increased sense of distension and bloating. This may well reflect increased tone with stimulation of tension receptors which determines perception of distension.21 The symptoms did not correlate with colonic volumes which are of course only indirectly related to colonic wall tension. An alternative explanation may be a direct sensitising effect on visceral afferents since CRH injections reduce the threshold for the sensation of distension during rectal distension in both humans22 and rodents.23
The cortisol levels in all treatments except the CRH injection showed the normal diurnal decrease through the study day.24 The raised levels at baseline may reflect the stress of coming in the early morning for an experimental study which applied to all the study arms. Despite the subjects experiencing the expected discomfort during the repeated immersions in iced water this intervention did not apparently cause any further stimulation of the hypothalamic pituitary axis as there was no measureable rise in salivary cortisol levels. Indeed there was a small but significant drop observed for reasons which are unclear. This is in contrast to previous studies where a small increase was observed.25,26 However, the injection of CRH did, as expected, lead to a large rise in average cortisol levels.27 The lack of significant changes in pulse and blood pressure levels in any of the study arms was unexpected, particularly given the high level of discomfort caused by the repeated ice immersions. The post injection pulse and blood pressure measurements were taken after the first scan and short-lived effects may have been missed.
The changes in AC volume after administration of the CRH seem to be consistent with the associated drop in the SBWC. Post injection values show an average (CRH vs saline) expansion of the AC of ∼25 mL compared with an average of drop in SBWC of ∼20 mL. This represents over 10% of the AC volume which may be sufficient to cause a change in sensation. One interpretation of this is that the CRH may have caused the small bowel to empty into the AC which may then contribute to an acceleration of colonic transit as is seen in the rat post CRH administration.28 The magnitude of this response is similar to the postprandial gastro-colic reflex seen previously in humans13 and in the saline, ice and warm treatments within this study (with a similar drop in SBWC and a rise in ACV).
Although there were no differences seen in the AC volume between the warm and ice in the cold pressor test the small reduction in the transverse volume seen is consistent with previous investigations where increased colonic tone in descending and transverse regions was observed29 (although in this case the AC was not studied).
However, there was no significant correlation seen between the changes in colon volume measured and the perceived symptoms.
As may be expected, the fullness scores were more influenced by consumption of the meal in all treatments although there was a small increase in the fullness immediately post CRH injection. The symptoms overall were much more affected by the CRH vs saline than the ice vs warm although there were small differences seen in distension and fullness in the ice/warm case.
One of the limitations in our study was the lack of a baseline MRI scan before any intervention so our first scan already showed the effect of the CRH injection which appears to be rapid. The lesser effect of the ice water stressor may relate to it being only experienced for approximately a minute every 45 min while the CRH effect may have been continuous.
Although one should be cautious in extrapolating results from HV to patients our results suggest that stress-related release of CRH could mediate the observed reduction in SBWC that we have previously reported in IBS-D patients.12 Studies of the CRH-1 antagonist, pexacerfont30 disappointingly showed no effect of blocking the CRH receptor on transit in IBS-D patients. Whether this reflects inadequate dosing, or the fact that factors other than CRH such as sympathetic tone are important in IBS, remains to be determined. Our new technique is highly patient acceptable and lends itself to future pharmacological studies of the underlying mechanism of stress-related small bowel abnormalities.
Acknowledgments
None.
Glossary
- AC
ascending colon
- MRI
magnetic resonance imaging
Funding
This study was partly funded from a UK NIHR Research for Patient Benefit (RfPB) grant and supported by the staff of the Nottingham Digestive Diseases Biomedical Research Unit funded by the UK NIHR. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Conflicts of Interest
RS has received grant support for research from Ironwood, Falk, Lesaffre, and Norgine Pharmaceuticals and acted on advisory boards for Danone, Ironwood, and Astellas. The other authors have no competing interests.
Author Contribution
Research was designed by RCS, PAG, LM, and CLH and performed by SEP, LM, KCG, WT, ER, and CC. It was analyzed by SEP, WT, ER, cortisol measurements by ML and RB. The paper was written by SEP, LM, PAG, and RCS.
References
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Spiller RC, Humes DJ, Campbell E, Hastings M, Neal KR, Dukes GE, Whorwell PJ. The Patient Health Questionnaire 12 Somatic Symptom scale as a predictor of symptom severity and consulting behaviour in patients with irritable bowel syndrome and symptomatic diverticular disease. Aliment Pharmacol Ther. 2010;32:811–20. doi: 10.1111/j.1365-2036.2010.04402.x. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, Creed FH, Olden KW, Svedlund J, Toner BB, Whitehead WE. Psychosocial aspects of the functional gastrointestinal disorders. Gut. 1999;45:25–30. doi: 10.1136/gut.45.2008.ii25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sykes MA, Blanchard EB, Lackner J, Keefer L, Krasner S. Psychopathology in irritable bowel syndrome: support for a psychophysiological model. J Behav Med. 2003;26:361–72. doi: 10.1023/a:1024209111909. [DOI] [PubMed] [Google Scholar]
- 5.Tache Y, Kiank C, Stengel A. A role for corticotropin-releasing factor in functional gastrointestinal disorders. Curr Gastroenterol Rep. 2009;11:270–7. doi: 10.1007/s11894-009-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marciani L, Foley S, Hoad CL, Campbell E, Totman JJ, Cox E, Gowland PA, Spiller RC. Abnormalities of small bowel and colonic water content in diarrohea-predominant irritable bowel syndrome: novel insights from magnetic resonance imaging. Gut. 2007;56:A65–A65. [Google Scholar]
- 7.Rao SSC, Hatfield RA, Suls JM, Chamberlain MJ. Psychological and physical stress induce differential effects on human colonic motility. Am J Gastroenterol. 1998;93:985–90. doi: 10.1111/j.1572-0241.1998.00293.x. [DOI] [PubMed] [Google Scholar]
- 8.Suarez-Hitz KA, Otto B, Bidlingmaier M, Schwizer W, Fried M, Ehlert U. Altered psychobiological responsiveness in women with irritable bowel syndrome. Psychosom Med. 2012;74:221–31. doi: 10.1097/PSY.0b013e318244fb82. [DOI] [PubMed] [Google Scholar]
- 9.Murray CDR, Flynn J, Ratcliffe L, Jacyna MR, Kamm MA, Emmanuel AV. Effect of acute physical and psychological stress on gut autonomic innervation in irritable bowel syndrome. Gastroenterology. 2004;127:1695–703. doi: 10.1053/j.gastro.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 10.Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845–9. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garsed K, Marciani L, Roberts E, Thongborisute W, Pritchard SE, Costigan C, Hoad CL, Lingaya M, et al. Stress reduces small bowel water content in healthy volunteers. Neurogastroenter Motil. 2010;22((S1)):21. doi: 10.1111/nmo.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marciani L, Cox EF, Hoad CL, Pritchard S, Totman JJ, Foley S, Mistry A, Evans S, et al. Postprandial changes in small bowel water content in healthy subjects and patients with irritable bowel syndrome. Gastroenterology. 2010;138:469–77. doi: 10.1053/j.gastro.2009.10.055. [DOI] [PubMed] [Google Scholar]
- 13.Pritchard SE, Marciani L, Garsed KC, Hoad CL, Thongborisute W, Roberts E, Gowland PA, Spiller RC. Fasting and postprandial volumes of the undisturbed colon: normal values and changes in diarrhea-predominant irritable bowel syndrome measured using serial MRI. Neurogastroenterol Motil. 2014;26:124–30. doi: 10.1111/nmo.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoad CL, Marciani L, Foley S, Totman JJ, Wright J, Bush D, Cox EF, Campbell E, et al. Non-invasive quantification of small bowel water content by MRI: a validation study. Phys Med Biol. 2007;52:6909–22. doi: 10.1088/0031-9155/52/23/009. [DOI] [PubMed] [Google Scholar]
- 15.Villanova N, Azpiroz F, Malagelada JR. Gastrogastric reflexes regulating gastric tone and their relationship to perception. Am J Physiol. 1997;36:G464–9. doi: 10.1152/ajpgi.1997.273.2.G464. [DOI] [PubMed] [Google Scholar]
- 16.Monnikes H, Tebbe JJ, Hildebrandt M, Arck P, Osmanoglou E, Rose M, Klapp B, Wiedenmann B, et al. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig Dis. 2001;19:201–11. doi: 10.1159/000050681. [DOI] [PubMed] [Google Scholar]
- 17.Cann PA, Read NW, Cammack J, Childs H, Holden S, Kashman R, Longmore J, Nix S, et al. Psychological stress and the passage of a standard meal through the stomach and small intestine in man. Gut. 1983;24:236–40. doi: 10.1136/gut.24.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ditto B, Miller SB, Barr RG. A one-hour active coping stressor reduces small bowel transit time in healthy young adults. Psychosom Med. 1998;60:7–10. doi: 10.1097/00006842-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Larauche M, Kiank C, Tache Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol. 2009;60(Suppl. 7):33–46. [PMC free article] [PubMed] [Google Scholar]
- 20.Coffin B, Lemann M, Flourie B, Picon L, Rambaud JC, Jian R. Ileal tone in humans: effects of locoregional distensions and eating. Am J Physiol. 1994;267:G569–74. doi: 10.1152/ajpgi.1994.267.4.G569. [DOI] [PubMed] [Google Scholar]
- 21.Serra J, Azpiroz F, Malagelada JR. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut. 2001;48:14–9. doi: 10.1136/gut.48.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lembo T, Plourde V, Shui Z. Fullerton S, Mertz H, Tache Y, Sytnik B, Munakata J, et al. Effects of the corticotropin-releasing factor (CRF) on rectal afferent nerves in humans. Neurogastroenterol Motil. 1996;8:9–18. doi: 10.1111/j.1365-2982.1996.tb00237.x. [DOI] [PubMed] [Google Scholar]
- 23.Tache Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol. 2004;141:1321–30. doi: 10.1038/sj.bjp.0705760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- 25.Geliebter A, Gibson CD, Hernandez DB, Atalayer D, Kwon A, Lee MI, Mehta N, Phair D, et al. Plasma cortisol levels in response to a cold pressor test did not predict appetite or ad libitum test meal intake in obese women. Appetite. 2012;59:956–9. doi: 10.1016/j.appet.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geliebter A, Carnell S, Gluck ME. Cortisol and ghrelin concentrations following a cold pressor stress test in overweight individuals with and without night eating. Int J Obes. 2013;37:1104–8. doi: 10.1038/ijo.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stalla GK, Stalla J, Schopohl J, von Werder K, Muller OA. Corticotropin-releasing factor in humans. I. CRF stimulation in normals and CRF radioimmunoassay. Horm Res. 1986;24:229–45. doi: 10.1159/000180562. [DOI] [PubMed] [Google Scholar]
- 28.Shannon MH, Bihm CC, Short WJ, Burks TF, Williams CL. Interactions of oxytocin and vasopressin with CRF on the rat colon. Neuropeptides. 1997;31:94–8. doi: 10.1016/s0143-4179(97)90027-5. [DOI] [PubMed] [Google Scholar]
- 29.Ford MJ, Camilleri M, Joyner MJ, Hanson RB. Autonomic control of colonic tone and the cold pressore test. Gut. 1996;39:125–9. doi: 10.1136/gut.39.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sweetser S, Camilleri M, Linker Nord SJ, Burton DD, Castenada L, Croop R, Tong G, Dockens R, et al. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in women with irritable bowel syndrome? Am J Physiol Gastrointest Liver Physiol. 2009;296:G1299–306. doi: 10.1152/ajpgi.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


