Abstract
Objectives:
Chronic pancreatitis (CP) may be difficult to diagnose in early stages. We aimed to measure pancreatic juice (PJ) prostaglandin E2 (PGE2) concentrations to determine whether they are elevated in CP and improve diagnosis of early disease.
Methods:
We measured PJ PGE2 in 10 patients with established CP, 25 patients who met criteria for “minimal change” chronic pancreatitis (MCCP), and 10 normal control participants.
Results:
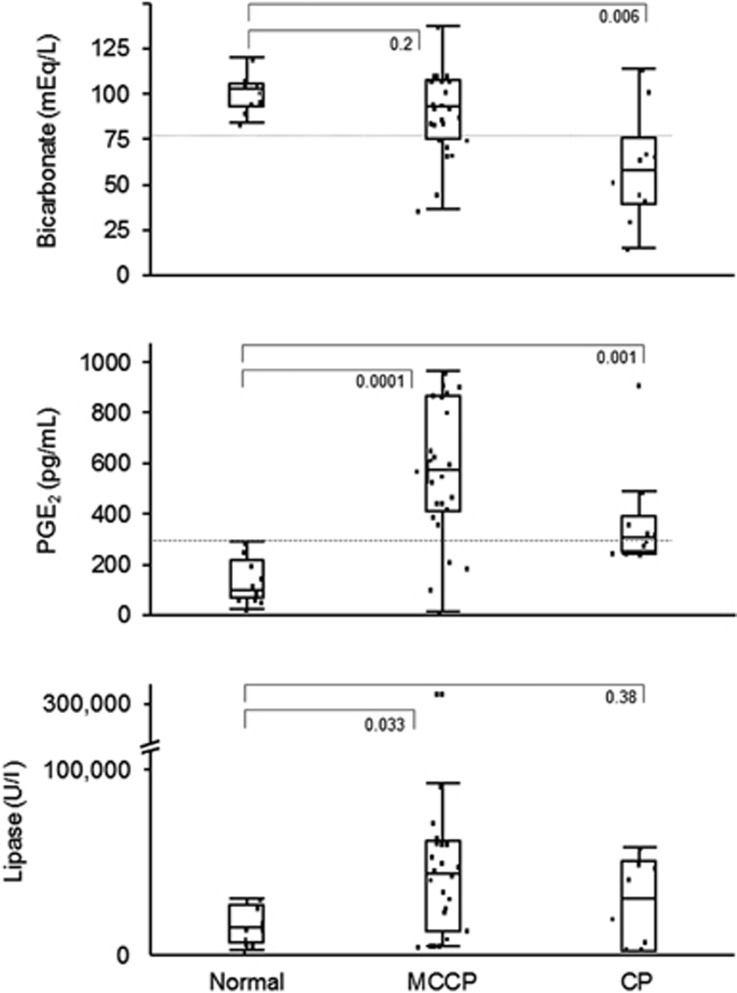
Median PJ PGE2 was elevated in CP (307 pg/ml, IQR (249–362)) and MCCP (568 pg/ml, (418–854)) compared with normal controls (104 pg/ml, (68–206)) (P≤ 0.001). Area under receiving operator curve (AUROC) for diagnosis of CP and MCCP was 0.9 and 0.62, respectively, for PJ bicarbonate concentration alone; AUROC was 1.0 and 0.94 for the combination of PJ bicarbonate and PGE2 concentrations.
Conclusions:
PJ PGE2 appears to be a biomarker for CP and is elevated in both established and “minimal change” chronic pancreatitis.
Introduction
Established chronic pancreatitis (CP) is marked by irreversible structural and functional changes and is readily diagnosed by imaging or functional testing.1 Early or “minimal change” chronic pancreatitis (MCCP) is a controversial entity because there is no diagnostic method that clearly identifies patients with early forms of CP. Patients with suspected MCCP often have non-diagnostic cross-sectional imaging of the pancreas and normal pancreatic function testing. Endoscopic ultrasound (EUS) frequently shows abnormalities in patients with suspected MCCP, but the significance of these findings is debated.2
Induction of the cyclooxygenase-2 (COX-2) enzyme increases production of prostaglandin E2 (PGE2) at sites of inflammation. COX-2 is overexpressed in pancreatic acini and ducts of patients with chronic pancreatitis.3, 4 PGE2 is a potent inflammatory mediator and also regulates pancreatic stellate cell profibrotic activity.5 In a rat model propagation of pancreatic inflammation is COX-2 dependent,6 and COX-2 inhibition inhibits pancreatic inflammation and fibrosis.7
We hypothesized that pancreatic juice (PJ) PGE2 concentrations would be elevated in patients with CP, and measured PJ PGE2 in both CP and MCCP to better understand the pathophysiology of chronic pancreatitis and to evaluate a potential biomarker for early disease.
Methods
Under approved IRB protocols we prospectively enrolled 27 participants at Mayo Clinic Rochester (MCR) and 18 participants at Brigham and Women's Hospital (BWH) between July 2009 and March 2012. Each participant was assigned to one of three groups (normal, MCCP, or CP). Normal participants were volunteers with no history of pancreatitis, abdominal pain, or diabetes. The CP group had definite chronic pancreatitis by the M-ANNHEIM criteria,8 with moderate-to-marked ductal changes of CP on cross-sectional imaging (contrast-enhanced computed tomography (CECT), magnetic resonance imaging (MRI), or magnetic resonance cholangiopancreatography (MRCP)). The MCCP group had normal or equivocal pancreatic CECT, MRI, or MRCP findings, but met criteria in one or more of the following domains: (A) ≥1 clinical risk factor for chronic pancreatitis (≥1 attack of unexplained acute pancreatitis documented biochemically or by cross-sectional imaging, history of heavy alcohol consumption (>10 drinks per week for at least 5 years), or family history of pancreatitis),9 (B) ≥3 of 9 EUS standard criteria of CP, or (C) peak PJ bicarbonate concentration <80 mEq/l. Steatorrhea was deemed present if either qualitative or quantitative fecal fat determination was abnormal.
All participants underwent an endoscopic pancreatic function test as previously described.10 In brief, PJ samples were suctioned from the lumen of the second portion of duodenum through an endoscope 30 to 45 min after administration of intravenous secretin 0.2 mcg/kg (ChiRhoStim, ChiRhoClin, Burtonsville, MD, USA). All samples were immediately placed on ice. An aliquot was assayed for bicarbonate concentration by an autoanalyzer.11 The remainder was frozen at −80 °C within 30 min of collection and batch-tested for PGE2 and lipase.
For PGE2 assays samples were thawed and centrifuged at 10,000 g for 5 min at 4 °C. Supernatants (500 μL) were diluted with enzyme immunoassay buffer (EIA kit Cat No. 514010, Cayman Chemical, Ann Arbor, MI, USA) and the samples were assayed at 1/1 (no dilution), 1/10, and 1/50 dilutions. The enzyme immunoassay is based on a competition between PGE2 and PGE2 acetyl cholinesterase (AChE) conjugate (PGE2 Tracer) for a limited amount of PGE2 monoclonal antibody after 18 h of incubation at 4 °C. Two hundred microliters of Ellman's reagent was used as the substrate for AChE. The proportion of the bound tracer was determined spectroscopically, and the concentration of PGE2 was determined using optimized standard curves from known concentrations. The product of this enzymatic reaction is determined using absorbance measured by a spectrophotometer. The least dilution yielding a result within the linear portion of the standard curves was considered most reliable, and was multiplied by the appropriate dilution factor to yield a final result.
Personnel blinded to the group assignment of subjects conducted all laboratory assays. Acute pancreatitis was defined as compatible acute symptoms with a ≥3-fold elevation of serum amylase or lipase above the upper limit of normal, and/or cross-sectional imaging evidence of pancreatic inflammation. We used Wilcoxon/Kruskal–Wallis tests to compare PGE2 levels among groups, multivariate linear regression analyses to adjust for potential confounders, and logistic regression analysis to generate receiver operator curves (ROC) and calculate area under the receiver operator curve (AUROC). For ROC analysis we used a binary PJ PGE2 cutoff of 300 pg/ml as this maximized sensitivity and specificity for detecting MCCP. All analyses were performed using SAS version 9.2 software (SAS Institute, Cary, NC, USA). Graphing was performed using JMP software (version 8; SAS Institute, Cary, NC, USA).
Results
Demographic and clinical data are shown in Table 1. Among the CP group all had moderate or marked changes of CP on cross-sectional imaging studies, five had pancreatic calcifications on plain radiography or CT, and eight had a peak PJ bicarbonate concentration <80 mEq/l.
Table 1. Comparison of the three groups.
| Normal (N=10) | MCCP (N=25) | CP (N=10) | P value | |
|---|---|---|---|---|
| Age (years) (mean, s.d.) | 29 (6) | 44 (16) | 52 (15) | 0.002 |
| Female (%) | 50% | 36% | 60% | 0.4 |
| Race | ||||
| White | 9 | 23 | 9 | 0.97 |
| None-white | 1 | 2 | 1 | |
| BMI (kg/m2) (mean, s.d.) | 24 (3.6) | 27 (6) | 27 (5.9) | 0.33 |
| Smokinga | ||||
| Never | 10 (100%) | 12 (48%) | 4 (40%) | 0.04 |
| Prior | 0 | 6 (24%) | 2 (20%) | |
| Current | 0 | 7 (28%) | 4 (40%) | |
| EtOH useb | ||||
| No | 10 (100%) | 16 (64%) | 6 (60%) | 0.07 |
| Yes | 0 | 9 (36%) | 4 (40%) | |
| Diabetes (%) | 0 | 12% | 20% | 0.28 |
| NSAID usec | ||||
| No | 9 (90%) | 17 (68%) | 6 (60%) | 0.29 |
| Yes | 1 (10%) | 8 (32%) | 4 (40%) | |
| History of acute pancreatitis | 0/10 (0%) | 11/25 (44%) | 5/10 (50%) | 0.03 |
| Number of episodes (median, range) | — | 5 (1–12) | 2.5 (1–5) | |
| Months between last attack and pancreatic juice collection (median, range) | — | 3 (1–72) | 3.5 (1–42) | |
| Chronic pain | 0 | 17 (68%) | 7 (70%) | 0.001 |
| Steatorrhea | 0 | 4 (16%) | — | |
| Family history of pancreatitis | 0 | 1 (4%) | 1 (10%) | 0.55 |
| Genetic abnormality | Not tested | 3 (12%) | 0 | |
| CFTR | 0 | 0 | ||
| SPINK-1 | 2 (8%) | 0 | ||
| PRSS-1 | 1 (4%) | 0 | ||
| Number of standard EUS criteriad of chronic pancreatitis (mean, s.d.) | — | 3.5 (1) | 4 (1.4) | 0.2 (between MCCP and CP) |
| M-ANNHEIM(8)a,e CT/MRCP criteria for chronic pancreatitis | ||||
| Normal | 10 (100%) | 22 (88%) | 0 | <0.001 |
| Equivocal | 2 (8%) | 0 | ||
| Mild | 1 (4%) | 0 | ||
| Moderate | 0 | 2 (20%) | ||
| Marked | 0 | 8 (80%) | ||
| Pancreatic juice lipase (U/I) (median, IQR) | 11,924 (3,850–25,465) | 43,230 (10,142–61,182) | 27,742 (2,468–48,455) | <0.001 |
| Pancreatic juice Bicarbonate (mEq/l) (median, IQR) | 103.5 (94–105) | 93 (75–108) | 58 (42–68) | <0.001 |
| Pancreatic juice PGE2 (pg/ml) (median, IQR) | 104 (68–206) | 568 (418–854) | 307 (249–362) | 0.001 |
Abbreviations: CP, chronic pancreatitis; IQR, interquartile range; MCCP, “minimal change” chronic pancreatitis.
Smoking: categorical variable with never-smoker defined as no current or previous history of smoking, prior smoker as quitting smoking ≥5 years previously, and current smoker as smoking within 5 years of PJ collection.
ETOH: binary variable for ethanol use, defined as regular ethanol use within 3 years of PJ collection.
NSAID use: binary variable with NSAIDs use defined as any use of aspirin, NSAIDs or Cox-2 inhibitors within 3 months of PJ collection.
Standard EUS criteria: continuous variable of the number of 9 standard EUS criteria of chronic pancreatitis present.
M-ANNHEIM: new international classification of chronic pancreatitis.
Of the 25 patients in the MCCP group, 6 had equivocal to mild changes of CP on CECT (2), MRI/MRCP (2), or pancreatography (2), including 3 with varying degrees of pancreatic atrophy. Eleven of the 25 had a history of idiopathic acute pancreatitis (median 5 episodes), 9 had a history of heavy alcohol consumption, and 1 had a family history of pancreatitis. Seven of the 25 had a peak PJ bicarbonate concentration <80 mEq/l, and 23 had ≥3 EUS criteria of CP. Five MCCP patients had clinical risk factors, EUS findings, and endoscopic pancreatic function test abnormalities consistent with MCCP; 15 met criteria in 2 of these three areas; and 5 met criteria in one area. EUS was the sole criterion for diagnosis of MCCP in three patients.
Figure 1 and Table 1 show pancreatic juice bicarbonate, PGE2, and lipase concentrations for the study groups. PJ PGE2 concentration was elevated in CP (median 307 pg/ml, IQR (249–362)) (P=0.001) and MCCP (median 568 pg/ml, IQR (418–854)) (P=0.0001) compared with healthy controls (median 104, IQR (68–206)). The association between chronic pancreatitis (both CP and MCCP) and higher PGE2 concentrations remained significant after adjusting for PJ lipase and bicarbonate concentrations, history of previous acute pancreatitis, and age (univariate β=0.51, P=0.004; multivariate β=0.48, P=0.005). After substitution of smoking for history of previous acute pancreatitis in the above multivariate model, the association between PGE2 level and chronic pancreatitis remained unchanged (univariate P=0.004, multivariate P=0.005).
Figure 1.
Pancreatic juice PGE2, bicarbonate, and lipase concentrations in the normal, MCCP, and CP groups. Medians and interquartile ranges are presented.
In secondary analyses of the MCCP cohort, PJ PGE2 concentrations in those with previous acute pancreatitis did not significantly differ from those with no previous acute pancreatitis (median 646 vs. 535, respectively) (P=0.09). Among the 25 participants with MCCP, the 5 who met diagnostic criteria in all three domains (clinical, EUS, and PJ bicarbonate) had median PJ PGE2 of 442 pg/ml, the 15 who met criteria in two domains had median PJ PGE2 of 590 pg/ml, and the 5 who met criteria in only one domain had median PJ PGE2 of 525 pg/ml (P=0.56 for comparison between the three groups).
For diagnosis of MCCP, AUROC for bicarbonate (<80 mEq/l) was 0.62, compared with 0.92 for PJ PGE2 (≥ 300 pg/ml). Combining PJ PGE2 and bicarbonate resulted in an AUROC for diagnosis of MCCP of 0.94. For the diagnosis of CP, AUROC for bicarbonate was 0.9, compared with 0.75 for PJ PGE2, and 1.0 for bicarbonate and PJ PGE2 combined.
Discussion
In this pilot study we assessed whether the concentration of an inflammatory mediator in pancreatic juice might be a biomarker for CP and have a role in diagnosis. Because of the risks and limitations of pancreatic biopsy, the current diagnostic paradigm for CP relies on cross-sectional imaging studies for detection of well-established morphologic changes of disease, and pancreatic function tests for detection of exocrine pancreatic insufficiency.1 These diagnostic methods are specific for CP but likely lack sensitivity for detection of early disease. Because chronic pancreatitis is a fibro-inflammatory process, high concentrations of inflammatory mediators might theoretically be present in pancreas juice in both early and advanced stages of disease.
Our finding of significant elevations in PJ PGE2 concentrations in MCCP and CP is biologically plausible.6, 7 The COX-2 enzyme, which produces PGE2, is overexpressed in the pancreatic ducts and acini of patients with chronic pancreatitis.3, 4 PGE2 is a potent inflammatory mediator, and also regulates pancreatic stellate cell profibrotic activity through the EP4 receptor.5 The pivotal role of PGE2 in pancreatitis is supported by the finding that COX-2 inhibition prevents post-ERCP pancreatitis.12
There is no consensus method of diagnosing “minimal change chronic pancreatitis”. The symptom overlap with other gastrointestinal disorders and the debatable accuracy of imaging and functional tests for early disease both contribute to the uncertainty that surrounds this diagnosis, which is associated with considerable morbidity and cost.13 Because the pathology of CP is characterized by fibrosis, acinar cell loss, and inflammation, pancreatic biopsy would seem to be a promising means of diagnosing early disease. However, biopsy has limited utility for diagnosis of MCCP for several reasons: there is histologic overlap between CP and age-related pancreatic fibrosis and acinar cell loss,14, 15 histologic features of CP may not be uniformly distributed in the gland, and pancreatic biopsy is prone to complications.16 EUS detects subtle morphologic changes of the pancreas and may detect MCCP when other tests are normal. However, the significance of EUS findings is debated, and there is concern that EUS may “over diagnose” CP in patients with dyspepsia.2 Newer imaging methods, such as EUS elastography and MR elastography, are promising but not well validated and may also have limited accuracy for early disease.17
Published data support the clinical, functional, and EUS criteria we used to diagnose MCCP. Among patients with normal or equivocal cross-sectional imaging findings, those with clinical risk factors for CP have higher EUS scores for CP and lower peak pancreas juice bicarbonate and lipase concentrations than those with chronic abdominal pain alone.9 Patients with chronic abdominal pain, normal cross-sectional imaging, and abnormal pancreas function test results are more likely to progress to advanced CP over time.18 In histologic correlation studies performed in patients undergoing pancreatic surgery, the finding of ≥3 (of 9) standard EUS features of CP has an overall accuracy of >80% for diagnosis of CP.19, 20, 21 However, these histology studies mainly included patients with CP (not MCCP), and there is only moderate interobserver agreement for EUS interpretation.22, 23 Currently MCCP is best diagnosed by taking clinical risk factors, PFT results, and EUS into account.9
The median PJ PGE2 concentration was higher in our MCCP group than in our CP group, suggesting that most of our MCCP patients had active pancreatic inflammation at the time of pancreas juice collection. It is unclear why PJ PGE2 concentrations were higher in MCCP than that in CP. This might be due to differences in degree of glandular fibrosis or inflammation, or differences in the relative contributions of exocrine and ductal secretions to pancreatic juice. A history of recent acute pancreatitis did not account for the overall elevation of PJ PGE2 levels in participants with CP or MCCP.
Our findings suggest that PJ PGE2 concentration is a biomarker for both CP and MCCP and may be useful for diagnosis of early disease. If validated in future studies, measurement of PJ PGE2 concentration might change the diagnostic paradigm for CP by adding detection of pancreatic inflammation to the current diagnostic tests, which detect morphologic and functional changes. Accurate detection of MCCP is important because invasive therapies are increasingly advocated for this condition. Early diagnosis might also provide opportunities to delay or interrupt the fibro-inflammatory process before non-reversible structural and functional changes occur.
Strengths of this study include its multicenter design comparing three well-defined groups separated by clear and established criteria that included clinical, structural, and functional pancreatic assessments. Limitations include a small sample size and lack of long-term follow-up of MCCP participants. Although age was not a significant determinant of PJ PGE2 concentration in multivariable analysis, the limited age range of our normal controls limits the utility of multivariable analysis. In addition, NSAID use was more common among CP and MCCP patients than normal controls (potentially biasing our results toward the null hypothesis), and we did not collect details of NSAID type and dose. Finally, we did not study important disease control groups such as patients with functional dyspepsia or healthy volunteers with a history of previous acute pancreatitis. For these reasons our findings require validation.
We conclude that PJ PGE2 concentrations are elevated in CP and MCCP. PJ PGE2 concentration may be useful diagnostically. In addition, our findings support the concept that COX-2 inhibition might modify disease progression at early stages.7
Study Highlights
Guarantor of the article: Mark Topazian, MD.
Specific author contributions: BK Abu Dayyeh: conception and design, data analysis, drafting of the manuscript. Darwin Conwell: data collection and analysis, critical revision of the manuscript. NS Buttar: data collection and analysis, critical revision of the manuscript. PA Hart, BL Bick, ST Chari, JE Clain, FC Gleeson, MJ Levy, RK Pearson, BT Petersen, S Suleiman, PA Banks, E Rajan, SS Vege: critical revision of the manuscript. V Kadilaya: data collection. LS Lee: data collection. S Chowdhary: data collection. M Topazian: conception and design, data collection, data analysis, critical revision of the manuscript, overall study supervision. All authors: approved the final version of the manuscript.
Financial support: Division of Gastroenterology and Hepatology, Department of Internal Medicine, Mayo Clinic, Rochester, MN; Harvard Digestive Diseases Center (DC, NIH 5 P30 DK034854-24); and NIH NIDDK (DC, 1R21 DK081703-01A2), Burrill Family Research Grant. These entities did not participate in the conception or design of the study, the analysis or interpretation of data, the writing of the manuscript, or approval of the final manuscript.
Potential competing interests: None.
References
- Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- Fusaroli P, Kypraios D, Caletti G, et al. Pancreatico-biliary endoscopic ultrasound: a systematic review of the levels of evidence, performance and outcomes. World J Gastroenterol. 2012;18:4243–4256. doi: 10.3748/wjg.v18.i32.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliopanos A, Friess H, Kleeff J, et al. Cyclooxygenase 2 expression in chronic pancreatitis: correlation with stage of the disease and diabetes mellitus. Digestion. 2001;64:240–247. doi: 10.1159/000048868. [DOI] [PubMed] [Google Scholar]
- Schlosser W, Schlosser S, Ramadani M, et al. Cyclooxygenase-2 is overexpressed in chronic pancreatitis. Pancreas. 2002;25:26–30. doi: 10.1097/00006676-200207000-00008. [DOI] [PubMed] [Google Scholar]
- Charo C, Holla V, Arumugam T, et al. Prostaglandin E2 regulates pancreatic stellate cell activity via the EP4 receptor. Pancreas. 2012;42:467–474. doi: 10.1097/MPA.0b013e318264d0f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LK, Reding T, Bain M, et al. Prostaglandin E2 modulates TNF-alpha-induced MCP-1 synthesis in pancreatic acinar cells in a PKA-dependent manner. Am J Physiol: Gastrointest Liver Physiol. 2007;293:G1196–G1204. doi: 10.1152/ajpgi.00330.2007. [DOI] [PubMed] [Google Scholar]
- Reding T, Bimmler D, Perren A, et al. A selective COX-2 inhibitor suppresses chronic pancreatitis in an animal model (WBN/Kob rats): significant reduction of macrophage infiltration and fibrosis. Gut. 2006;55:1165–1173. doi: 10.1136/gut.2005.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Lohr JM, Singer MV. The M-ANNHEIM classification of chronic pancreatitis: introduction of a unifying classification system based on a review of previous classifications of the disease. J Gastroenterol. 2007;42:101–119. doi: 10.1007/s00535-006-1945-4. [DOI] [PubMed] [Google Scholar]
- Stevens T, Dumot JA, Zuccaro G, Jr, et al. Evaluation of duct-cell and acinar-cell function and endosonographic abnormalities in patients with suspected chronic pancreatitis. Clin Gastroenterol Hepatol. 2009;7:114–119. doi: 10.1016/j.cgh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Wu B, Conwell DL. The endoscopic pancreatic function test. Am J Gastroenterol. 2009;104:2381–2383. doi: 10.1038/ajg.2008.181. [DOI] [PubMed] [Google Scholar]
- Zhong N, Saenger AK, Topazian M, et al. An automated analyzer provides clinically concordant results to manual back titration for quantitation of bicarbonate in pancreatic juice. Pancreas. 2011;40:422–425. doi: 10.1097/MPA.0b013e318204e89a. [DOI] [PubMed] [Google Scholar]
- Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414–1422. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner TB, Kennedy AT, Gelrud A, et al. Chronic pancreatitis and its effect on employment and health care experience: results of a prospective American multicenter study. Pancreas. 2010;39:498–501. doi: 10.1097/MPA.0b013e3181c5c693. [DOI] [PubMed] [Google Scholar]
- Martin E.Different pathomorphological aspects of pancreatic fibrosis, correlated with etiology: anatomical study of 300 cases Pancreatitis Concepts and Classification, Proceedings of the Second International Symposium on the Classification of Pancreatitis Elsevier Science: Philadelphia; 1984. p77–82. [Google Scholar]
- Pitchumoni CS, Glasser M, Saran RM, et al. Pancreatic fibrosis in chronic alcoholics and nonalcoholics without clinical pancreatitis. Am J Gastroenterol. 1984;79:382–388. [PubMed] [Google Scholar]
- DeWitt J, McGreevy K, LeBlanc J, et al. EUS-guided Trucut biopsy of suspected nonfocal chronic pancreatitis. Gastrointest Endoscopy. 2005;62:76–84. doi: 10.1016/s0016-5107(05)00504-3. [DOI] [PubMed] [Google Scholar]
- Gupte AR, Forsmark CE. Chronic pancreatitis. Curr Opin Gastroenterol. 2014;30:500–505. doi: 10.1097/MOG.0000000000000094. [DOI] [PubMed] [Google Scholar]
- Ketwaroo G, Brown A, Young B, et al. Defining the accuracy of secretin pancreatic function testing in patients with suspected early chronic pancreatitis. Am J Gastroenterol. 2013;108:1360–1366. doi: 10.1038/ajg.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong AK, Hawes RH, Hoffman BJ, et al. Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc. 2007;65:808–814. doi: 10.1016/j.gie.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Varadarajulu S, Eltoum I, Tamhane A, et al. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc. 2007;66:501–509. doi: 10.1016/j.gie.2006.12.043. [DOI] [PubMed] [Google Scholar]
- Albashir S, Bronner MP, Parsi MA, et al. Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: correlation in chronic pancreatitis. Am J Gastroenterol. 2010;105:2498–2503. doi: 10.1038/ajg.2010.274. [DOI] [PubMed] [Google Scholar]
- Wallace MB, Hawes RH, Durkalski V, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc. 2001;53:294–299. doi: 10.1016/s0016-5107(01)70401-4. [DOI] [PubMed] [Google Scholar]
- Gardner TB, Gordon SR. Interobserver agreement for pancreatic endoscopic ultrasonography determined by same day back-to-back examinations. J Clin Gastroenterol. 2011;45:542–545. doi: 10.1097/MCG.0b013e3181f42d69. [DOI] [PubMed] [Google Scholar]