Abstract
Chronic pancreatitis (CP) is a debilitating disease that leads to varying degrees of pancreatic endocrine and exocrine dysfunction. One of the most difficult symptoms of CP is severe abdominal pain, which is often challenging to control with available analgesics and therapies. In the last decade, total pancreatectomy with autologous islet cell transplantation has emerged as a promising treatment for the refractory pain of CP and is currently performed at approximately a dozen centers in the United States. While total pancreatectomy is not a new procedure, the endocrine function-preserving autologous islet cell isolation and re-implantation have made the prospect of total pancreatectomy more acceptable to patients and clinicians. This review will focus on the current status of total pancreatectomy with autologous islet cell transplant including patient selection, technical considerations, and outcomes. As the procedure is performed at an increasing number of centers, this review will highlight opportunities for quality improvement and outcome optimization.
INTRODUCTION
Chronic pancreatitis (CP) is an often painful, debilitating condition that accounts for more than 56,000 hospitalizations annually in the United States.1 However, despite the more widespread availability of diagnostic techniques that allow for earlier identification of disease (such as endoscopic ultrasound and pancreatic function testing), the treatment of CP has not changed appreciably in decades. Historically, treatment has involved medical management, drainage procedures, and in severe cases, total or subtotal pancreatectomy.
However, there is still no pharmacologic therapy that specifically targets the inflammation and/or fibrosis, which leads to the often recalcitrant pain of this disease, although medication such as antioxidants and pancreatic enzyme replacement therapy (PERT) are sometimes used to help treat pain.2 In addition, although endoscopic and surgical drainage can have value for patients with intraductal stones, most patients with CP do not have lesions amenable to a drainage procedure. Segmental pancreatic resection is limited by the usual diffuse parenchymal involvement of CP and total pancreatectomy leads to immediate lifetime dependence on PERT and the development of type 3c diabetes with loss of both insulin and the counter-regulatory hormone glucagon. As a result, most patients with CP have few pharmacologic, endoscopic, or surgical options and many eventually become dependent on chronic opiate therapy to manage their pain symptoms.2
As a means of reducing the risk of type 3c diabetes following total pancreatectomy, in 1977 researchers at the University of Minnesota School of Medicine pioneered the first Total Pancreatectomy with Islet Autologous Transplant (TP-IAT) for the treatment of CP.3 At that time, islet cell isolation techniques, which had been pursued to treat insulin-dependent diabetes via allotransplant, yielded variable results and raised uncertainty regarding the future efficacy of TP-IAT.4 Since then, advances in isolation and purification have improved islet transplant outcomes, and the practice of TP-IAT has expanded.5, 6, 7, 8 In the United States, there are currently approximately 12 centers performing TP-IAT, with 1–2 centers annually establishing programs; there is no available information on the worldwide use of this procedure.
However, despite significant progress, today TP-IAT remains associated with variable outcomes in pain reduction and endocrine functionality. Bearing in mind the risk and irreversible nature of pancreatic resection, the burden of lifelong PERT, potential long-term complications such as gastrointestinal dysmotility, and the variable nature of the pain and islet cell response, physicians must balance several considerations in determining which patients are optimal candidates for TP-IAT. Herein we provide recommendations for patient selection, a detailed review of the procedure, and opportunities for further quality improvement and implementation.
DETERMINING PATIENT ELIGIBILITY
Arguably, the most difficult aspect of the TP-IAT process is determining which patients are likely to benefit from the procedure. Given the risks, irreversible consequences, and variable outcomes of TP-IAT, this determination can often be challenging for the clinician. In fact, selecting patients for an elective TP-IAT represents the penultimate in balancing risk/reward. It is standard of care that a multidisciplinary team comprising of at minimum a medical pancreatologist, pancreatic surgeon, and endocrinologist evaluates each patient and help to determine eligibility. At many centers, pain management, psychiatry, and nutritional expertise is also utilized. We have recently employed laparoscopic pancreas core biopsy as a minimally invasive procedure to establish a histological diagnosis of pancreatitis in select patients where the diagnosis is not firmly established.
Of note, there are no standardized patient selection guidelines endorsed by any of the major gastroenterology, endocrinology, transplant, or surgical societies. However, a consensus conference was held at Pancreasfest 2014, which provided guidance statements around three areas in regard to TP-IAT: (i) Indications and contraindications, (ii) Evaluation and timing of the procedure, and (iii) Following patients after TP-IAT.9 Pertinent recommendations of the committee are shown in Table 1. Note however, that both the evidence level and grade of recommendation for each guidance statement were generally poor.
Table 1. Recommendations from Pancreasfest in regard to indications, contraindications, evaluation, and timing for TP-IAT 9 .
| Guidance statement | Evidence level a | Grade of recommendation |
|---|---|---|
| The primary indication for TP-IAT is to treat intractable pain in patients with impaired quality of life due to CP or RAP in whom medical, endoscopic, or prior surgical therapy have failed | 2a | B |
| TP-IAT should not be performed in patients with active alcoholism, active illicit substance abuse, or untreated/uncontrolled psychiatric illness that could be expected to impair the patient's ability to adhere to a complicated medical management plan…Patients with poor support networks have a relative contraindication due to the cost and complexity of managing diabetes and pancreatic enzyme replacement therapies | 5 | D |
| TP-IAT should not be performed in patients with specific medical conditions, including: c-peptide negative diabetes, type 1 diabetes, portal vein thrombosis, portal hypertension, significant liver disease, high-risk cardio-pulmonary disease, or known pancreatic cancer | 5 | D |
| There are no studies that specifically evaluate contraindications to this procedure. However, TP and TP-IAT are major surgical procedures, with potential operative complications, a prolonged surgical recovery, and an intensive post-operative regimen that includes management of diabetes mellitus and lifelong enzyme therapy for pancreatic enzyme insufficiency | 5 | D |
| The severity, frequency, and duration of pain symptoms, narcotic requirements, disability/impaired quality of life, residual islet function, rate of disease progression, and age of the patient should be considered in timing of the procedure | 5 | D |
| Patients who meet the inclusion criteria (see above) and who are not excluded should be evaluated by a multi-disciplinary team who will review alternative interventions, assess the likelihood of success in reducing pain and preventing or minimizing diabetes, follow the patient through the procedure and provide guidance for long-term care | 5 | D |
| Evaluation should include confirming that pancreatitis is the primary diagnosis, determining that the pain is of pancreatic origin, monitoring the presence of diabetes, assessing beta-cell mass, and assessing the patency of the portal venous system, evaluating for liver disease, and determining immunization status | 5 | D |
CP, chronic pancreatitis; RAP, recurrent acute pancreatitis; TP-IAT, Total Pancreatectomy with Islet Autologous Transplant.
Methods of developing consensus based on the Grading of Recommendations, Assessment, Development, and Evaluation Grid.10
The major indications currently employed by United States TP-IAT centers are discussed below. These apply only in patients with confirmed diagnoses of CP, recurrent acute pancreatitis (RAP), and/or hereditary pancreatitis.
Unresponsive to maximal medical and/or surgical therapy
Before considering TP-IAT, all applicable less-invasive therapies should be attempted. Depending on the patient and the spectrum of disease, management using antioxidant supplementation, pancreatic enzyme replacement, endoscopic decompression and/or stenting, celiac plexus nerve block, and/or opiate regimens can sufficiently relieve symptoms in some patients.11, 12, 13, 14, 15, 16 Only after failure of these therapies should TP-IAT be considered as TP-IAT is often considered as a choice of last resort.
Techniques involving partial resection of the pancreas may successfully treat pain, but should be evaluated with caution, as loss of pancreatic tissue may reduce the islet yield and negatively impact the success of a future TP-IAT.17 Like resection procedures, decompressive procedures such as pancreaticojejunostomy (Puestow) may also relieve pain, but may negatively impact islet yield in those that subsequently undergo TP-IAT.17, 18
Metabolic status—adequate islet function
In addition to exhausting alternative therapies, potential candidates for TP-IAT should undergo metabolic assessment to validate islet function. Patients must be non-diabetic or have C-peptide positive diabetes to retain islet function following TP-IAT.9 So far, attempts to reliably predict post-TP-IAT endocrine function have been unsuccessful, although studies have observed positive correlations between greater islet cell yield and insulin independence. Ahmad et al.17 reported that insulin-independent TP-IAT patients received a significantly higher mean islet yield (6,635 islet equivalents (IEQ)/kg) compared with that of their insulin-dependent group (3,799 IEQ/kg, P=0.04).17 Sutherland et al.19 observed a similar trend, where 72% of patients receiving >5,000 IEq/kg were insulin independent after 3 years, compared with only 22% in a group that received 2,501–5,000 IEq/kg, and 12% in those receiving <2,500 IEq/kg. It should be noted that patients who achieved only partial insulin independence, and thus still benefited from auto-islet transplant, were not included in these statistics. Other factors that have been shown to impact islet yield and may warrant further investigation include gender, alcoholic etiology, and pre-op insulin and C-peptide responses.17, 20, 21
Quality of pain
The etiology of pain in pancreatitis is multifactorial and may originate from acinar necrosis, pseudocyst formation, ductal hypertension, bile duct obstruction, and/or neurogenic inflammation; as such, the presentations of pain vary among patients.2, 9 TP-IAT should be reserved only for those patients with severe episodes of debilitative pain. From an anecdotal perspective, we have seen a much better pain response in patients with intermittent severe (type A) pain rather than chronic daily (type B) pain.
Quality of life
TP-IAT is indicated only in those for whom the post-operative sequelae cannot further diminish their Quality of Life (QOL). This determination is particularly difficult to make, because even mild pancreatitis will negatively impact patients' QOL.22, 23 For this reason, it is recommended that each patient be evaluated in this regard on a case-by-case basis by a multidisciplinary TP-IAT team. Guidelines for defining a severe QOL impairment would include loss of job, inability to attend work or school, or frequent hospitalization.9
The SF-36 and SF-12 questionnaires can be useful both pre- and post-TP-IAT to quantify patients' QOL, and are divided into a physical component summary and mental component summary score. The mean score for healthy individuals is 50, with a standard deviation of 10. Numerous studies have used this scoring system to demonstrate QOL improvement post-TP-IAT (Table 2).
Table 2. Quality of life changes in recent TP-IAT series.
| Source | Patient | n | Survey |
Baseline
|
1-Year
|
||
|---|---|---|---|---|---|---|---|
| PCS | MCS | PCS | MCS | ||||
| Bellin et al.59 | Pediatric | 19 | Sf-36 | 30 | 34 | 50 | 46 |
| Sutherland et al.19 | Adult | 70 | Sf-36 | 29 | 38 | 39 | 47 |
| Morgan et al.42 | Adult | 33 | Sf-12 | 25 | 32 | 36 | 44 |
MCS, mental composite score; PCS, physical composite score; TP-IAT, Total Pancreatectomy with Islet Autologous Transplant.
CONTRAINDICTIONS FOR TP-IAT
Upon meeting the criteria indicating TP-IAT, the ideal TP-IAT candidate must not have any of the following contraindications:
Active substance abuse
Recent estimates indicate that alcohol is the primary etiologic factor in 44–53% of CP cases in the United States.24, 25 It is the second most common factor (after gallstones) in cases of acute pancreatitis, accounting for roughly 36% of cases.26 Therefore, abstinence from alcohol is often a first step in the management of pancreatitis, as its consumption will worsen the condition by a variety of mechanisms, regardless of the initial etiology.27
In addition, the rate of death among alcoholic CP patients is nearly three times higher in those who continue to abuse alcohol after their diagnosis (67 vs. 23%).28 Furthermore, it has even been suggested that those with alcoholic pancreatitis may fare worse than others undergoing TP-IAT in measures of QOL, islet yield, and insulin dependence.20 Thus, to maximize the benefit of TP-IAT, it is critical for the patient to cease alcohol consumption. While no “required” duration of abstinence to qualify for TP-IAT has been defined, many centers have adopted 6 months as a standard based on criteria for liver transplantation.9
Illicit drug use is contraindicated because it may contribute to a patients' inability to comply with or manage the complex post-operative regimen. Additionally, drug-induced pancreatitis is a rare but genuine phenomenon, with poorly understood toxicity mechanisms.29 While almost all cases reports of drug-induced pancreatitis come from patients using prescription medications, a few case reports have strongly linked heavy cannabis use to the onset of acute pancreatitis.30, 31
Pancreatic malignancy
Balzano et al.32 published an international study of 34 patients in which TP-IAT was performed for reasons other than CP, calling for the expansion of indications to include malignancy. Traditionally, this application has been avoided because islet isolation in the presence of multifocal pancreatic cancers could lead to reintroduction of malignant cells.32, 33 Longitudinal research regarding the recurrence rates of pancreatic cancers following autologous islet transplant is lacking, and would be needed to safely validate use of TP-IAT in cases of cancer. For this reason, pancreatic cancer, as well as premalignant cystic disease such as intraductal papillary mucinous neoplasm, remains an absolute contraindication for TP-IAT.
Poorly controlled psychiatric illness
Following TP-IAT, patients may require a complex regimen of enzyme therapy, insulin dependence, and nutritional monitoring. Ensuring the patient is mentally fit to manage the post surgical management process is critical for improving their QOL and health. A strong support network often helps in this regard. The TP-IAT team should take care not to overlook this criterion, as both suicide and narcotic overdose have been reported post-TP-IAT.17 At our center, psychiatric evaluation before TP-IAT is mandatory for all patients.
Cost
Recently, Wilson et al.34 evaluated the cost effectiveness of TP-IAT in patients with minimal change CP, justifying the substantial upfront costs of the procedure. It was found that with TP-IAT, the total cost averaged $153,575 with a survival of 14.9 quality adjusted life years. Comparatively, medical management totaled $196,042 with a survival of only 11.5 quality adjusted life years. However, despite the lower cost and improved survival associated with TP-IAT, many state entitlement programs do not reimburse for the procedure. As such Medicare and Medicaid patients are not accepted at many programs due to insufficient reimbursement.
TP-IAT OPERATIVE PROCEDURE
Pancreatic resection and reconstruction
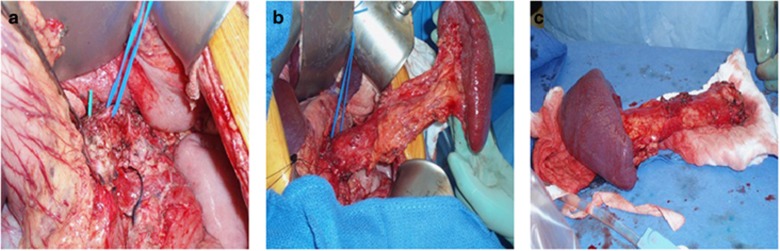
The pancreatectomy procedure will vary depending on the previous surgical history of the patient and the condition of the pancreas, as many patients will have undergone partial resections before completion pancreatectomy. At our institution, we often begin laproscopically to establish pancreatic mobilization, and convert to an open procedure for pancreas explant. For those undergoing a complete total pancreatectomy, the surgical principal is to maintain arterial inflow and venous outflow on the specimen during the dissection to optimize islet perfusion and to avoid warm ischemia. This is accomplished by preservation of the splenic artery and vein and the gastroduodenal artery (GDA) during the resection. Instead of dividing the neck of the pancreas attempts are made to preserve the entire pancreas intact. This is facilitated by developing the plane between the portal vein and the neck of the pancreas to the right of the GDA. The “hanging maneuver” can be employed to provide lateral traction on the head during the uncinate dissection with preservation of the GDA inflow by placing a quarter inch Penrose drain beneath the neck bringing it up superior to the pancreas to the right of the GDA. Once the entire specimen is mobilized from the retroperitoneum and the bile duct, duodenum/stomach and proximal jejunum are divided the splenic artery and vein and GDA are ligated, divided and the specimen is passed off the field for back table preparation (Figure 1).
Figure 1.
Operative pancreatectomy. (a) Intra-operative identification of the splenic artery. (b) Mobilization of the pancreas and spleen. (c) Complete pancreatic explantation.
Regarding reconstruction, a pylorus preserving pancreaticoduodenectomy is preferred to enable direct duodenal anastomosis with the distal segment of duodenum. For patients with significant duodenal resection, roux-en-y choledochojejunostomy or hepaticojejunostomy with gastrostomy is often used alternatively.
Islet cell isolation
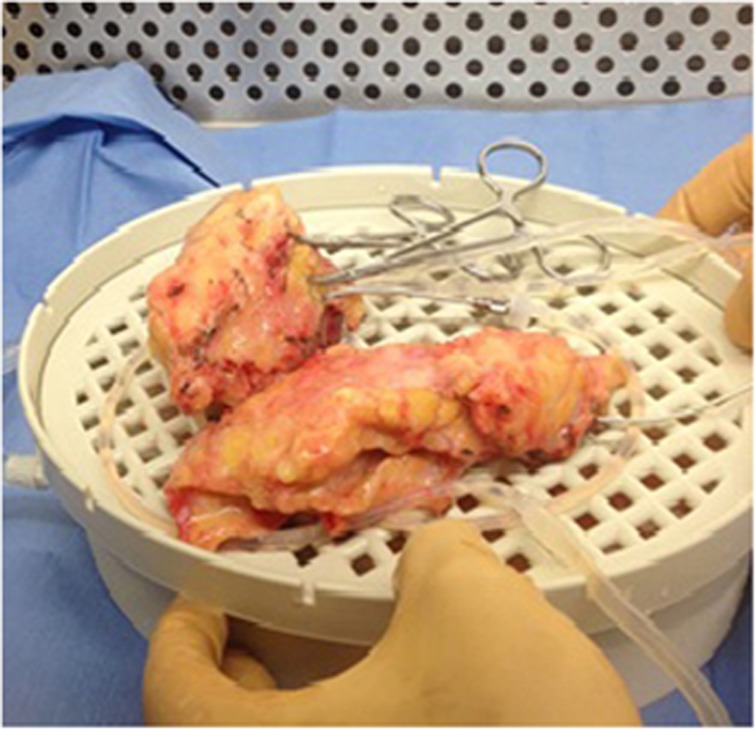
The explanted pancreas is placed immediately into an ice-cold, antibiotic infused, static preservation solution (SPS-1) bath on ice. The splenic artery, GDA, and pancreatic ducts are flushed with SPS-1. Next, the intra-ductal distension method is used to initiate digestion; a warm mixed enzyme solution of collagenase, buffers, and proteases are infused directly into the cannulated pancreatic duct. The parenchyma is then repeatedly injected with mixed enzyme solution under manual pressure generated by a 60 cc syringe to monitor the gland for optimal distension and distribution of the enzyme solution throughout the parenchyma. Next, the gland is manually dissociated using scissors to generate small 5-mm-sized chunks of tissue (Figure 2). The entire collected tissue with enzyme solution is placed into a Ricordi digestion chamber at 37°C. The chamber is either manually or mechanically “shaken” as it is brought to 37°C to initiate further mechanical dissociation and activate enzymatic digestion. Serial samples are taken every 5 min for microscopy inspection to assess islet number, size, and morphology using dithizone staining.
Figure 2.
The pancreas explant after it has been infused with buffering solution and is ready to undergo mechanical digestion.
Upon completion of the digest, the Ricordi system is cooled to 4°C and the digest is collected for centrifugation. Human serum albumin is added to the collection bottle to quench the enzyme preparation, and a series of centrifugations and re-suspensions with Hank's balanced salt solution are used to wash the islets. In cases where islet volume is large, a purification step can be added to reduce the final volume, but this may also reduce the final islet yield. The final pellet is then suspended with 5% human serum albumin and 35 units of Heparin per kilogram of patient body weight.
Islet cell infusion
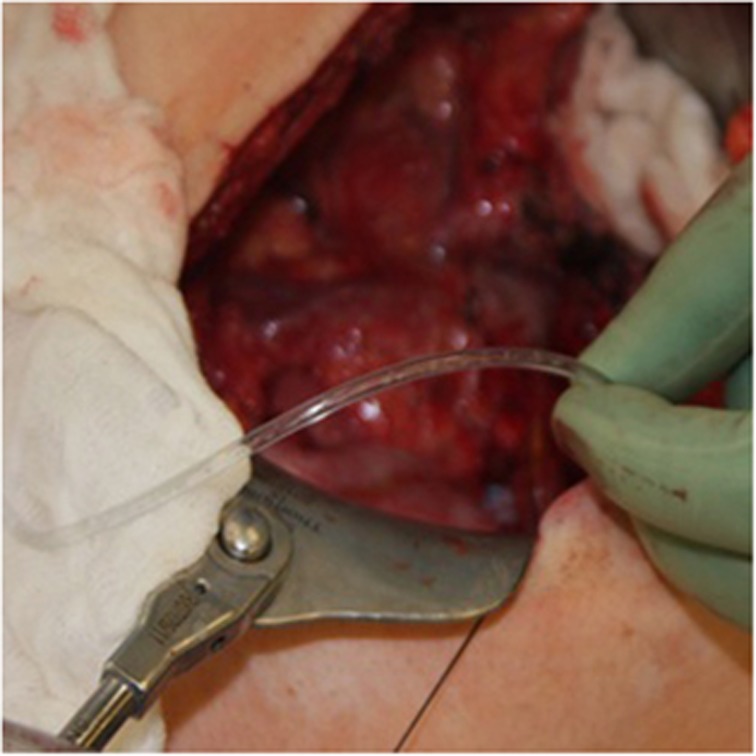
Most centers infuse the islet cells via catheter into the portal vein or mesenteric venous tributaries for engraftment into the liver (Figure 3). Other sites, such as the peritoneum, subcutaneous tissue and kidney capsule, have been considered and are under investigation; however, portal vein infusion has remained the gold standard to date.35, 36, 37 To reduce complication rates of acute portal hypertension and thrombosis, an endotoxin free, low-volume, homogenous cell suspension is infused while the patient is given intravenous heparin.38 We generally give the patient 35 U/kg intravenously and 35 U/kg of Heparin with the islets during portal venous infusion; the patient receives a therapeutic dose of 70 U/kg of heparin. Portal pressures are closely monitored during infusion, as it has been recently demonstrated that the risk of thrombosis increases tenfold (1.52–15.2%) in those with portal pressure changes greater than 25 cm H2O.39
Figure 3.
Islets being infused via arterial-line tubing into the superior mesenteric vein.
Operative risks
The reported complications and their frequencies within recently published TP-IAT series are displayed in Table 3. TP-IAT entails major surgery with risks that may impede post-operative quality of life; i.e., in the largest published series of TP-IAT procedures to date, 15.9% of patients required reoperation.19 Major causes included bleeding (9.5%), anastomotic leak (4.2%), gastrointestinal distress (4.7%), and intra-abdominal infection (1.9%).19 Furthermore, following TP-IAT the patient will have complete pancreatic exocrine insufficiency, necessitating life-long PERT.
Table 3. Post-operative complications in recent TP-IAT series.
| Series | n | Complications reported (n) | |
|---|---|---|---|
| Ahmad et al.17 | 45 | Delayed gastric emptying (4) | Intra-abdominal hematoma (3) |
| Deep vein thrombosis (4) | Pneumonia (1) | ||
| Pulmonary embolism (3) | Intra-abdominal abscess (1) | ||
| Urinary tract infection (3) | Pneumothorax (1) | ||
| Central line infection (3) | Neuropraxia (1) | ||
| Death (3a) | |||
| Morgan et al.42 | 33 | Pneumonia (6) | Respiratory failure (1) |
| Wound infection (4) | Acute renal failure (1) | ||
| Urinary tract infection (3) | Biliary stricture (1) | ||
| Intra-abdominal abscess (2) | Hepatic artery pseudoaneurysm (1) | ||
| Deep vein thrombosis (2) | Portal vein thrombosis (1) | ||
| Sepsis (1) | |||
| Sutton et al.43 (Pediatric) | 16 | Pneumonia (3) | |
| Delayed gastric emptying (1) | |||
| Wilson et al.41 (Pediatric) | 14 | Acute respiratory distress (1) | [Central line associated] bacteremia (1) |
| Pneumonia (1) | |||
| Urinary tract infection (1) | |||
TP-IAT, Total Pancreatectomy with Islet Autologous Transplant.
1 Hepatic failure, 1 narcotic overdose, and 1 suicide.
PATIENT OUTCOMES
Pain
The primary goal of TP-IAT is to control the pain of CP and prevent further episodes of pancreatitis in those with RAP. When compared with other surgical management techniques such as pancreaticoduodenectomy, distal pancreatectomy, or pancreaticojejunostomy, TP-IAT has demonstrated excellent outcomes in regard to pain relief (Table 4). Still, depending on the center performing the surgery, an estimated 10–20% of TP-IAT patients will not achieve significant long-term pain control.9, 40 Moreover, a small subset of patients will report their pain has worsened following TP-IAT; Sutherland et al.19 reported this finding in 3–6% of their cohort.
Table 4. Pain relief following pancreatic resection.
| Source | Procedure | n | % Achieving pain relief (complete+partial) |
|---|---|---|---|
| Jimenez et al.60 | Standard pancreaticoduodenectomy | 33 | 70 |
| Pylorus-preserving pancreaticoduodenectomy | 39 | 60 | |
| Hutchins et al.61 | Distal pancreatectomy | 84 | 57 |
| Bradley62 | Lateral pancreaticojejunostomy | 42 | 66 |
| Caudal pancreaticojejunostomy | 18 | 34 | |
| Beger et al.63 | Duodenum-preserving pancreatic head resection | 479 | 91 |
| Sutherland et al.19 | TP-IAT | 207 | 94 |
TP-IAT, Total Pancreatectomy with Islet Autologous Transplant.
A useful metric in evaluating pain relief is the post-operative reduction in opiate use. Nearly, all TP-IAT patients are chronic opiate users pre-operatively, and there is wide variation in use depending on the patient population and the duration of their pancreatitis. For example, a recent study by Wilson et al.41 found that before operation, their pediatric cohort averaged 32.7 mean morphine equivalents per day. In comparison, Morgan et al.42 published results from an adult cohort where patients averaged 357 mean morphine equivalents per day. Fortunately, for nearly all patients, this level of intensive narcotic use will decrease post-TP-IAT, with 23–82% achieving complete independence9, 19, 41, 42, 43, 44, 45, 46 (Table 5). For some patients, the inability to fully wean from narcotics has been attributed to opiate-induced hyperalgesia, neurological central sensitization, gastrointestinal dysmotility, and/or chronic post surgical pain.9, 19 Factors that have been found to positively correlate with narcotic independence include lower preoperative use and preadolescence (age <13).17, 47 However, a multicenter, prospectively designed modeling tool has never been constructed to pinpoint the patient and disease characteristics that predict the most favorable and negative outcomes.
Table 5. Daily morphine equivalents (ME) pre-TP-IAT and narcotic use post-TP-IAT.
| Source | Year | n | Patient type | Pre-TP-IAT mean ME | Post-TP-IAT % narcotic free |
|---|---|---|---|---|---|
| Sutherland et al.19 | 2012 | 207 | Mixed | N/A | 46% at 1 year |
| Ahmad et al.17 | 2005 | 45 | Mixed | 206 | 58% at last follow-up |
| Morgan et al.42 | 2012 | 33 | Adult | 357 | 23% at 1 year |
| Walsh et al.45 | 2012 | 20 | Adult | 89.2 | 30% at last follow-up |
| Rilo et al.46 | 2003 | 22 | Adult | 78.4 | 82% at last follow-up |
| Sutton et al.43 | 2010 | 16 | Genetic CP | 185 | 63% at last follow-up |
| Wilson et al.41 | 2013 | 14 | Pediatric | 32.7 | 79% at 6 months |
| Chinnakolta et al.44 | 2014 | 75 | Pediatric | N/A | >80% at 1 year |
CP, chronic pancreatitis; TP-IAT, Total Pancreatectomy with Islet Autologous Transplant.
Endocrine function
With regard to endocrine functionality, it is estimated that following autologous islet transplant, about one-third of patients become insulin free and one-third will have partial islet function, requiring only minimal insulin. The remaining third will require standard daily insulin, and about 10% of islet transplants will result in complete graft failure, potentially leaving the patient with unstable type 3c diabetes.21, 48 The unstable “brittle” diabetic state can be especially detrimental to the patient, predisposing them to episodes of wide glycemic excursions and ketoacidosis.
Additionally, the long-term durability of beta cell function remains uncertain. In the University of Minnesota series, among patients who initially achieved insulin independence, nearly half (46%) were no longer independent after 5 years.19 Within this group, higher rates of independence correlated positively with the quantity of islets per kilogram transplanted. Of those with partial post-operative graft function, many regressed to a state of complete dependence over time (58% had partial function at 6 months, while only 33% have partial function at 3 years).19
FUTURE DIRECTIONS
Predictive modeling
Currently, there is no predictive model to determine which patients will have a successful short- or long-term outcome following TP-IAT. While there have been recommendations from Pancreasfest and single institutions, a prospectively derived model needs to be developed.9, 49 In addition, validated shared decision making aids could prove to be invaluable resources for patients in succinctly outlining the risks and benefits of the procedure.
“Remote” TP-IAT isolation
“Remote TP-IAT Isolation” refers to the concept of performing the TP-IAT with collaboration from two sites; one to perform the pancreatectomy, and another to perform the islet cell isolation. This enables centers not equipped with islet isolation facilities to offer TP-IAT to their patients, but comes with several potential drawbacks. Most importantly, it requires that two surgeries be conducted instead of one, greatly increasing total procedure length, sometimes to more than 24 h. Not only is this challenging to coordinate, but the additional transit time is associated with longer periods of cold ischemia and thus potentially a decrease in islet viability.50, 51 Finally, outsourcing the islet isolation is an expensive endeavor due to transportation and laboratory fees in the realm of $50,000.
Intra-operative isolation
In an effort to reduce the reliance of TP-IAT on an FDA-approved isolation facility, several centers have begun performing islet cells intra-operatively. The advantage to this method is that costs are considerably lower per isolation and there is a not the need to maintain an FDA-approved alloislet facility. Comparative effectiveness studies in regard to islet yields and patient outcomes have yet to be performed comparing standard and intra-operative isolation.
Investigational therapeutics to maximize islet yield
Interleukin-8 receptor inhibition
It is believed that intraportal micro-thrombosis or transient ischemia following islet infusion may contribute significantly to graft loss due to the secretion of tissue factor by the islet cells in response to the clotting reaction.52 This process has been termed instant blood mediated inflammatory injury, and has been estimated to account for the loss of up to 60–80% of transplanted islets.53 The drug Reparixin, an Interleukin-8 (CXCL8) receptor inhibitor, is currently in phase III clinical trials and targets this inflammatory pathway to improve islet engraftment. In its recent phase II trial, patients receiving allogenic islet transplants with Reparixin demonstrated significantly higher C-peptide levels, higher islet estimated function, and lower insulin requirements compared with the control group.54
Glucagon-like peptide 1 analogs
Glucagon-like peptide 1 (GLP-1) analogs have been pursued for many years as adjuvant therapy to boost transplanted islet function. It is hypothesized that the denervation of islets during the isolation procedure is responsible for a reduced response to incretins like GLP-1, and that exposure to GLP-1 analogs early in transplant therapy may improve function in islet transplant recipients.55 Specifically, Exenatide, an inhibitor of Dipeptidyl peptidase 4, acts to prevent degradation of GLP-1 and has been shown to stimulate insulin secretion, protect beta cells from apoptotic mechanisms, and even promote beta cell regeneration; it has thus been proposed as a adjunctive pre-treatment in islet cell transplantation and is still under investigation.56
Non-hepatic sites of implantation
The peritoneum
It has been previously suggested that recipients of intrahepatic islet transplant do not respond to hypoglycemia with adequate glucagon secretion by alpha cells, placing recipients at risk for severe hypoglycemia unawareness.57 Perhaps, the most accepted theory that could explain a subpar alpha response is that hypoglycemia-induced glycogen breakdown increases glucose locally within the liver, and thus could negate systemic signals of hypoglycemia to alpha cells residing in the hepatic parenchyma. However, recent work by these groups has also shown intra-hepatic alpha cells to respond sufficiently to intravenous arginine stimulation, calling the functionality of alpha cells post-TP-IAT into question.58
In light of this issue, the peritoneum is currently under investigation as a promising secondary transplant site for islet recipients. Bellin et al.59 found that in patients in whom a portion of islets were transplanted into the peritoneal cavity, acute glucagon responses to arginine stimulation were comparable to those of a normal control, whereas the response was completely absent in patients that received only intra-hepatic transplant.
CONCLUSION
Although first performed in 1977, it is only in the last decade that TP-IAT has gained worldwide attention and adoption as a therapy for painful CP. Despite very promising results in certain series, it is a drastic intervention that should only be considered in highly selected patients. While progress has been made to refine operative technique, islet manipulation and patient identification, there is still much progress to be made. Currently, many patients are rendered insulin dependent following TP-IAT and up to 15% have no improvement in their pain. This is a procedure for patients who have few other options, and who must be prepared to trade one disease (chronic pain) for potentially another (diabetes).
Study Highlights
Guarantor of the article: Timothy B. Gardner, MD, MS.
Specific author contributions: All authors had an equal role in conceiving, initiating, and writing up the research project.
Financial support: None.
Potential competing interests: None.
References
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- Forsmark CE. Management of chronic pancreatitis. Gastroenterology. 2013;144:1282–1291. doi: 10.1053/j.gastro.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am. 1978;58:365–382. doi: 10.1016/s0039-6109(16)41489-1. [DOI] [PubMed] [Google Scholar]
- Sutherland DE, Steffes MW, Bauer GE, et al. Isolation of human and porcine islets of Langerhans and islet transplantation in pigs. J Surg Res. 1974;16:102–111. doi: 10.1016/0022-4804(74)90017-1. [DOI] [PubMed] [Google Scholar]
- Linetsky E, Bottino R, Lehmann R, et al. Improved human islet isolation using a new enzyme blend, liberase. Diabetes. 1997;46:1120–1123. doi: 10.2337/diab.46.7.1120. [DOI] [PubMed] [Google Scholar]
- Nano R, Clissi B, Melzi R, et al. Islet isolation for allotransplantation: variables associated with successful islet yield and graft function. Diabetologia. 2005;48:906–912. doi: 10.1007/s00125-005-1725-3. [DOI] [PubMed] [Google Scholar]
- O'Gorman D, Kin T, Imes S, et al. Comparison of human islet isolation outcomes using a new mammalian tissue-free enzyme versus collagenase NB-1. Transplantation. 2010;90:255–259. doi: 10.1097/TP.0b013e3181e117ce. [DOI] [PubMed] [Google Scholar]
- Balamurugan AN, Loganathan G, Bellin MD, et al. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplantation. 2012;93:693–702. doi: 10.1097/TP.0b013e318247281b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellin MD, Freeman ML, Gelrud A, et al. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology. 2014;14:27–35. doi: 10.1016/j.pan.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke R, Guyatt GH, Dellinger P, et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ. 2008;337:a744. doi: 10.1136/bmj.a744. [DOI] [PubMed] [Google Scholar]
- Burton F, Alkaade S, Collins D, et al. Use and perceived effectiveness of non-analgesic medical therapies for chronic pancreatitis in the United States. Aliment Pharmacol Ther. 2011;33:149–159. doi: 10.1111/j.1365-2036.2010.04491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstead NS, Wilcox CM. Clinical trials of pancreatic enzyme replacement for painful chronic pancreatitis—a review. Pancreatology. 2009;9:344–350. doi: 10.1159/000212086. [DOI] [PubMed] [Google Scholar]
- Whitcomb DC, Lehman GA, Vasileva G, et al. Pancrelipase delayed-release capsules (CREON) for exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatic surgery: a double-blind randomized trial. Am J Gastroenterol. 2010;105:2276–2286. doi: 10.1038/ajg.2010.201. [DOI] [PubMed] [Google Scholar]
- Clarke B, Slivka A, Tomizawa Y, et al. Endoscopic therapy is effective for patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2012;10:795–802. doi: 10.1016/j.cgh.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman M, Singh G, Das S, et al. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127–134. doi: 10.1097/MCG.0b013e3181bb854d. [DOI] [PubMed] [Google Scholar]
- Toouli J, Brooke-Smith M, Bassi C, et al. Guidelines for the management of acute pancreatitis. J Gastroenterol Hepatol. 2002;17:S15–S39. doi: 10.1046/j.1440-1746.17.s1.2.x. [DOI] [PubMed] [Google Scholar]
- Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg. 2005;201:680–687. doi: 10.1016/j.jamcollsurg.2005.06.268. [DOI] [PubMed] [Google Scholar]
- Gruessner RW, Sutherland DE, Dunn DL, et al. Transplant options for patients undergoing total pancreatectomy for chronic pancreatitis. J Am Coll Surg. 2004;198:559–567. doi: 10.1016/j.jamcollsurg.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214:409–424. doi: 10.1016/j.jamcollsurg.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunderdale J, McAuliffe JC, McNeal SF, et al. Should pancreatectomy with islet cell autotransplantation in patients with chronic alcoholic pancreatitis be abandoned. J Am Coll Surg. 2013;216:591–596. doi: 10.1016/j.jamcollsurg.2012.12.043. [DOI] [PubMed] [Google Scholar]
- Lundberg R, Beilman G, Dunn T, et al. Metabolic assessment prior to total pancreatectomy and islet autotransplant: utility, limitations and potential. Am J Transplant. 2013;13:2664–2671. doi: 10.1111/ajt.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokrowiecka A, Pinkowski D, Malecka-Panas E. Assessment of quality of life in patients with chronic pancreatitis. Med Sci Monit. 2011;17:CR583–CR588. doi: 10.12659/MSM.881985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzilli R, Morselli Labate A, Ceciliato R, et al. Quality of life in patients with chronic pancreatitis. Dig Liver Dis. 2005;37:181–189. doi: 10.1016/j.dld.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Yadav D, Timmons L, Benson JT, et al. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol. 2011;106:2192–2199. doi: 10.1038/ajg.2011.328. [DOI] [PubMed] [Google Scholar]
- Coté GA, Yadav D, Slivka A, et al. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2011;9:266–273. doi: 10.1016/j.cgh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frossard J, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- LaRusch J, Solomon S, Whitcomb DC, et al. Pancreatitis overviewGeneReviews®. [Internet]. Seattle (WA): University of Washington, Seattle1993–2014. [PubMed]
- Ammann RW, Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology. 1999;116:1132–1140. doi: 10.1016/s0016-5085(99)70016-8. [DOI] [PubMed] [Google Scholar]
- Badalov N, Baradarian R, Iswara K, et al. Drug-induced acute pancreatitis: an evidence-based review Clin Gastroenterol Hepatol 20075648–661.e3. [DOI] [PubMed] [Google Scholar]
- Wargo KA, Geveden BN, McConnell VJ. Cannabinoid-induced pancreatitis: a case series. JOP. 2007;8:579–583. [PubMed] [Google Scholar]
- Grant P, Gandhi P. A case of cannabis-induced pancreatitis. JOP. 2004;5:41–43. [PubMed] [Google Scholar]
- Balzano G, Piemonti L. Autologous islet transplantation in patients requiring pancreatectomy for neoplasm. Curr Diab Rep. 2014;14:1–10. doi: 10.1007/s11892-014-0512-2. [DOI] [PubMed] [Google Scholar]
- Dudeja V, Beilman GJ, Vickers SM. Total pancreatectomy with islet autotransplantation in patients with malignancy: are we there yet. Ann Surg. 2013;258:219–220. doi: 10.1097/SLA.0b013e31829c4a1b. [DOI] [PubMed] [Google Scholar]
- Wilson GC, Ahmad SA, Schauer DP, et al. Cost-effectiveness of total pancreatectomy and islet cell autotransplantation for the treatment of minimal change chronic pancreatitis. J Gastrointest Surg. 2014. pp. 1–10. [DOI] [PubMed]
- Merani S, Toso C, Emamaullee J, et al. Optimal implantation site for pancreatic islet transplantation. Br J Surg. 2008;95:1449–1461. doi: 10.1002/bjs.6391. [DOI] [PubMed] [Google Scholar]
- Mellgren A, Landström AS, Petersson B, et al. The renal subcapsular site offers better growth conditions for transplanted mouse pancreatic islet cells than the liver or spleen. Diabetologia. 1986;29:670–672. doi: 10.1007/BF00869269. [DOI] [PubMed] [Google Scholar]
- Gores P, Rabe F, Sutherland D. Prolonged survival of intraportal versus subrenal capsular transplanted islet allografts. Transplantation. 1987;43:747–749. [PubMed] [Google Scholar]
- Casey JJ, Lakey JR, Ryan EA, et al. Portal venous pressure changes after sequential clinical islet transplantation. Transplantation. 2002;74:913–915. doi: 10.1097/00007890-200210150-00002. [DOI] [PubMed] [Google Scholar]
- Wilhelm JJ, Bellin M, Dunn T, et al. Proposed thresholds for pancreatic tissue volume for safe intraportal islet autotransplantation after total pancreatectomy. Am J Transplant. 2013;13:3183–3191. doi: 10.1111/ajt.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farney AC, Sutherland DE. Islet autotransplantation. Pancreas Islet Stem Cell Transplant Diab. 2010. p. 413.
- Wilson GC, Sutton JM, Salehi M, et al. Surgical outcomes after total pancreatectomy and islet cell autotransplantation in pediatric patients. Surgery. 2013;154:777–784. doi: 10.1016/j.surg.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Morgan K, Owczarski SM, Borckardt J, et al. Pain control and quality of life after pancreatectomy with islet autotransplantation for chronic pancreatitis. J Gastrointest Surg. 2012;16:129–134. doi: 10.1007/s11605-011-1744-y. [DOI] [PubMed] [Google Scholar]
- Sutton JM, Schmulewitz N, Sussman JJ, et al. Total pancreatectomy and islet cell autotransplantation as a means of treating patients with genetically linked pancreatitis. Surgery. 2010;148:676–686. doi: 10.1016/j.surg.2010.07.043. [DOI] [PubMed] [Google Scholar]
- Chinnakotla S, Bellin MD, Schwarzenberg SJ, et al. Total pancreatectomy and islet autotransplantation in children for chronic pancreatitis: indication, surgical techniques, postoperative management, and long-term outcomes. Ann Surg. 2014;260:56–64. doi: 10.1097/SLA.0000000000000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh R, Saavedra JA, Lentz G, et al. Improved quality of life following total pancreatectomy and auto-islet transplantation for chronic pancreatitis. J Gastrointest Surg. 2012;16:1469–1477. doi: 10.1007/s11605-012-1914-6. [DOI] [PubMed] [Google Scholar]
- Rilo HLR, Ahmad SA, D'Alessio D, et al. Total pancreatectomy and autologous islet cell transplantation as a means to treat severe chronic pancreatitis. J Gastrointest Surg. 2003;7:978–989. doi: 10.1016/j.gassur.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Bellin MD, Carlson AM, Kobayashi T, et al. Outcome after pancreatectomy and islet autotransplantation in a pediatric population. J Pediatr Gastroenterol Nutr. 2008;47:37–44. doi: 10.1097/MPG.0b013e31815cbaf9. [DOI] [PubMed] [Google Scholar]
- Sutherland DE, Gruessner AC, Carlson AM, et al. Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation. 2008;86:1799–1802. doi: 10.1097/TP.0b013e31819143ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clin Gastroenterol Hepatol. 2011;9:793–799. doi: 10.1016/j.cgh.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pileggi A, Ribeiro M, Hogan A, et al. Effects of pancreas cold ischemia on islet function and quality. Transplant Proc. 2009;41:1808–1809. doi: 10.1016/j.transproceed.2009.03.075. [DOI] [PubMed] [Google Scholar]
- Lakey JR, Warnock GL, Rajotte RV, et al. Variables in organ donors that affect the recovery of human islets of langerhans1. Transplantation. 1996;61:1047–1053. doi: 10.1097/00007890-199604150-00010. [DOI] [PubMed] [Google Scholar]
- Moberg L, Johansson H, Lukinius A, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360:2039–2045. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- Citro A, Cantarelli E, Piemonti L. Anti-inflammatory strategies to enhance islet engraftment and survival. Curr Diab Rep. 2013;13:733–744. doi: 10.1007/s11892-013-0401-0. [DOI] [PubMed] [Google Scholar]
- Citro A, Cantarelli E, Maffi P, et al. CXCR1/2 inhibition enhances pancreatic islet survival after transplantation. J Clin Invest. 2012;122:3647–3651. doi: 10.1172/JCI63089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vethakkan SR, Walters JM, Gooley JL, et al. The incretin response after successful islet transplantation. Transplantation. 2014;97:e9–e11. doi: 10.1097/01.TP.0000437565.15965.67. [DOI] [PubMed] [Google Scholar]
- Buss JL, Rajab A, Essig ED, et al. Exenatide pretreatment improved graft function in nonhuman primate islet recipients compared to treatment after transplant only. J Transplant. 2012;2012 doi: 10.1155/2012/382518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paty BW, Ryan EA, Shapiro AM, et al. Intrahepatic islet transplantation in type 1 diabetic patients does not restore hypoglycemic hormonal counterregulation or symptom recognition after insulin independence. Diabetes. 2002;51:3428–3434. doi: 10.2337/diabetes.51.12.3428. [DOI] [PubMed] [Google Scholar]
- Robertson RP, Bogachus LD, Oseid E, et al. Assessment of beta-cell mass and alpha- and beta-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes. 2014. [DOI] [PMC free article] [PubMed]
- Bellin M, Parazzoli S, Oseid E, et al. Defective glucagon secretion during hypoglycemia after intrahepatic but not nonhepatic islet autotransplantation. Am J Transplant. 2014;14:1880–1886. doi: 10.1111/ajt.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez RE, Fernandez-del Castillo C, Rattner DW, et al. Outcome of pancreaticoduodenectomy with pylorus preservation or with antrectomy in the treatment of chronic pancreatitis. Ann Surg. 2000;231:293–300. doi: 10.1097/00000658-200003000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins RR, Hart RS, Pacifico M, et al. Long-term results of distal pancreatectomy for chronic pancreatitis in 90 patients. Ann Surg. 2002;236:612–618. doi: 10.1097/00000658-200211000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley EL., III Long-term results of pancreatojejunostomy in patients with chronic pancreatitis. Am J Surg. 1987;153:207–213. doi: 10.1016/0002-9610(87)90816-6. [DOI] [PubMed] [Google Scholar]
- Beger HG, Schlosser W, Friess HM, et al. Duodenum-preserving head resection in chronic pancreatitis changes the natural course of the disease: a single-center 26-year experience Ann Surg 19992305129; discussion 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]